Abstract
We describe the highly conserved sequence 56–68 of the HIV Nef protein as the first promiscuous HLA-DQ HIV-derived peptide. The Nef peptide exhibits an albeit rare capacity to bind 6 different HLA-DQ molecules whereas no binding is observed with the 10 HLA-DR molecules tested. In agreement with these data, after immunization with the Nef peptide, HLA-DQ transgenic Aβ° mice display a vigorous cellular and humoral response while the specific immune response of HLA-DR expressing mice is minimal. The promiscuous potentiality of the Nef 56–68 peptide in humans has been confirmed by ex vivo immunization experiments with CD4+ T cells from 14 healthy donors expressing different HLA genotypes. Nef 56–68 specific CD4+ T cells rapidly acquire a memory cell phenotype and are characterized by the preferential usage of the TCR Vβ 6·1 gene segment and predominant production of IFN-γ. Taken together, these data indicate that the Nef 56–68 peptide constitutes an attractive component of vaccines aiming at inducing or enhancing HIV-specific T cell immunity.
Keywords: epitope, HLA-transgenic mice, human, Th1 cells, T cell receptor
INTRODUCTION
Combination chemotherapy referred to as highly active antiretroviral treatment (HAART) dramatically suppresses human immunodeficiency virus type 1 replication and has thereby contributed substantially to reducing AIDS-related opportunistic infections and death [1]. However, recent studies have shown that, even in the presence of optimal HAART, when the virus is undetectable for several years in plasma, replication-competent provirus persists in ‘resting ‘memory T cells, with continuous transcription and evolution of HIV [2–5]. Moreover, since HAART regimens are complex and frequently associated with side-effects, long-term compliance with treatment is difficult, increasing the risk of development of drug-resistant virus. So, effective cellular and/or humoral immune responses against HIV-1 need to be actively maintained as a form of adjunctive therapy to HAART.
Protective immunity will probably depend upon both the production of neutralizing antibodies and the generation of HIV-specific cytotoxic T cells. In HIV-infected patients, however, CTL function is lost as the disease progresses, possibly because of the absence of HIV-specific CD4+ T cell help [6]. In the rare cases in which the CD4+ response to HIV antigens is persistently detectable, strong anti-HIV CTL responses are maintained and are associated with the control of viral replication and a good prognosis [7,8]. Although the majority of HIV+ patients progress towards AIDS, a minority does not show signs of disease and maintains stable CD4 counts despite a long-lasting infection [9]. The observation of relatively strong HIV-specific CD4+ T cell responses in these long-term nonprogressors compared with responses in progressive disease [8] has confirmed the idea that such a response may be the critical missing link in progressive infection. Thus, induction or restoration of HIV-specific CD4+ T cell responses in infected patients might be important for both treatment and prevention of HIV disease.
Much effort has been put into the identification of universal T helper (Th) epitopes capable of enhancing the induction of T cell immunity in a wide variety of subjects displaying different HLA types [10–12]. But the advantage in using epitopes from HIV itself is that natural boosting can occur early after exposure, whereas this might not occur if the helper epitopes are not virus-specific. Several conserved HIV-specific HLA-DR-restricted T helper epitopes exhibiting both high affinity and immunogenicity have been identified [13–15]. However, the targeting of HLA-DQ-restricted response also represents an interesting complementary approach in the identification of vaccine candidates. Regarding the Nef regulatory protein, T and B cell epitopes have previously been described particularly in the region 56–68 [16,17]. The ability of this peptide to bind HLA class II DQ and DR molecules and to recruit naïve T cells by immunization in vivo in HLA-DQ and -DR transgenic Aβ° mice and ex vivo using CD4+ T cells from HLA-typed healthy donors was evaluated. We describe a new HIV-derived peptide, presented by at least six different HLA-DQ molecules, that is capable of priming an HIV-specific Th1 response. Moreover, our ex vivo immunization protocol allowed us to rapidly induce memory, IFN-γ producing, CD4+ T helper cells. Consequently, our search for Nef-derived Th epitopes resulted in the identification of an interesting candidate for vaccine strategy and/or cellular immunotherapy in HIV-infected patients.
MATERIALS AND METHODS
Peptides
Nef 56–68 (AWLEAQEEEEVGF), TT 830–846 (QYIKANSK FIGITELKK), MHC I α 46–63 (EPRAPWIEQEGPEYWDQE), DQB 45–57 (ADVEVYRAVTPLGPPD), Ig 44–60 (DTLRSYY ADWYQQKPG), INS 1–15 A (FVNQHLAGSHLVEAL), B7 150–164 (LNEDLRSWTAADTAA) peptides were synthesized on an Advanced ChemTech model 357 MPS Synthesizer (Advanced Chemtech Europe, Brussels, Belgium) as previously described [18]. Homogeneity was confirmed by analytical HPLC.
HLA class II/peptide binding assays
EBV homozygous cell lines were used as source of human HLA class II molecules [19]. As previously described [20], purified HLA-DR and HLA-DQ molecules were incubated with a referenced biotinylated peptide in the presence of serial dilutions of Nef 56–68 competitor peptide. Data are expressed as the peptide concentration that prevented binding of 50% of the labelled peptide (IC50). Average and SE values were deduced from at least three independent experiments.
