Abstract
To study the activation states and cytokine profiles of pulmonary T cells in corticosteroid-resistant and corticosteroid-sensitive interstitial pneumonitis (IP) in dermatomyositis (DM)/polymyositis (PM), we examined the activation markers and cytokine profiles of T cells in bronchoalveolar lavage fluids (BALF) from patients with IP in DM/PM before prednisolone therapy and then compared the activation states of T cells according to the therapeutic response of IP to prednisolone therapy. CD25+ CD4+ T cells in BALF were significantly increased in both corticosteroid-resistant and corticosteroid-sensitive IP in DM/PM as compared with those in controls without IP. Furthermore, CD25+ CD4+ T cells in BALF were significantly more increased in corticosteroid-resistant IP than those in cortico teroid- sensitive IP. Moreover, CD25+ CD8+ T cells in BALF were significantly increased only in corticosteroid-resistant IP, but not in corticosteroid-sensitive IP or controls without IP. IFN-γ mRNA was detected in BALF T cells in corticosteroid-resistant and corticosteroid-sensitive IP but not in controls without IP, whereas IL-4 mRNA was virtually undetected in BALF T cells in both the IP groups. However, there were no significant differences in CD4/CD8 ratio of BALF T cells, HLA-DR+ BALF T cells or CD25+ and HLA-DR+ peripheral blood T cells between the two IP groups. These results indicate that activated Th1-type pulmonary T cells play an important role in the development of corticosteroid- resistant IP in DM/PM and that the increase in CD25+ CD8+ T cells in BALF is a useful indicator for corticosteroid-resistant IP in DM/PM and hence may be an indicator for early use of cyclosporin.
Keywords: Corticosteroid-resistant interstitial pneumonitis, dermatomyositis/polymyositis, CD25+ T cells, IFN-γ producing T cells, bronchoalveolar lavage fluids
INTRODUCTION
Interstitial pneumonitis (IP) is the most frequent and serious pulmonary involvement in patients with dermatomyositis (DM)/polymyositis (PM) [1–8]. In one study representing the largest series of autopsy cases with DM/PM, the mean survival of patients with IP was shown to reduce to only 8 months after the onset of pulmonary symptoms [5]. We also showed that IP developed in 32% of 111 DM/PM patients and that 35% of DM/PM patients with IP died of respiratory failure in a year [8]. Moreover, it has been shown that 44–60% of IP in DM/PM, especially in DM, is resistant to high-dose corticosteroid therapy and the corticosteroid-resistant IP patients die of respiratory failure in a relatively short period [6–8]. Furthermore, we and others have recently shown that the corticosteroid-resistant IP is successfully treated with a T cell-specific immunosuppressant cyclosporin [8–10], suggesting that the activation of T cells is importantly involved in the development of corticosteroid-resistant IP in DM/PM.
Therefore, in order to determine the difference in T cell activation states between corticosteroid-resistant and corticosteroid-sensitive IP in DM/PM, we studied the activation markers and cytokine profiles of T cells in bronchoalveolar lavage fluids (BALF) from patients with IP in DM/PM before prednisolone therapy and then compared the activation states of T cells according to the therapeutic response of IP to prednisolone therapy. Our results indicate that activated Th1-type pulmonary T cells are importantly involved in the development of corticosteroid- resistant IP in DM/PM and that the increase in CD25+ CD8+ T cells in BALF is a useful indicator for corticosteroid-resistant IP in DM/PM.
METHODS
Patients
Twenty-two DM or PM patients associated with IP who had been admitted to Chiba University Hospital from 1996 through 1999 were studied (Table 1). Diagnosis of DM/PM was made according to the criteria by Bohan and Peter [11] when clinical data fulfilled 4 or 5 of the criteria. Patients were 13 DM (6 males and 7 females) aged 46·2 years on average and 9 PM (9 females) aged 50·6 years on average. As a control, four DM patients without IP and eight patients with solitary lung adenocarcinoma who had normal chest radiographs except for the solitary lesions and no history of smoking were entered in the study. Informed consent was obtained from each patient in written forms.
Table 1.
