Abstract
Dysregulation of apoptosis through the Fas-Fas ligand pathway is relevant in autoimmune disease onset. We recently reported elevated serum levels of sFas in patients with silicosis, systemic sclerosis (SSC) and systemic lupus erythematosus (SLE), and proposed a block of apoptosis in the pathogenesis. The disturbance of apoptosis in lymphocytes including autoreactive clones could induce autoantibody production. Since autoantibodies directed against unknown antigens are present in the sera of these patients, the sera samples were examined for the presence of autoantibodies directed to caspase-8.
Using Western blotting, autoantibodies against caspase-8 were detected in healthy individuals and in over 60% of patients. Using epitope mapping employing 12 amino acid polypeptides with SPOTs system, a minimum of 4 epitopes and a maximum of 13 were found, which implied that epitope spreading was in progress. It is noteworthy that two important catalytic cystein residues were included within the epitopes; firstly the active site cystein Cys287, and secondly Cys360 located in the unique pentapeptide motif QACQG.
Using recombinant human caspase-8 linked protein chip array, autoantibodies were identified and molecular weight determined. The antibodies were mainly IgG; 80% were subclass IgG1λ; 20% were IgG4κ. Despite the ratio of human light chain κ:λ = 2:1, the predominance of IgG1λ is noticeable.
Anti-caspase-8 autoantibodies are detectable in healthy individuals and in patients suffering silicosis, SSc or SLE. A few epitopes were detected in healthy individuals compared to those suffering autoimmune diseases, indicating the intramolecular epitope spreading. Relationship of autoantibodies and the clinical background of the patients requires clarification.
Keywords: caspase-8, autoantibody, epitope mapping
INTRODUCTION
Dysfunction of apoptosis through the Fas-Fas ligand pathway is related to the onset of autoimmune disease. Previously, we reported elevated serum levels of sFas in patients suffering silicosis, systemic sclerosis (SSC) and systemic lupus erythematosus (SLE) and proposed that the blocking of apoptosis is involved in the pathogenesis of these diseases [1].
Since autoantibodies against unknown antigens are present in the serum of these patients, their sera have now been analysed for autoantibodies directed against caspase enzymes, and we now report the detection of anti-caspase-8 autoantibodies. In healthy individuals, caspase-8 is recruited to the ‘death-inducing signal complex’ (DISC), a multiprotein complex that forms rapidly on the cytoplasmic portion of the Fas/APO-1/CD95 receptor [2]. However, patients with inherited mutations in CD95 demonstrate the resultant defects in caspase activation that causes them to suffer autoimmune lymphoproliferative syndrome (ALPS) [3,4].
SPOTs system is a helpful technology that allows the solid-phase synthesis of extensive series of short peptides for the systematic analysis of antibody epitopes [5]. In the present study we used the SPOTs system to achieve epitope mapping of anti-caspase-8 autoantibodies, and a total of 14 epitopes were detected on the human caspase-8 molecule.
ProteinChip surface enhanced laser desorption/ionization (SELDI) mass spectrometry can be used to detect a few femtomoles of a specific protein from a crude solution and determine its molecular mass with an error rate of less than 0·2%[6]. Recombinant human caspase-8 was linked to the PS2 chips then autoantibodies were captured from the serum samples and detected. To our knowledge, these findings represent the first demonstration of anti-caspase-8 autoantibody epitope mapping. Furthermore, we have shown that intramolecular epitope spreading detectable among anti-caspase-8 autoantibodies in the sera of patients suffering silicosis, SSc and SLE and in healthy individuals.
MATERIALS AND METHODS
Patients
A total of 91 serum samples were obtained from silicosis patients lacking clinical symptoms (61 samples), SLE and SSc patients (10 samples each) and agematched healty volunteers (10 samples), then the sera were analysed for anti-caspase-8 autoantiboeies. Serum samples were obtained only after informed consent had been given. These experimets were ethicaly approved by the Research Commitee of Kawasaki Medical School.