HLA-transgenic Aβ° mice
Mice expressing different HLA alleles (HLA-DR2, HLA-DQ6 and HLA-DQ8) and deficient in murine class II molecules (Aβ°) were a kind gift of Dr Ch. David (Mayo Clinic Rochester, MI, USA) [21]. Mice expressing the HLA-DR1 transgene on an FVB/N background were kindly provided by Dr D. Altmann (Hammersmith Hospital, London, UK) [22] and backcrossed with Aβ° mice [23]. HLA-transgenic Aβ° mice were immunized s.c. with Nef 56–68 peptide (50 μg) in CFA (Sigma-Aldrich, Saint Quentin Fallavier, France) and two booster injections with peptide (25 μg) in IFA (Sigma-Aldrich) at 2 weekly intervals were performed. The proliferative response was measured as previously described [24] by incubating 5 × 105 splenic or lymph node cells, removed seven days after the last injection, with an optimal concentration of Nef 56–68 peptide (25 μg/ml) and testing the cell culture supernatants for cytokine release.
Antibody and cytokine detection
The quantification was performed by ELISA as previously described [24]. Mouse sera were diluted 1/100 for IgG1 and 1/10 for IgG2a and IgG2b detection and peroxidase labelled antimouse IgG1(dilution 1/3000) or IgG2a (dilution 1/2000) were provided by Diagnostic Pasteur (Marnes-la-Coquette, France). IL-4 and IFN-γ in the sera (dilution 1/10) and IL-2, IL-4, IL-5, IL-10 and IFN-γ in the supernatants were detected using sandwich ELISA. The antibody pairs used for the detection of mouse and human IL-2, IL-4, IL-5, IL-10 and IFN-γ were provided by BD PharMingen (San Diego, CA, USA). Absorbances at 492 nm were measured using a multichannel spectrophotometer (Titertek Multiskan MCC 1340). Results were expressed as the mean of duplicate wells after subtraction of the background.
Blood donors
Blood was collected from healthy, adult HIV-uninfected individuals. Donors were informed of the details of the study and signed an appropriate consent form according to the guidelines for research volunteers. HLA typing was performed by E.T.S. (Lille, France) using standard serotyping assays.
Dendritic cell generation
PBMCs were isolated from heparinized whole blood, CD14+ cells separated by high gradient magnetic sorting (VARIOMACS, Miltenyi Biotech GmbH, Bergish Gladbach, Germany) [25] and cultured for 5 days at a cell density of 1 × 106 cells/ml in a cell culture bag (AFC, Columbia, MA, USA) in RPMI 1640 supplemented with 10% human AB+ serum (pool of 3 sera, E.T.S). Differentiation into dendritic cells (DC) was obtained by addition of rhIL-4 (1000 U/ml) and rhGM-CSF (800 U/ml) (Peprotech Inc, Rocky Hill, NJ, USA). After 5 days, the cells expressed a typical dendritic cell phenotype with high levels of CD11c (99%), HLA class II (99%), CD86 (99%) and CD40 (76%) molecules. They showed very limited expression of CD1a (2%), CD80 (10%) and the CD83 maturation marker (6%) and no longer expressed the monocyte lineage marker CD14 (0·5%).
Ex vivo immunization of CD4+ T cells
CD4+ T cells were separated by negative selection using the VARIOMACS technique (Miltenyi) from CD14− cells cultured for 5 days in RPMI 1640 supplemented with 10% human AB+ serum. 1 × 106 cells were immunized ex vivo with Nef 56–68 peptide (50 μg/ml) in the presence of DCs (1 × 106). After 15 days of culture in RPMI 1640 supplemented with 10% human AB+ serum, 15 day cycles of restimulation with the peptide were performed using as APCs, first DCs and then B cells.
Naive (CD45RA) and memory (CD45RO) T cells were isolated from 10 day peptide-immunized CD4+ T cells by depletion using the VARIOMACS technique (Miltenyi), stimulated with Nef 56–68 peptide (50 μg/ml) for 48 h and the culture supernatants were tested for cytokine production.
Cytofluorimetric cell-surface phenotyping
Cell staining was performed using FITC- or PE-conjugated and affinity purified mouse moAb for direct labelling. For DC phenotyping, moAb anti-CD1a, anti-CD11c, anti-CD14, anti-CD40, anti-CD80, anti-CD83, anti-CD86 and anti-HLA-DR were purchased from BD Pharmingen. For T cell phenotyping, moAb anti-CD3, anti-CD25, anti-CD28, anti-CD30, antiCD40L, anti-CD45RA, anti-CD45RO, anti-CD62L and anti-CD69 were obtained from Caltag (Burlingame, CA, USA).
T cell clones
A cloning feeder cell mixture was prepared by adding together 5 × 105 cells/ml of irradiated (4000 rad) PBMC from any healthy donor, 5 × 104 cells/ml of an irradiated (5000 rad) EBV-LCL, and 50 ng/ml PHA and used for serial dilutions of Nef 56–68 specific T cells, recovered 10 days after the first restimulation with the peptide, in 96-well round-bottom plates as previously described [26]. Culture medium containing rIL-2 (20 U/ml)(Peprotech Inc) was added after 5 days of culture. After identification of growing T-cell clones, usually after 10–14 days of culture, the cells were transferred to a 24-well plate in the presence of fresh culture medium and with a 2× feeder cell mixture to expand the cells.
TCR analysis
Total cellular RNA was isolated from CD4+ T cell lines or clones and then reverse transcribed to cDNA by MMLV reverse transcriptase (Gibco BRL, France). PCR analysis of the TCR Vβ repertoire was accomplished as previously described by Genevée et al.[27].