Profiles of dermatomyositis (DM)/polymyositis (PM) patients with corticosteroid-resistant and corticosteroid-sensitive interstitial pneumonitis (IP)
| Corticosteroid- resistant IP† (n = 6) | Corticosteroid- sensitive IP† (n = 16) | P-value | |
|---|---|---|---|
| Age (years) | 49·0 ± 15·9 | 49·1 ± 9·1 | NS |
| Sex (male/female) | 2/4 | 4/12 | NS |
| DM/PM | 6/0 | 7/9 | 0·003 |
| Disease duration (weeks)‡ | 5·5 ± 3·5 | 7·8 ± 4·5 | NS |
| CT findings§ | |||
| Reticular opacities | 6 (100%) | 16 (100%) | NS |
| Ground-glass opacities | 6 (100%) | 16 (100%) | NS |
| Alveolar opacities | 4 (67%) | 12 (75%) | NS |
| Honeycombing | 1 (17%) | 2 (13%) | NS |
| Pulmonary function tests§ | |||
| FVC (% predicted) | 72·3 ± 5·5 | 73·6 ± 21·7 | NS |
| FEV1 (% predicted) | 89·1 ± 3·5 | 82·8 ± 10·0 | NS |
| DLCO (% predicted) | 51·8 ± 3·1 | 59·8 ± 17·0 | NS |
| PaO2 (mmHg) | 75·7 ± 8·0 | 72·8 ± 10·3 | NS |
| AaDO2 (mmHg) | 30·5 ± 8·1 | 32·6 ± 12·6 | NS |
| Changes after 4-week corticosteroid therapy† | |||
| PaO2 (mmHg) | −9·5 ± 5·5 | 12·2 ± 5·7 | <0·001 |
| AaDO2 (mmHg) | 11·7 ± 8·7 | −15·7 ± 6·7 | <0·001 |
| Survival after 1 years (%)¶ | 5 (83%)¶ | 16 (100%) | NS |
Data are means ± SD. NS, not significantly different between the two IP groups (P > 0·05).
Corticosteroid-resistant IP was defined as a progression of IP despite administration of 1 mg/kg/day prednisolone for more than 4 weeks.
Periods from the onset of dyspnea and dry cough to the admission.
CT findings and pulmonary functions are the data on admission.
Survivals after one-year prednisolone therapy. Patients with corticosteroid-resistant IP were treated with cyclosporin in addition to prednisolone. One patient died of progression of IP in spite of cyclosporin therapy.
IP was diagnosed by a reticulonodular pattern on the chest radiograph and computed tomography, by decreased PaO2 levels (less than 80 mmHg) without PaCO2 elevation in arterial blood, and by a restrictive pattern and a decrease in diffusing capacity for carbon monoxide in pulmonary function tests (Table 1) [12]. All IP patients showed the decrease in PaO2 levels by more than 15 mmHg within less than 3 months before corticosteroid therapy. The diagnosis was confirmed by histopathologic findings of transbronchial lung biopsy samples in 19 patients and by increased numbers of lymphocytes and macrophages in BALF in all 22 patients [12]. In addition, no causative microorganisms including pneumocystis carinii, fungi, and cytomegalovirus or drugs were identified in the IP patients.
Treatment of IP in DM/PM with corticosteroids and evaluation of therapeutic response
All patients with DM/PM associated with IP received corticosteroid therapy, prednisolone (1 mg/kg/day) daily as the initial dosage for at least 4 weeks, and then the dosage gradually tapered as muscle weakness, skin rashes, serum creatine phosphokinase (CPK) and aldolase levels, and IP improved with the therapy.
We periodically evaluated the therapeutic response of IP as follows [8]: the treatment was considered to be effective if PaO2 levels and the reticulonodular shadow (especially, ground-glass and/or alveolar opacities) on the computed tomography improved; and ineffective if these findings progressed. Chest computed tomography findings were evaluated by a radiologist in a single-blinded manner. Corticosteroid-resistant IP was defined as a progression of IP despite administration of 1 mg/kg/day prednisolone for more than 4 weeks (Table 1). Corticosteroid-resistant IP was then treated with cyclosporin in addition to prednisolone. Cyclosporine was initially administered at a dose of 150–200 mg/day orally in 2 divided doses to corticosteroid-resistant IP. After 1 week, the dose of cyclosporin was increased to 250–300 mg/day to maintain serum trough levels between 160 and 200 ng/ml to avoid nephrotoxicity [13].