Western blotting analysis
Active fragments of recombinant human caspase-8 (Calbiochem, La Jolla, CA, USA) were separated by 15% SDS–polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane (Amersham, Buckinghamshire, UK). The membrane was blocked for 3 h using 10% Block ace (Dainihon Pharm Co, Osaka, Japan) in phosphate buffered saline containing 0·05% Tween-20 (PBS-T), washed with PBS-T and incubated at room temperature for 1 h with a 1: 50 dilution of sera from the patients or the healthy volunteers. The blots were washed with PBS-T, incubated at room temperature for 1 h with a 1: 5000 dilution of horse radish peroxidase (HRP)-conjugated goat anti-human IgG (Amersham, Buckinghamshire, UK), washed with PBS-T, and finally the bound antibodies were detected by enhanced chemiluminescence method (Amersham, Buckinghamshire, UK).
Epitope mapping
Oligopepties were synthesized on activated membranes using the SPOTs system (Sigma-Genosys, Cambridge, UK) [5]. The peptides were 12 amino acids in length and had a sequential overlap of 9 amino acids. The SPOTs spanned the caspase-8 amino acid sequence from residues 1–479 (numbering according to the SWISS-PROT database entry q14790, caspase-8 precursor). The membranes with immobilized peptides attached were blocked overnight at 4°C in 10% concentrated blocking buffer (Sigma-Genosys) dissolved in Tris buffered saline containing 0·05% Tween 20 (TBS-T). The SPOTs were incubated at 4°C for 4 h with a 1: 100 dilution of the patients’ or the healthy volunteers’ sera. The membranes were washed twice for 10 min with TBS-T, then the bound antibodies were detected using the secondary antibodies, β-galactosidase-conjugated goat anti-human Ig (American Qualex Antibodies, San Clemente, CA, USA) and the substrate 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside, according to the manufacturer's instructions.
SELDI ProteinChip analysis
ProteinChip surface enhanced laser desorption/ionization (SELDI) mass spectrometry was performed to detect and quantify the autoantibodies captured on the antigen coated ProteinChip arrays [6]. NP (normal phase) and PS2 (epoxy) ProteinChipR (Ciphagen Biosystems, Fremount, CA, USA) were used for the analysis. The protocol details for individual arrays were based on the manufacturer's recommendations. Briefly, caspase-8 (100 pg/2μl) was loaded to the spots of a preactivated SELDI ProteinChip and incubated at room temperature for 1 h. The chip was blocked for 20 min at room temperature with 1 m ethanolamine and washed 3 times with PBS-T; 6 μl of patients’ serum or 6 μl of control antibody was applied to the spots and the chips were incubated over night at 4°C. The whole chip array was washed 3 times with PBS-T, twice with distilled water, air dried, then 0·5 μl of saturated sinapinic acid (an energy absorbing molecule) in 50% acetonitrile containing 0·5% trifluoroacetic acid was applied. Following a final air drying, the chip was analysed using SELDI ProteinChip System (PBS-II, Ciphergen Biosystems). Data were normalized with internal standards before making a comparison between the spots.
Autoantibody immunoglobulin subclass specificity
Monoclonal antibodies that were representative of the different human IgG subclasses including IgG1, IgG2, IgG3 and IgG4 (κ light chain), and IgG1, IgG2, IgG3 and IgG4 (λ light chain) were purchased from Sigma (St.Louis, MO, USA). Protein G (1μg/1μl) was conjugated to the PS2 chips, which were then blocked using 1 m ethanolamine. Each chip was incubated with antibodies of a particular IgG subclass, treated with sinapinic acid, and the molecular mass was analysed using the SELDI ProteinChip system. A total of 14 oligopeptides were synsethized (as above) containing the amino acid sequences of the caspase-8 epitopes, and these were used to determine the IgG subclass specificity of the autoantibodies. Mouse monoclonal antibodies directed against human IgG1, IgG2, IgG3 and IgG4 (Immunotech-A Coulter Company, Marseille, France), and then β-galactosidase-conjugated antimouse IgG antibodies (American Qualex Antibodies, San Clemente, Ca., USA) with the substrate 5-bromo-4-chloro-3-indoyl-β-D-galactopyranoside were also used. Autoantibodies were also analysed for the κ or λ isotype light chain by Western blotting using anti-caspase-8 positive human sera and mouse monoclonal antibodies directed against the human κ or λ chain (ICN Pharmaceuticals Inc., Aurora, OH, USA) followed by detection using enhanced chemiluminescence.