RESULTS
Conservation of the Nef 56–68 sequence
As shown in Table 1, the 56–68 region of the HIV Nef protein corresponds to a region conserved among various HIV isolates referred to in the Data Bank (GenBank). Indeed, the native sequence is represented at a frequency of about 55% in the 620 sequences of Nef evaluated and in the other cases only single amino acid mutations were observed.
Table 1.
Sequences of Nef 56–68 peptide published in databases*
| A | W | L | E | A | Q | E | E | – | E | E | V | G | F | 55.3% |
| X | 9.2% | |||||||||||||
| D | – | 7% | ||||||||||||
| – | D | 1.8% | ||||||||||||
| D | – | D | 1.4% | |||||||||||
| Others | each < 1.4% |
research of homologies on 620 sequences published in PubMed
High binding capacity of Nef 56–68 peptide to HLA-DQ alleles
To first evaluate the potency of the peptide 56–68 to stimulate T cells in humans, we investigated its capacity to bind a large number of HLA class II molecules. More precisely, it was submitted to binding assays specific for 10 HLA-DR and 7 HLA-DQ alleles which are all frequently encountered in the Caucasian population (Table 2). Data were expressed as IC50 but also as relative activity. The latter is the ratio between the peptide and a highly active peptide (reference peptide). It therefore allows a comparison between the binding activity to relevant peptides, such as T cell epitopes, and naturally processed peptides. The lower the ratio is, the closer the Nef peptide is to the reference peptide and hence the higher is its affinity. In this context, we observed that the Nef 56–68 peptide exhibited good binding to 6/7 HLA-DQ alleles. It was especially active towards DQ2 and DQ8 since it was more active for these alleles than the corresponding reference peptide and presented low IC50, which are close to or below 100 nm. Only the DQ7 allele bound at a very high concentration and hence with low efficiency. Low binding efficiency was also observed for the HLA-DR molecules. IC50 were mostly greater than 10 000 nm and the relative activities were at least higher by a factor of 77. The Nef peptide was only moderately active on HLA-DR11, its mid-activity being in the micromolar range and one hundred fold less active than the reference peptide. Taken together, the results suggest that Nef 56–68 peptide appears to be a promiscuous ligand for HLA-DQ molecules preferentially.
Table 2.
Binding capacities of Nef 56–68 peptide to HLA class II molecules
| Alleles | Allelic frequencies (%) | Referenced peptide | Referenced peptide IC50 (nM) | Nef 56–68 peptide IC50 (nM) | Ratio | Binding | |
|---|---|---|---|---|---|---|---|
| DQ2 | DQA1*0201/DQB1*0201 | 12·4/23·1 | MHC I α46–63 | 620 (±150) | 1100 (±188) | 1·8 | + |
| DQ2 | DQA1*0501/DQB1*0201 | 27·4/23·1 | MHC I α46–63 | 340 (±160) | 180 (±30) | 0·5 | +*Τ*+ |
| DQ7 | DQA1*0501/DQB1*0301 | 27·4/18·5 | DQB45–57 | 60 (±20) | 31000 (±18000) | 517 | − |
| DQ8 | DQA1*0301/DQB1*0302 | 14·2/8·1 | DQB45–57 | 1150 (±350) | 44 (±9) | 0·04 | +*Τ*+ |
| DQ5 | DQA1*0101/DQB1*0505 | 17/14·9 | Ig44–60 | 620 (±150) | 3500 (±1800) | 5·6 | + |
| DQ6 | DQA1*0102/DQB1*0602 | 15·8/9·8 | INS1–15A | 170 (±35) | 3100 (±760) | 18 | + |
| DQ6 | DQA1*0103/DQB1*0603 | 6·2/5·8 | B7150–164 | 2200 (±760) | 24000 (±8660) | 11 | + |
| DR1 | DRB1*0101 | 9·3 | HA306–318[20] | 6 (±1) | >10000 | >1666 | − |
| DR3 | DRB1*0301 | 10·9 | MT2–16[20] | 120 (±40) | >10000 | >84 | − |
| DR4 | DRB1*0401 | 5·6 | HA306–318[20] | 30 (±30) | >10000 | >333 | − |
| DR7 | DRB1*0701 | 14·0 | YKL [20] | 130 (±40) | >10000 | >77 | − |
| DR11 | DRB1*1101 | 9·2 | HA306–318[20] | 20 (±4) | 2200 (±*Τ*350) | 110 | − |
| DR13 | DRB1*1301 | 6·0 | B121–36[20] | 1000 (±0) | >100000 | >100 | − |
| DR15 | DRB1*1501 | 8·0 | A3152–166[20] | 30 (±17) | >10000 | >333 | − |
| DR51 | DRB5*0101 | 7·9 | HA306–318[20] | 10 (±0) | >10000 | >1000 | − |
| DR52 | DRB3*0101 | 9·2 | 14·166 [20] | 10 (±0) | >10000 | >1000 | − |
| DR53 | DRB4*0101 | 28·4 | E2/E7 [20] | 4 (±1) | >10000 | >2500 | − |
Nef 56–68 peptide is highly immunogenic in HLA-DQ transgenic Aβ° mice
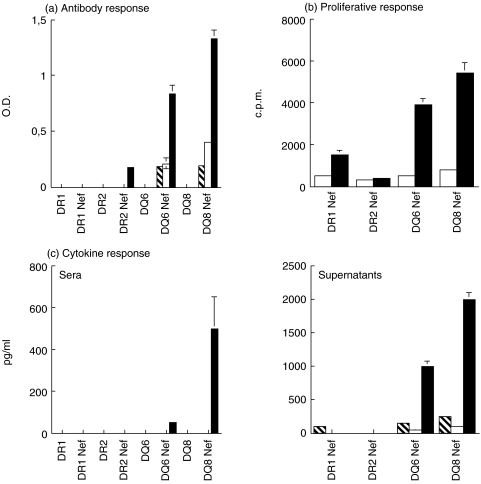
In agreement with the binding data, HLA-DQ (in particular DQ8) transgenic (Tg) Aβ° mice showed vigorous cellular and humoral responses after immunization with the Nef 56–68 peptide while HLA-DR Aβ° mice exhibited only a very limited responses (Fig. 1).