Bronchoalveolar lavage
Prior to corticosteroid therapy, bronchoalveolar lavage fluid (BALF) examination was performed according to the guidelines described previously [14]. Briefly, patients were premedicated with atropine and hydroxyzine intramuscularly, and lidocaine was aerosolized into the upper airways and instilled into the larynx and lower airways. After the fiberoptic bronchoscope (Olympus BF-20; Olympus Optical Co., Tokyo, Japan) was wedged into the right middle lobe, 50 ml of prewarmed, sterile saline was instilled through the channel of bronchoscope and immediately aspirated into plastic tubes with gentle suction and this procedure was repeated three times.
Isolation of cells in BALF and peripheral blood lymphocytes
BALF was strained through sterile gauze to remove debris and centrifuged at 400 × g for 10 min at 4°C. Cells were washed twice with phosphate-buffered saline (PBS) and then suspended in 10 ml of PBS, and cell differentials were determined by counting 500 cells on cytocentrifuge slides stained with Wright-Giemsa solution. A part of the cells were subjected to flow cytometric analysis for T cell surface phenotyping and to cytokine mRNA analysis in T cells.
Peripheral blood lymphocytes (PBL) were isolated from heparinized peripheral venous blood by Ficoll-Paque (Pharmacia Fine Chemicals, Uppsala, Sweden) density gradient centrifugation.
Flow cytometric analysis
Cells (1 × 106) from BALF and peripheral blood were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, or peridinin chloropyll protein (PerCP)-conjugated antibodies in PBS containing 1% fetal calf serum for 30 min at 4°C. The following FITC-, PE- or PerCP-conjugated monoclonal antibodies were used: CD4, CD8, CD3, CD25, CD45RO, and HLA-DR (Becton Dickinson, Mountain View, CA, USA). Stained cells were washed twice with PBS, resuspended in PBS containing 1% fetal calf serum and analysed by FACSCalibur (Becton Dickinson) using Cell Quest program. CD25+ or HLA-DR+ cells were defined as the cells that were stained with anti-CD25 or anti-HLA-DR mAb at the fluorescent intensity greater than that of cells stained with isotype-matched control mAb.
Detection of cytokine mRNA by RT-PCR
IFN-γ and IL-4 mRNA expression was detected by PCR amplification of complementary DNA (cDNA) from BALF T cells by using specific primers. Briefly, T cells were purified from BALF by Ficoll-Paque density gradient centrifugation and by a subsequent magnetic cell sorting using MACS anti-CD3 microbeads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) according to manufacturer's instruction (>95% pure T cells). Total RNA was prepared from BALF T cells by the method of acid guanidinium thiocyanate/phenol/chloroform extraction using Isogen solution (Nippon Gene Co., Tokyo, Japan). The first strand cDNA was then synthesized from 0·1 to 1 μg of total RNA in 20 μl of reaction buffer containing oligo-dT primer using avian myeloblastosis virus reverse transcriptase (1st Strand cDNA Synthesis Kit; Boehringer Mannheim GmbH, Mannheim, Germany).
PCR amplification of the cDNA from BALF T cells was performed with Taq DNA polymerase (Boehringer Mannheim GmbH) in the presence of IFN-γ (5′-TGTTACTGCCAGGACC CATAT-3′ and 5′-ACTCTTTTGGATGCTCTGGTC-3′), IL-4 (5′-CTTCCCCCTCTGTTCTTCCT-3′ and 5′-TTCCTGTCGAG CCGTTTCAG-3′), IL-5 (5′-GAGTTTGCTAGCTCTTGGAGC-3′ and 5′-GCACAGTTTGACTCTCCAGAT-3′), or TCR Cβ (5′-CCCACACCCAAAAGGCCA-3′ and 5′-CATAGAGGATG GTGGCAG-3′)-specific primers. The denaturing step was done at 94°C for 1·5 min, the annealing step at 55°C for 1 min, and the extension step at 72°C for 1 min, for 35 cycles on a DNA thermal cycler (Perkin-Elmer Corp., Norwalk, CT, USA). The PCR-amplified sample was run on 1·5% agarose gel, and visualized using ethidium bromide.
Data analysis
Data are summarized as mean ± SD. The statistical analysis of the results was performed by the analysis of variance using Fisher's least significant difference test for multiple comparisons and by chi-square test. P-values < 0·05 were considered significant.