RESULTS
Detection of autoantibodies against human caspase-8 by immunoblotting
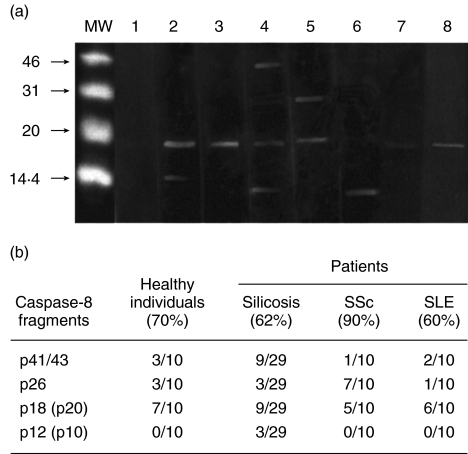
Western blotting was performed using the active fragments of recombinant human caspase-8, serum samples and detection by enhanced chemiluminescence. By this method, anti-caspase-8 antibodies were detected in the sera of patients with silicosis, SSc and SLE, as well as in healthy volunteers. Antibodies against p20(p18), a caspase-8 fragment that contains the cystein protease catalytic pentapeptide QACQG [7], were detected most frequently in the sera tested. Autoantibodies against p12(p10) containing the substrate specificity determinant RNPAEGTW [7] were detected in silicosis patients. Antibodies directed against the p26 fragment which contains two death effector domains (DEDs) and a caspase-8 linker region [7] were detected in silicosis, SSc and SLE patients as well as in healthy individuals (Fig. 1a,b).
Fig. 1.
Detection of anti-caspase-8 autoantibodies by Western blotting analysis. (a) Molecular weight markers (MW), negative serum of a silicosis patient (lane 1), positive sera from silicosis patients (lane 2–6), positive serum of a SSc patient (lane 7), positive serum of a SLE patient (lane 8). (b) Frequencies of anti-caspase-8 autoantibodies detected by Western blotting analysis.
Identification of amino acids involved in antibody binding
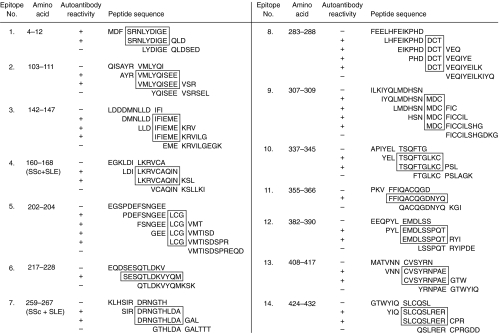
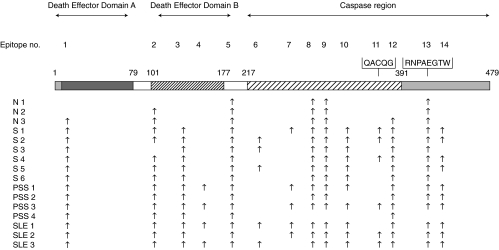
On activated membranes with SPOTs system [4], we synthesized oligopeptides which were 12 amino acids in length and had a partial overlap of 9 amino acids. The SPOTs spanned the amino acid sequence of the caspase-8 molecule from residues 1–479. The number of epitopes recognized by the autoantibodies were 14 sites altogether (Fig. 2). It is noticeable that the amino acid sequences of the epitopes recognized by the autoantibodies contained residues involved in key biological functions of caspase-8. The location of each epitope on the caspase-8 molecule and reactive autoantibodies is summarized in Fig. 3.
Fig. 2.
Epitope mapping of autoantibodies directed against human caspase-8. Each epitope recognized by the autoantibodies were indicated by the boxes.