Fig. 1.
Response of HLA-transgenic Aβ° mice to Nef 56–68 peptide. (a) Antibody isotypes were determined in pools of mouse sera (n = 5) 35 days after immunization with or without 50 μg of Nef 56–68 peptide.  IgG1, □ IgG2a, ▪ IgG2b. (b) Proliferative response of splenic cells recovered 35 days after immunization with 50 μg of Nef 56–68 peptide and stimulated in vitro with 25 μg/ml of Nef 56–68 peptide (▪), control without in vitro stimulation with Nef peptide (□). (c) Cytokine (
IgG1, □ IgG2a, ▪ IgG2b. (b) Proliferative response of splenic cells recovered 35 days after immunization with 50 μg of Nef 56–68 peptide and stimulated in vitro with 25 μg/ml of Nef 56–68 peptide (▪), control without in vitro stimulation with Nef peptide (□). (c) Cytokine ( IL-2; □ IL-4; ▪ IFN-γ) secretion was evaluated in pools of mouse sera (n = 5) 35 days after immunization with or without 50 μg of Nef 56–68 peptide and in 48 h culture supernatants of splenic cells recovered 35 days after immunization and stimulated in vitro with 25 μg/ml of Nef 56–68 peptide. n = 3 series of experiments.
IL-2; □ IL-4; ▪ IFN-γ) secretion was evaluated in pools of mouse sera (n = 5) 35 days after immunization with or without 50 μg of Nef 56–68 peptide and in 48 h culture supernatants of splenic cells recovered 35 days after immunization and stimulated in vitro with 25 μg/ml of Nef 56–68 peptide. n = 3 series of experiments.
So, Nef 56–68 immunized-DQ6 and -DQ8 Tg mice responded with the predominant production of IgG2b Abs (Fig. 1a), no antibody production being observed in the control non-immunized mice. The cells from immunized DQ Tg mice showed a significant proliferative response associated with predominant IFN-γ secretion after in vitro specific restimulation with the Nef 56–68 peptide (Fig. 1b,c) whereas cells from control non-immunized mice did not (data not shown). The presence of IFN-γ was also detected in the sera of Nef-peptide-immunized DQ8 and DQ6 Tg mice (Fig. 1c)
In contrast, after immunization with the Nef 56–68 peptide, only small amounts of IgG2b Abs were detected in the sera of DR2 Tg mice (Fig. 1a) and a weak peptide-specific proliferative response, associated with minimal production of IL-2, was observed in DR1 Tg mice (Fig. 1b).
HLA-DQ dependent induction of memory CD4 Th1 cells
As a preclinical study, we performed ex vivo immunizations of CD4+ T cells from a cohort of 14 HLA-typed healthy donors with Nef 56–68 peptide in the presence of autologous DCs as antigen presenting cells and cytokine production was evaluated.
All the donors, expressing various HLA genotypes, showed a response to the Nef 56–68 peptide demonstrating its promiscuous potential in humans. Thus, 48 h after immunization with the peptide, CD4+ T cells displayed a Th1 phenotype with a predominant production of IFN-γ associated either with or without IL2 production. IL-4 and IL-5 secretions were never observed (Table 3). This profile was conserved after specific restimulation of CD4+ T cells with Nef 56–68 peptide 15 days later (data not shown). Confirming in the human model the DQ dependence of the response to the Nef 56–68 peptide which was suspected in the mouse model, this production of Th1 cytokines was inhibited by about 80% when ex vivo immunizations with peptide were performed in the presence of an anti HLA-DQ antibody (20 μg/ml) whereas an anti HLA-DR antibody was without effect (data not shown).
Table 3.