RESULTS
Cellular components of BALF in DM/PM patients associated with IP
In order to determine the difference in T cell activation states between corticosteroid-resistant and corticosteroid-sensitive IP in DM/PM, we examined the activation markers and cytokine profiles of T cells in bronchoalveolar lavage fluids (BALF) from patients with IP in DM/PM before prednisolone therapy and then compared the activation states of T cells according to the therapeutic response of IP to prednisolone therapy (1 mg/kg/day for 4 weeks). The therapeutic response of IP to prednisolone was classified into corticosteroid-resistant and corticosteroid-sensitive IP according to the criteria described in the Methods.
Twenty-two DM or PM patients with IP were enrolled in this study, and 6 DM patients with IP were classified as corticosteroid-resistant IP and 16 DM or PM patients (7 DM and 9 PM) as corticosteroid-sensitive IP (Table 1). In addition, 3 out of 6 DM patients with corticosteroid-resistant IP and 2 out of 7 DM patients with corticosteroid-sensitive IP showed normal CPK levels at the onset of IP. The 6 DM patients with corticosteroid-resistant IP were then treated with cyclosporin in addition to prednisolone and 5 of the 6 patients with corticosteroid resistant-IP were improved by additional cyclosporin therapy.
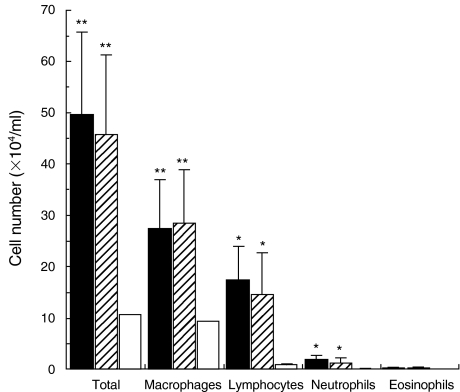
In both corticosteroid-resistant and corticosteroid-sensitive IP, lymphocytes, macrophages, and neutrophils in BALF were significantly increased as compared with those in controls without IP (n = 12) that consisted of 4 DM patients without IP and 8 lung cancer patients without IP (P < 0·005 to 0·05) (Fig. 1). However, there were no significant differences in these cellular components between the two IP groups (Fig. 1).
Fig. 1.
Cellular components of bronchoalveolar lavage fluid (BALF) in dermatomyositis (DM)/polymyositis (PM) patients with corticosteroid-resistant and corticosteroid-sensitive interstitial pneumonitis (IP). Prior to corticosteroid therapy, BALF was obtained from DM/PM patients with corticosteroid-resistant IP (n = 6, ▪) and corticosteroid-sensitive IP (n = 16, ) and from controls without IP including DM patients without IP (n = 12, □), and the number of total cells, lymphocytes, macrophages, neutrophils, and eosinophils in BALF was evaluated. Data are means ± SD in each group. Significant different from the mean values in controls without IP, *P < 0·05, **P < 0·005.
) and from controls without IP including DM patients without IP (n = 12, □), and the number of total cells, lymphocytes, macrophages, neutrophils, and eosinophils in BALF was evaluated. Data are means ± SD in each group. Significant different from the mean values in controls without IP, *P < 0·05, **P < 0·005.
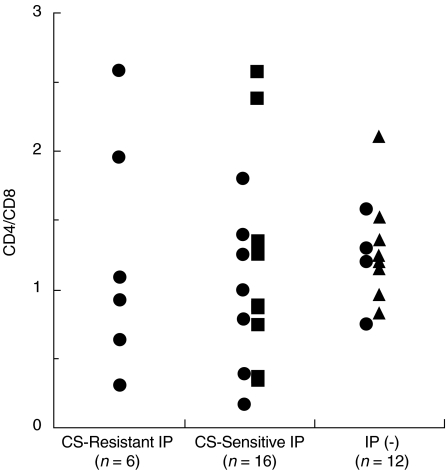
We found that there was no significant difference in CD4/ CD8 ratio of BALF T cells among corticosteroid-resistant IP, corticosteroid-sensitive IP, or controls without IP (Fig. 2). In addition, most of BALF T cells (more than 95%) in the three groups expressed CD45RO, a marker of memory T cells (data not shown).
Fig. 2.