Fig. 3.
The location of each epitope on the caspase-8 molecule and reactive autoantibodies.
DEDs in both caspase-8 and FADD function as domains for homophilic interaction between proteins. A BLAST search revealed that residues 7–75 and 101–169 of caspase-8 had homologuey with the DED of FADD [7]. These caspase-8 residues included epitope 1 located in DED-A, and epitopes 2, 3 and 4 located in DED-B. The herpes virus type 2 E8 protein inhibits caspase-8 by a competitive interaction with DED-B [8,9] thus preventing the binding interaction between caspase-8 and FADD. The E8 binding sites of on DED-B were located in epitopes 3 and 4. Interestingly, epitope 4 was recognized only by SLE or SSc patients, but not by silicosis patients or healthy volunteers. Epitope 5 was localized in a linker region. On the basis of the X-ray crystal structure of the ICE family, the caspase-8 amino acid residues His242, Ser338 and Cys287 are likely to be involved in catalysis [10,11]. It is therefore relevant that the active site cystein Cys287 [12] was included in epitope 8. Epitope 10 included Ser338, located in the caspase-8 active site. Caspase-8 contains a unique pentapeptide motif QACQG [13], including the catalytic cysteine Cys360. This pentapeptide constitutes the central part of epitope 11, and it is important to note that autoantibodies directed against this epitope were not detected in healthy individuals. The caspase-8 substrate specificity determinant RNPAEGTW [13] was included in the sequence of epitope 13. The number of epitopes detected by the autoantibodies was lowest in healthy individuals, especially in N1, increased in the patients with silicosis, and was more extensive in the patients with SSc and SLE. The serum of SLE2 contained autoantibodies against 13 epitopes on the caspase-8 molecule. These epitope mapping data demonstrate that the immune system of each individual recognizes the caspase-8 antigenic sites in specifically linked sets rather than detecting each site at random. The antigen presenting cells may present a large conformation of epitopes to the immune system, displaying them in a cooperative manner.
Capture of anti-caspase-8 autoantibodies by ProteinChip analysis
The autoantibodies were examined next by SELDI ProteinChip analysis [6]. ProteinChip arrays represent a new technology, therefore the accuracy of the technique was confirmed prior to the collection of any experimental data. Recombinant human caspase-8 was linked to the NP1 chip, and a purity check of the antigen was performed (data not shown). Recombinant human caspase-8 was linked to PS2 chip, and incubated with polyclonal goat anti-human caspase-8 IgG. The chip was treated with the energy-absorbing molecule sinapinic acid, and analysed with SELDI ProteinChip system.
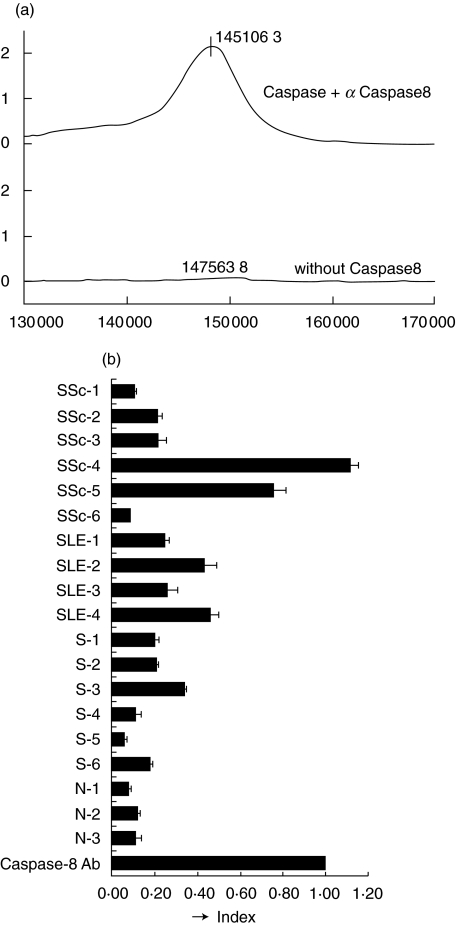
To prove a negative control, another PS2 chip was treated in the same way, but without the antigen. Captured antibodies were detectable, and average molecular weight of the IgG in main peak was about 148 000 kD (Fig. 4a). These preliminary data confirmed that the technique is valid for the detection of autoantibodies in serum samples. The sera from healthy individuals (3 samples), silicosis, SSc and SLE patients (6, 4 and 3 samples, respectively) were analysed for anti-caspase-8 autoantibodies using PS2 chips. These sera had also been analysed previously using the SPOTs system.