Type 1 cytokine secretion after ex vivo immunization with Nef 56–68 peptide
| Cytokines (pg/ml) | ||||||||
|---|---|---|---|---|---|---|---|---|
| IL-2 | IL-4 | IL-5 | IFN-γ | |||||
| Donor | – | Peptide | – | Peptide | – | Peptide | – | Peptide |
| 1. DR1/DR1; DQ6/DQ3 | <250 | 1000 | <250 | <250 | <250 | <250 | <250 | 2300 |
| 2. DR1/DR3; DQ5/DQ2 | <250 | <250 | <250 | <250 | <250 | <250 | <250 | 2500 |
| 3. DR3/DR4; DQ2/DQ3 | <250 | 2400 | <250 | <250 | <250 | <250 | <250 | <250 |
| 4. DR3/DR11; DQ2/DQ3 | <250 | <250 | <250 | <250 | <250 | <250 | <250 | 7500 |
| 5. DR3/DR11; DQ2/DQ3 | <250 | <250 | <250 | <250 | <250 | <250 | <250 | 2500 |
| 6. DR4/DR7; DQ2/DQ5 | <250 | <250 | <250 | <250 | <250 | <250 | <250 | 2000 |
| 7. DR4/DR13; DQ3/DQ6 | <250 | 2400 | <250 | <250 | <250 | <250 | <250 | 1800 |
| 8. DR4/DR13; DQ3/DQ3 | <250 | 7500 | <250 | <250 | <250 | <250 | <250 | 10000 |
| 9. DR7/DR9; DQ2/DQ3 | <250 | 5000 | <250 | <250 | <250 | <250 | <250 | 20000 |
| 10. DR13/DR8; DQ6/DQ4 | <250 | 8500 | <250 | <250 | <250 | <250 | <250 | 2500 |
| 11. DR13/DR11; DQ3/DQ3 | <250 | <250 | <250 | <250 | <250 | <250 | <250 | 2600 |
| 12. DR15/DR3; DQ6/DQ2 | <250 | <250 | <250 | <250 | <250 | <250 | <250 | 3100 |
| 13. DR15/DR4; DQ6/DQ3 | <250 | 7000 | <250 | <250 | <250 | <250 | <250 | 10000 |
| 14. DR15/DR13; DQ6/DQ6 | <250 | <250 | <250 | <250 | <250 | <250 | <250 | 8000 |
IL-2, IL-4, IL-5 and IFN-γ productions were evaluated in 48 h-culture supernatant of CD4+ T cells immunized or not ex vivo with 50 μg/ml of Nef 56–68 peptide in the presence of autologous DCs as APCs (n = 14 donors).
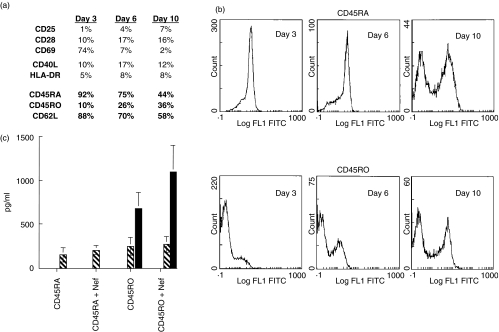
Three days after ex vivo immunization with the Nef 56–68 peptide, CD4+ T cells from naïve donors expressed the CD69, CD28 and CD40L activation markers (Fig. 2a). From day 6 after activation, we observed a strong decrease in CD62L expression and the induction of the CD45RO molecule, which was expressed on 36% of CD4+ T cells at day 10 (Fig. 2a,b), whereas the ratio CD45RA/CD45RO was not modified in unstimulated cells (data not shown). So, a memory T cell response was rapidly (under 10 days) induced after stimulation with the Nef 56–68 peptide. At this time of culture, after separation of CD45RA+ and CD45RO+ T cells, production of IFN-γ was observed only in the memory population and amplified after specific restimulation with the Nef 56–68 peptide (Fig. 2c).
Fig. 2.
Human memory CD4+ T cell specific for Nef 56–68 peptide. (a) Phenotypic characteristics of CD4+ T cells from HLA-typed healthy donors (n = 6) 3, 6 and 10 days after ex vivo immunization with 50 μg/ml of Nef 56–68 peptide in the presence of DCs as APCs. This phenotype is representative for the 6 donors. (b) Expression of CD45RA+ and CD45RO+ populations in Nef 56–68 specific CD4+ T cells from 3 to 10 days after ex vivo immunization. Representative profile for the 6 donors. (c) Cytokine secretion ( IL-2; □ IL-4;
IL-2; □ IL-4;  IL-5;
IL-5;  IL-10; ▪ IFN-γ) in Nef 56–68 specific CD45RA+ and CD45RO+ CD4+ T cells stimulated with or without 50 μg/ml of peptide 10 days after ex vivo immunization.
IL-10; ▪ IFN-γ) in Nef 56–68 specific CD45RA+ and CD45RO+ CD4+ T cells stimulated with or without 50 μg/ml of peptide 10 days after ex vivo immunization.
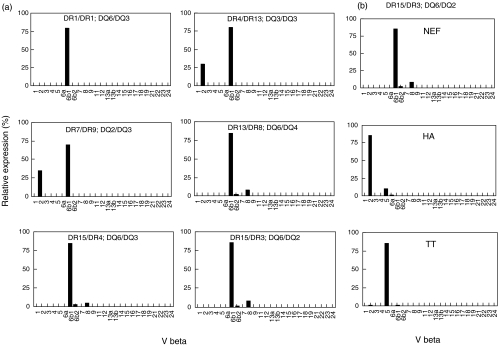
Preferential usage of Vβ6·1 by Nef 56–68 specific T cell clones
Finally, we derived Nef 56–68 specific T cell clones from 6 of the 14 HLA-typed healthy donors. These T cell clones exhibited the same characteristic phenotype and Th1 cytokine profile described above (data not shown). They strikingly expressed the same TCR Vβ6·1 gene segment although they were derived from donors expressing different HLA genotypes (Fig. 3a). This Vβ6·1 recruitment was, however, specific for the Nef 56–68 peptide since using the same experimental procedure, Th1 clones specific for two other promiscuous peptides (HA 307–319 and TT 830–846) expressed different TCR Vβ usage (Vβ2 and Vβ5, respectively) (Fig. 3b).
Fig. 3.
Restricted usage of Vβ6·1 by Nef 56–68 T cell clones Amplification of specific Vβ gene segments was performed by PCR. (a) In Nef 56–68 specific CD4+ clones derived from 6 different HLA-typed healthy donors. (b) In Nef 56–68, HA 307–319 and TT 830–846 specific CD4+ clones derived from the same donor. The relative amount of TCR Vβ gene transcripts was described as the ratio (%) of each Vβ to total Vβ.