CD4/CD8 ratio of BALF T cells in DM/PM patients with corticosteroid-resistant and corticosteroid-sensitive IP. BALF cells from DM/ PM patients with corticosteroid-resistant IP (n = 6) and corticosteroid- sensitive IP (n = 16) and from controls without IP (n = 12) were stained with anti-CD4-FITC, anti-CD8-PerCP, and anti-CD3-PE antibodies and analysed by FACS. • DM patients, ▪ PM patients, ▴ lung cancer patients without IP.
Increase in CD25+ T cells in BALF in corticosteroid-resistant IP
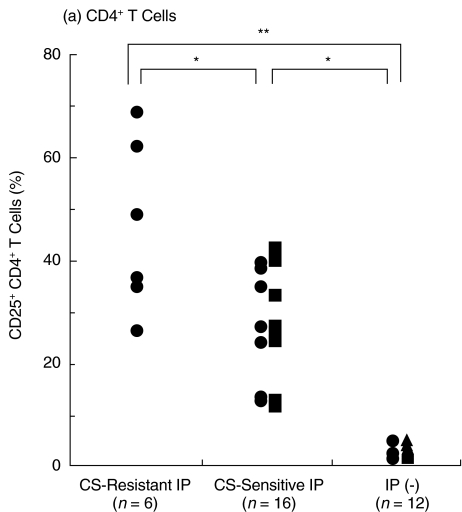
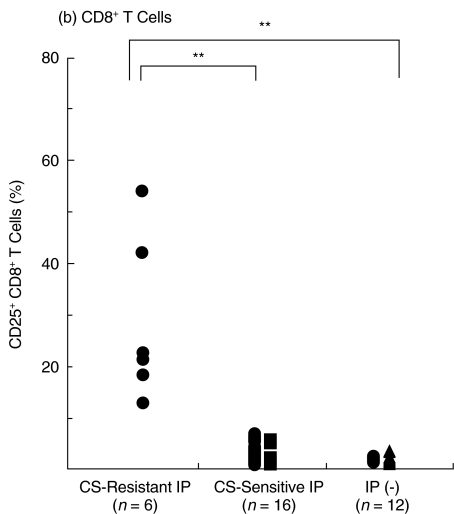
We then examined the expression of T cell activation markers CD25 and HLA-DR [15] on BALF T cells from the two IP groups. CD25 was expressed on few BALF CD4+ and CD8+ T cells from controls without IP including DM patients without IP (3·0 ± 1·1% and 1·2 ± 0·8%, respectively, n = 12) (Fig. 3). CD25+ CD4+ T cells were significantly increased in both corticosteroid-resistant and corticosteroid-sensitive IP as compared with those in controls without IP (P < 0·001) (Fig. 3). Moreover, CD25+ CD4+ T cells were significantly more increased in corticosteroid-resistant IP (46·4 ± 16·6%, n = 6, p <0·005) than those in corticosteroid- sensitive IP (28·2 ± 10·7%, n = 16) (Fig. 3). In addition, there was no significant difference in CD25+ CD4+ T cells of corticosteroid-sensitive IP between DM and PM (Fig. 3), suggesting that the difference of CD25+ CD4+ T cells between corticosteroid-resistant and corticosteroid-sensitive IP was not due to the difference between DM and PM.
Fig. 3.
Increase in CD25+ T cells in BALF in DM/PM patients with corticosteroid-resistant IP. BALF cells from DM/PM patients with corticosteroid-resistant IP (n = 6) and corticosteroid-sensitive IP (n = 16) and from controls without IP (n = 12) were stained with (a) anti-CD4-FITC and (b) anti-CD8-PerCP and anti-CD25-PE antibodies and analysed by FACS. • DM patients, ▪ PM patients, ▴ lung cancer patients without IP. *P < 0·005, **P < 0·001.
Furthermore, CD25+ CD8+ T cells were significantly increased only in corticosteroid-resistant IP (28·5 ± 15·0%, n = 6, P <0·001) as compared with those in corticosteroid-sensitive IP or controls without IP, whereas CD25+ CD8+ T cells were not significantly different between corticosteroid-sensitive IP (3·0 ± 2·1%; n = 16) and controls without IP (Fig. 3). All IP in patients whose CD25+ CD8+ BALF T cells were increased more than 9·3% (mean + 3 SD of CD25+ CD8+ BALF T cells in corticosteroid-sensitive IP) were resistant to corticosteroid therapy. These results indicate that pulmonary T cells are activated in both corticosteroid-resistant and corticosteroid-sensitive IP and that more pulmonary T cells in corticosteroid-resistant IP are activated than in corticosteroid-sensitive IP, suggesting that CD25 expression on BALF T cells, especially on CD8+ T cells, could be useful for prediction for responsiveness of IP to corticosteroid therapy.