Fig. 4.
Surface enhanced laser desorption/ionization (SELDI) ProteinChip analysis of anti-caspase-8 autoantibodies. (a) Recombinant human caspase-8 was loaded on the spots of a preactivated SELDI ProteinChip, and the chip captured goat polyclonal anti-human caspase-8 globulins. Data were normalized with internal standards before making a comparison between the spots. (b) The integrated total peak height for each sample calculated from the graph obtained as in (a). The index was based on the peak height gained with the goat anti-caspase-8 polyclonal antibody.
Autoantibodies directed against recombinant human caspase-8 were captured on the PS2 chips. The total integrated peak height was calculated to determine the strength of the interaction between the antibodies in the sample and the caspase-8 antigen (Fig. 4b). Anti-caspase-8 autoantibodies were detected at high levels in the sera of patients with SSc or SLE, and at moderate levels in silicosis patients. Anti-caspase-8 autoantibodies were also observed in the healthy individuals, but only at low levels.
Autoantibody immunoglobulin subclass specificity
The IgG subclass specificity of the anti-caspase-8 autoantibodies was then determined. A preliminary analysis was performed using monoclonal antibodies of four different IgG subclasses that had either κ or λ light chains (data not shown). Some differences in molecular weight between antibodies of the same subclass with the same light chain isotype were detected by the ProteinChip analysis. This was due to small diffrences in the amino acid sequence of the variable chain that is specific to each antibody.
The major IgG subclass of the anti-caspase-8 autoantibodies was estimated from the results of the ProteinChip array in which peak height was normalized schematically, Western blotting and the SPOTs system analysis. The major peak in 12 of the 16 samples was considered to be IgG1 and with the λ light chain, although the average κ to λ ratio is 2:1 in humans [14] (data not shown).
DISCUSSION
A significant finding was the location of the caspase-8 active site cystein residue Cys287 within one of the caspase-8 autoantibody epitopes. The catalytic property defining the caspase is their ability to cleave the substrate after the aspartic acid in position P1. This is combined with an equally stringent requirement for a particular amino acid conformation in front of the cleavage site [12]. Peptide-based inhibitors of ICE family, such as the irreversible inhibitors z-VAD-fmk (benzyloxycarbonyl-Val-Ala- Asp-fluoromethylketone) and z-DEVD-fmk (benzyloxycarbonyl-asp-Clu-Val-Asp-fluoromethylketone), bind to the active site cysteine Cys287 of caspase-8. This is achieved through covalent bond formation and the displacement of fluoromethylketone to form a thiomethylketone with the cystein residue of the active-site [10,11]. Cytokine response modifier A (Crm A), a product of the cowpox virus and a reversible inhibitor of caspase-8, compete with the substrate at LVAD thus reducing its chances of interaction with the active site cystein of caspase-8 molecule.
Intramolecular epitope spreading [15] may be a mechanism by which multiple autoantibodies are generated. Our results suggest a stepwise progression in the maturation of the humoral immune response to caspase-8, which is consistent with this concept [16]. Autoimmune epitope spreading has been described in patients with SLE, multiple sclerosis, and bullous pemphigoid [17–19], and it is reported to be B7-1 dependent, playing a major pathologic role in experimental autoimmune encephalomyelitis in mice [18]. By the time a patient is diagnosed with an autoimmune disease, significant tissue destruction has already occurred, making it difficult to identify the antigen against which the autoimmune response is directed [13]. It is noticeable that the epitope No.11 which includes the active site cystein Cys287 of caspase-8 has not been recognized by healthy individuals, although some of the sera from the healthy individuals reacted with recombinant human caspase-8 by Western blotting analysis. Our results, especially those in the healthy individual N1, suggest that epitope 5, 8, 9 or 13 could contain the primary epitope, and that intramolecular epitope spreading occurs not only in autoimmune patients, but also in healthy individuals.