DISCUSSION
In this study, we gave particular interest to the sequence 56–68 of the Nef regulatory protein which exhibited both antigenic and immunogenic properties for T and B cells as described in rodents (mice and rat) and in nonhuman primates (chimpanzee) [16,17]. Interestingly, this sequence is a conserved region of the HIV Nef protein represented among various HIV strains. Our search for Nef-derived Th epitopes, identified the peptide 56–68 as the first promiscuous HLA-DQ HIV-derived peptide capable of priming an HIV-specific Th1 response. The promiscuous peptides described so far, such as TT 830–846, HA 307–319 or other HIV-derived peptides [13–15,28,29] are restricted to HLA-DR molecules. In fact a large number of HLA-DR molecules are known to share common criteria regard their interaction with antigenic peptides [30] which may account for the existence of promiscuous HLA-DR epitopes. Similarities have been already highlighted for the HLA-DQ molecules [31,32] but none of the previously described peptides bound as many HLA-DQ molecules as the Nef 56–68 peptide. In particular, this peptide is more active than the reference peptides in binding to HLA-DQ2 and HLA-DQ8 molecules, demonstrating a high affinity for these molecules.
This wide specificity for HLA-DQ molecules may result from the presence of four consecutive glutamic acid residues within the nef peptide sequence. Indeed, glutamic acid, situated at the C- terminal part of the peptide, may represent an advantageous anchor candidate for the P6/7 to P9 pocket of different HLA-DQ alleles [31,32]. Such a pattern strongly suggests the existence of an HLA-DQ supermotif. In contrast, the glutamic residue generally appears to be a deleterious residue for P6 to P9 HLA-DR contact and hence may dramatically diminish the capacity of Nef 56–68 to bind HLA-DR molecules [30].
As a result, this peptide led to a vigorous cellular and humoral response in HLA-DQ6 and DQ8 mice while the response was very weak in HLA-DR transgenic mice. Accordingly, similar conclusions emerged from ex vivo immunization with the Nef 56–68 peptide using cells from 14 healthy donors expressing different HLA genotypes. All immunizations led to proliferation mediated by HLA-DQ molecules. Interestingly, the Nef 56–68 specific T cells displayed a type 1 cytokine secretion phenotype. This type 1 profile, obtained systematically, was assigned to the usage of DCs derived from peripheral blood monocytes: in the human system, myeloid DCs have been shown to generate Th1 responses whereas lymphoid/plasmacytoid DCs generate Th2 responses [33]. However, it has recently been reported that, depending on the DC culture conditions and activation signals, DCs may acquire the capacity to induce either Th1, Th2 or Th0/Tr1 (T regulatory) T-cell responses [34,35]. This seems to indicate that the type of the T-cell response induced by DCs depends on the nature of the DC-activating stimulus, and less on their ontogeny. Consequently, this Th1-biased production was probably favoured by our experimental conditions and the use of immature DCs.
The Nef 56–68-specific cells rapidly display a memory T cell phenotype, quantified by a strong diminution of CD62L and the appearance of the CD45RO marker. They also produced IFN-γ after restimulation with Nef 56–68 and could be considered as ‘effector memory’ T cells [36]. These cells lack lymph node homing receptors but express receptors to enter into inflamed tissues; therefore, their function is to provide immediate protection to contain pathogens in peripheral tissues.
Surprisingly, all T cell clones obtained after ex vivo immunization with Nef 56–68 were found to express the same TCR Vβ6·1 gene segment, despite the fact that they were derived from donors expressing different HLA genotypes. This preferential usage was, however, specific for the Nef 56–68 peptide as T cell clones specific for two other peptides, also described as promiscuous (HA 307–319 and TT 830–846), expressed different TCR Vβ usage. Previous studies in both mice and humans have frequently shown a limited usage of V gene segments in TCR that recognize defined peptide/MHC complexes. In some cases usage was correlated with MHC restriction or antigen specificity [37–39]. It seemed difficult to associate the preferential Vβ6·1 expression of the Nef-specific clones with the DQ promiscuous property of the peptide since the 6 donors showed various DQ-genotypes. This biased TCR gene usage may reflect a relatively low precursor frequency of high avidity T cells [40]. Furthermore, in peptide-specific T cell responses, TCR have often been found with CDR3 loops that are similar in both length and amino acid composition, suggesting that particular junctional sequences have an important role in determining the specificity for individual peptide/MHC complexes. It is possible that the peptide influences V gene usage, while the MHC may be flexible enough to interact with either the V and/or CDR3 region of the TCR.
The aim of this work was to identify an HIV-derived peptide able to react with several HLA molecules and therefore be potentially immunogenic in numerous individuals. We identified peptide 56–68 from the Nef protein as a promiscuous HLA-DQ peptide that could potentially be used as a component of a multiepitopic vaccine for the treatment of HIV infection, in addition to CTL epitopes for example. Moreover we were able to induce HIV-specific memory CD4+ T cells producing IFN-γ in all the healthy donors tested and, in the context of HIV-infection, this appears to be of considerable interest. Although HAART appears to partially reconstitute immunity to microbial antigens no effect on enhancing immune responses to HIV antigens in chronic infection [41] has so far been demonstrated. Moreover a dominant Th1 cytokine profile is associated with a lack of progression in HIV infection [42]. We propose, using an ex vivo immunization protocol with the Nef 56–68 peptide, to induce/reconstitute an HIV-specific protective response in HIV-infected patients. Studies will be underway shortly with a cohort of HIV+ patients who have stable CD4 counts and in whom the T cell response seems not to differ from that of healthy donors. Consequently, we could envisage the adoptive transfer of autologous CD4+ T cells bearing the protective phenotype in addition (or alternatively) to HAART as an alternative strategy for treatment of HIV infection.
Acknowledgments
We thank Dr H. Groux for critical review of the manuscript. This work was supported by the Centre National de la Recherche Scientifique, the Pasteur Institute of Lille, the University of Lille II and by ANRS grants.
REFERENCES
- 1.Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 3.Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active anti-retroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 4.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1991;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finzi D, Blankson J, Siliciano JD, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nature Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 6.Musey LK, Krieger JN, Hugues JP, Schacker TW, Corey L, McElath MJ. Early and persistent human immunodeficiency virus type 1 (HIV-1) -specific T helper dysfunction in blood and lymph nodes following acute HIV-1 infection. J Infect Dis. 1999;180:278–84. doi: 10.1086/314868. [DOI] [PubMed] [Google Scholar]
- 7.Harrer T, Harrer E, Kalams SA, et al. Strong cytotoxic T cell and weak neutralizing antibody responses in a subset of persons with stable non-progressing HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:585–92. doi: 10.1089/aid.1996.12.585. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg ES, Billingsley JM, Caliendo AM, Boswell SL, Sax PE, Kalams SA, Walker BD. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 9.Levy JA. HIV pathogenesis and long term survival. AIDS. 1993;7:1401–18. doi: 10.1097/00002030-199311000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Panina-Bordignon P, Tan A, Termijtelen A, Demotz S, Corradin G, Lanzavecchia A. Universally immunogenic T cell epitopes: promiscuous binding to human MHC class II and promiscuous recognition by T cells. Eur J Immunol. 1989;19:2237–42. doi: 10.1002/eji.1830191209. [DOI] [PubMed] [Google Scholar]
- 11.Alexander J, Sidney J, Southwood S, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–61. doi: 10.1016/s1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 12.Calvo-Calle JM, Hammer J, Sinigaglia F, Clavijo P, Moya-Castro ZR, Nardin EH. Binding of malaria T cell epitopes to DR and DQ molecules in vitro correlates with immunogenicity in vivo: identification of a universal T cell epitope in the Plasmodium falciparum circumsporozoite protein. J Immunol. 1997;159:1362–73. [PubMed] [Google Scholar]
- 13.Berzofsky JA, Pendleton CD, Clerici M, Ahlers J, Lucey DR, Putney SD, Shearer GM. Construction of peptides encompassing multideterminant clusters of human immunodeficiency virus envelope to induce in vitro T cell responses in mice and human of multiple MHC types. J Clin Invest. 1991;88:876–84. doi: 10.1172/JCI115389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Burg SH, Kwappenberg KMC, Geluk A, et al. Identification of a conserved universal Th epitope in HIV-1 reverse transcriptase that is processed and presented to HIV-specific CD4+ T cells by at least four unrelated HLA-DR molecules. J Immunol. 1999;162:152–60. [PubMed] [Google Scholar]
- 15.Wilson CC, Palmer B, Southwood S, et al. Identification and antigenicity of broadly cross-reactive and conserved human immunodeficiency virus type 1-derived helper T lymphocyte epitopes. J Virol. 2001;75:4195–207. doi: 10.1128/JVI.75.9.4195-4207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estaquier J, Boutillon C, Ameisen JC, et al. T helper cell epitopes of the human Immunodeficiency virus (HIV-1) nef protein in rats and chimpanzees. Molec Immunol. 1992;29:489–99. doi: 10.1016/0161-5890(92)90006-j. [DOI] [PubMed] [Google Scholar]
- 17.Estaquier J, Boutillon C, Ameisen JC, et al. Determination of B-cell epitopes of nef HIV-1 protein: immunogenicity related to their structure. Molec Immunol. 1992;29:1337–45. doi: 10.1016/0161-5890(92)90170-3. [DOI] [PubMed] [Google Scholar]
- 18.Gausepohl H, Kraft M, Boulin C, Frank RW. Frmoc solid phase peptide synthesis. In: Rivier JE, Marshall GR, editors. Proceedings of the 11th American Peptide Symposium. Leiden: ESCOM; 1990. pp. 1003–4. [Google Scholar]
- 19.Gorga JC, Horejsi V, Johnson DR, Raghupathy R, Strominger JL. Purification and characterization of class II histocompatibility antigens from a homozygous human B cell line. J Biol Chem. 1987;262:16087–94. [PubMed] [Google Scholar]
- 20.Texier C, Pouvelle S, Busson M, Herve M, Charron D, Menez A, Maillère B. HLA-DR restricted peptide candidates for bee venom immunotherapy. J Immunol. 2000;164:3177–84. doi: 10.4049/jimmunol.164.6.3177. [DOI] [PubMed] [Google Scholar]
- 21.Taneja V, David CS. HLA transgenic mice as humanized mouse models of disease and immunity. J Clin Invest. 1998;101:921–6. doi: 10.1172/JCI2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosloniec EF, Brand DD, Myers LK, et al. An HLA-DR1 transgene confers susceptibility to collagen-induced arthritis elicited with human type II collagen. J Exp Med. 1997;185:1113–22. doi: 10.1084/jem.185.6.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cosgrove D, Gray D, Dierich A, Kaufman J, Lemeur M, Benoist C, Mathis D. Mice lacking MHC Class II molecules. Cell. 1991;66:1051–66. doi: 10.1016/0092-8674(91)90448-8. [DOI] [PubMed] [Google Scholar]