Many traditional experimental systems are limited in their sensitivity or scale. In this study we employed the SELDI ProteinChip technology to determine the anti-caspase-8 antibody profiles of different serum samples. Through the use of this technology, it was possible to detect a few femtomoles of protein and determine its molecular mass with considerable precision. These features of the ProteinChip system allowed us to determine the main IgG subclass of the anti-caspase-8 autoantibodies.
A significant finding was that the frequency of the autoantibody κ or λ light chains differed from the average distribution in humans, although no functional difference has been detected between antibodies with different light chains [14]. A case report in which the patient had a high level of IgG4 λ antiplatelet autoantibody without thrombocytopenia suggested that IgG1 and IgG4 autoantibodies may have different pathogenetical effects [20]. Since the IgG4 subclass is incapable of fixing complement via the classical complement pathway, IgG4 is considered a noninflammatory antibody [21,22]. Another report concerning pemphigus vulgaris or pemphigus foliaceus indicated that most polyclonal autoantibodies in patients suffering acute onset or active disease were of the IgG4 subclass, while patients in remission had mainly IgG1 autoantibodies [23].
The present study provides evidence that anti-caspase-8 autoantibodies occur in the patients with silicosis, SSc and SLE as well as in healthy individuals. At least 14 epitopes on the caspase-8 molecule were recognized by the autoantibodies, and these epitopes included amino acid residues involved in the key biological functions of the protein. The main IgG subclass in almost all cases was IgG1λ but in some cases it was IgG4κ. These findings represent (to our knowledge) the first example of anti-caspase-8 autoantibody epitope mapping. In addition, intramolecular epitope spreading was demonstrated in the anti-caspase-8 autoantibodies of the patients with silicosis, SSc and SLE as well as healthy individuals. The relationship between the autoantibodies and the pathogenesis of these autoimmune diseases requires further clarification, but it might be possible that these autoantibodies could enter into cells and interact with autoantigens as soon as the apoptotic signal started and the intracellular condition changed.
Acknowledgments
This work was supported by a Kawasalki Medical School Grant and by a grant-in/aid for scientific research from the Japanese Ministry of Education, Science, Sports and Culture. The authors thank Ms Kazuko Hikasa for her excellent assistance.
REFERENCES
- 1.Tomokuni A, Aikoh T, Matsuki T, et al. Elevated soluble Fas/APO-1 (CD95) levels in silicosis patients without clinical symptoms of autoimmune diseases or malignant tumors. Clin Exp Immunol. 1997;110:303–9. doi: 10.1111/j.1365-2249.1997.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medema JP, Scaffidi C, Kischkel FC, et al. FLICE is activated by association with the CD95 death-inducing signaling complex (DISC) EMBO J. 1997;16:2794–804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher GH, Rosenberg FJ, Straus SE, et al. Dominant interfering Fas gene mutations impair apoptosis in a human autoimmune lymphoproliferative syndrome. Cell. 1995;81:935–45. doi: 10.1016/0092-8674(95)90013-6. [DOI] [PubMed] [Google Scholar]
- 4.Rieuz-Iaucat F, Le Deist F, Hivroz C, et al. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–9. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 5.Frank R. Spot synthesis. an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron. 1992;48:9217–32. [Google Scholar]