- 24.Pancré V, Delacre M, Herno J, Auriault C. Schistosomal egg antigen-responsive CD8 T cell population in Schistosoma mansoni-infected BALB/c mice. Immunol. 1999;98:525–34. doi: 10.1046/j.1365-2567.1999.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pickl WF, Majdic O, Stockl J, Riedl E, Scheinecker C, Bello- Fernandez C, Knapp W. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996;157:3850–8. [PubMed] [Google Scholar]
- 26.Yssel H. Generation and cloning of antigen-specific human T cells. In: Jones GE, editor. Methods in Molecular Medical Human Cell Culture Protocols. Totowa, NJ: Humana Press Inc; 1996. pp. 121–35. [DOI] [PubMed] [Google Scholar]
- 27.Genevée C, Diu A, Nierat J, et al. An experimentally validated panel of subfamily-specific oligonucleotide primers (Vα1-w29/ Vβ1-w24) for the study of human T cell receptor variable V gene segment usage by polymerase chain reaction. Eur J Immunol. 1992;22:1261–9. doi: 10.1002/eji.1830220522. [DOI] [PubMed] [Google Scholar]
- 28.O'Sullivan D, Sydney J, Del Guercio MF, Colon SM, Sette A. On the interaction of promiscuous antigenic peptides with different DR alleles. Identification of common structural motifs. J Immunol. 1991;147:2663–9. [PubMed] [Google Scholar]
- 29.Hammer J, Valsasnini P, Tolba K, Bolin D, Higelin J, Takacs B, Sinigaglia F. Promiscuous and allele-specific anchors in HLA-DR binding peptides. Cell. 1993;74:197–203. doi: 10.1016/0092-8674(93)90306-b. [DOI] [PubMed] [Google Scholar]
- 30.Sturniolo T, Bono E, Ding J, et al. Generation of tissue-specific and promiscuous HLA ligand databases using DNA microarrays and virtual HLA class II matrices. Nat Biotechnol. 1999;17:555–61. doi: 10.1038/9858. [DOI] [PubMed] [Google Scholar]
- 31.Khalil-Daher I, Boisgerault F, Feugeas JP, Tieng V, Toubert A, Charron D. Naturally processed peptides from HLA-DQ7 (alpha1*0501-beta1*0301). influence of both alpha and beta chain polymorphism in the HLA-DQ peptide binding specificity. Eur J Immunol. 1998;28:3840–9. doi: 10.1002/(SICI)1521-4141(199811)28:11<3840::AID-IMMU3840>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 32.Johansen BH, Vartdal F, Eriksen JA, Thorsby E, Sollid LM. Identification of a putative motif for binding of peptides to HLA-DQ2. Int Immunol. 1996;8:177–82. doi: 10.1093/intimm/8.2.177. [DOI] [PubMed] [Google Scholar]
- 33.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 34.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–12. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 35.Kapsenberg ML, Kalinski P. The concept of type 1 and type 2 antigen-presenting cells. Immunol Lett. 1999;69:5–6. doi: 10.1016/s0165-2478(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 36.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: Intermediates, Effectors and Memory cells. Science. 2000;290:92–7. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 37.Boitel B, Ermonval M, Panina-Bordignon P, Mariuzza RA, Lanzavecchia A, Acuto O. Preferential Vβ usage and lack of junctional sequence conservation among human T cell receptors specific for a tetanus toxin-derived peptide: evidence for a dominant role of germline-encoded V region in antigen/major histocompatibility complex recognition. J Exp Med. 1992;175:765–77. doi: 10.1084/jem.175.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones CM, Lake RA, Lamb JR, Faith A. Degeneracy of T cell receptor recognition of an influenza virus hemagglutinin epitope restricted by HLA-DQ and – DR class II molecules. Eur J Immunol. 1994;24:1137–42. doi: 10.1002/eji.1830240519. [DOI] [PubMed] [Google Scholar]
- 39.Hawes GE, Struyk L, Beacock-Sharp H, Henwood J, Hill Gaston JS, van den Elsen PJ. Conserved TCR beta chain usage for a single MHC class II-restricted heat shock protein peptide. Immunogenetics. 1998;48:196–201. doi: 10.1007/s002510050423. [DOI] [PubMed] [Google Scholar]
- 40.Yassine-Diab B, Carmichael P, L’Faqihi FE, Lombardi G, Deacock S, de Preval C, Coppin H, Lechler RI. Biased T-cell receptor usage is associated with allelic variation in the MHC class II peptide binding groove. Immunogenetics. 1999;49:532–40. doi: 10.1007/s002510050531. [DOI] [PubMed] [Google Scholar]
- 41.Rinaldo CR, Liebmann JM, Huang XL, et al. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J Infect Dis. 1999;179:329–36. doi: 10.1086/314599. [DOI] [PubMed] [Google Scholar]
- 42.Clerici M, Balotta C, Meroni L, et al. Type 1 cytokine production and low prevalence of viral isolation correlate with Long-Term Nonprogression in HIV-infection. AIDS Res Hum Retroviruses. 1996;12:1053–61. doi: 10.1089/aid.1996.12.1053. [DOI] [PubMed] [Google Scholar]