- 6.Austen BM, Frears ER, Davies H. The use of Seldi ProteinChip™ arrays to monitor production of Alzheimer's β-amyloid in transfected cells. J Peptide Sci. 2000;6:459–69. doi: 10.1002/1099-1387(200009)6:9<459::AID-PSC286>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 7.Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–27. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 8.Bertin J, Armstrong RC, Ottilie S, et al. Death effector domain- containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1- induced apoptosis. Proc Natl Acad Sci USA. 1997;94:1172–6. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh A, Ni J, Aggarwal BB. Death domain receptors and their role in cell demise. J Interferon Cytokine Res. 1998;18:439–50. doi: 10.1089/jir.1998.18.439. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard H, Donepudi M, Tschopp M, et al. Caspase-8 specificity probed at subsite S4. Crystal structure of the caspase-8-z-DEVD-cho complex. J Mol Biol. 2000;302:9–16. doi: 10.1006/jmbi.2000.4041. [DOI] [PubMed] [Google Scholar]
- 11.Blanchard H, Kodandapani L, Mittl PR, et al. The three dimensional structure of caspase-8: an initiator enzyme in appotosis. Structure. 1999;7:1125–33. doi: 10.1016/s0969-2126(99)80179-8. [DOI] [PubMed] [Google Scholar]
- 12.Thornberry NA, Miller DK, Nicholson DW. Interleukin-1-β- converting enzyme and related proteases as potential targets in inflammation and apoptosis. Perspective Drug Discovery Design. 1994;2:389–99. [Google Scholar]
- 13.Grenet J, Teitz T, Wei T, et al. Structure and chromosome localization of the human CASP8 gene. Gene. 1999;226:225–32. doi: 10.1016/s0378-1119(98)00565-4. [DOI] [PubMed] [Google Scholar]
- 14.Morrison S. The immune system in health and desease. In: Trarers CA, Walport P, Shomchik M, editors. Immunobiology. 5. 2001. pp. 131–56. [Google Scholar]
- 15.Lehmann PV, Forsthuber T, Miller A, et al. Spreading of T cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 16.Goebels N, Hofstetter H, Schmidt S, et al. Repertoire dynamics of autoreactive T cells in multiple sclerosis patients and healthy subjects. Epitope spreading versus clonal persistence. Brain. 2000;123:508–18. doi: 10.1093/brain/123.3.508. [DOI] [PubMed] [Google Scholar]
- 17.Arbuckle MR, Reichlin M, Harley JB, et al. Shared early autoantibody recognition events in the development of anti-Sm B/B′ in human Lupus. Scad J Immunol. 1999;50:447–55. doi: 10.1046/j.1365-3083.1999.00640.x. [DOI] [PubMed] [Google Scholar]
- 18.Vanderlugt CL, Neville KL, Nikcevich KM, et al. Pathologic role and temporal appearance of newly emerging autoepitopes in relapsing experimental autoimmune encephalomyelitis. J Immunol. 2000;164:670–8. doi: 10.4049/jimmunol.164.2.670. [DOI] [PubMed] [Google Scholar]
- 19.Perriard J, Jaunin F, Favre B, et al. IgG autoantibodies from bullous pemphigoid (BP) patients bind antigenic sites on both the extracellular and the intracellular domains of the BP antigen 180. J Inves Dermatol. 1999;112:141–7. doi: 10.1046/j.1523-1747.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- 20.McMillan R, Bowditch RD, Tani P, et al. A non-thrombocytopenic bleeding disorder due to an IgG4-kappa anti-GPIIb/IIIa autoantibody. Br J Haematol. 1996;95:747–9. doi: 10.1046/j.1365-2141.1996.d01-1957.x. [DOI] [PubMed] [Google Scholar]
- 21.Modre B, Allen J, Wojnarowska F. Does class switching contribute to remissiong in bullous pemphigoid? Acta Derm Venerol. 1999;79:127–31. doi: 10.1080/000155599750011345. [DOI] [PubMed] [Google Scholar]
- 22.Garcia BE, Sanz ML, Gato JJ, et al. IgG4 blocking effect on the release of antigen-specific histamine. J Invest Allergol Clin Immunol. 1993;3:26–33. [PubMed] [Google Scholar]
- 23.Hertl M, Veldman C. Pemphigus-paradigm of autoantibody-mediated autoimmunity. Skin Pharmacol Appl Skin Physiol. 2001;14:408–18. doi: 10.1159/000056375. [DOI] [PubMed] [Google Scholar]