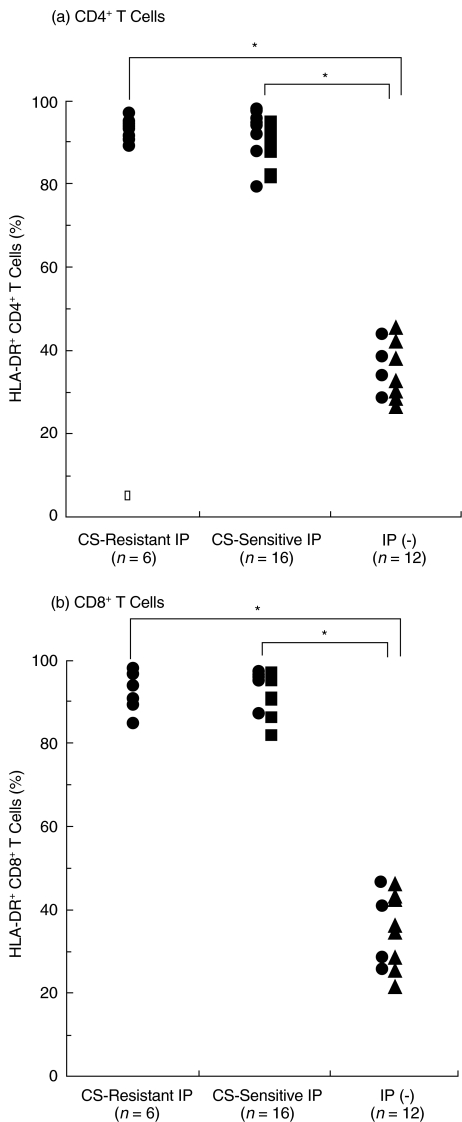
In contrast, HLA-DR+ CD4+ and CD8+ T cells were similarly increased in both corticosteroid-resistant and corticosteroid- sensitive IP as compared with those in controls without IP (P < 0·001), and there were no significant differences in HLA-DR+ CD4+ or CD8+ T cells between the two IP groups (Fig. 4).
Fig. 4.
Increase in HLA-DR+ T cells in BALF in DM/PM patients with corticosteroid-resistant and corticosteroid-sensitive IP. BALF cells from DM/PM patients with corticosteroid-resistant IP (n = 6) and corticosteroid-sensitive IP (n = 16) and from controls without IP (n = 12) were stained with (a) anti-CD4-FITC and (b) anti-CD8-PerCP, and anti-HLA-DR-PE antibodies and analysed by FACS. • DM patients, ▪ PM patients, ▴ lung cancer patients without IP. *P < 0·001.
CD25+ and HLA-DR+ T cells in peripheral blood in corticosteroid-resistant IP
We examined the expression of CD25 and HLA-DR on peripheral blood T cells from corticosteroid-resistant and corticosteroid-sensitive IP in order to determine whether CD25+ and HLA-DR+ peripheral blood T cells reflect pulmonary T cell activation in the two IP groups. We found that CD25+ CD4+ peripheral blood T cells were not significantly different among corticosteroid- resistant IP, corticosteroid-sensitive IP, and controls without IP (28·8 ± 8·3%, 33·3 ± 11·8%, and 29·6 ± 9·3% of peripheral blood CD4+ T cells, respectively). CD25+ CD8+ peripheral blood T cells were not significantly different among the three groups (4·3 ± 1·8%, 3·7 ± 1·9%, and 2·4 ± 1·3% of peripheral blood CD8+ T cells, respectively), either. There were also no significant differences in HLA-DR+ CD4+ or CD8+ peripheral blood T cells among the three groups (data not shown). Thus, CD25+ and HLA-DR+ peripheral blood T cells do not reflect pulmonary T cell activation in IP in DM/PM.
Increase in IFN-γ-producing T cells in BALF in both corticosteroid-resistant and corticosteroid-sensitive IP
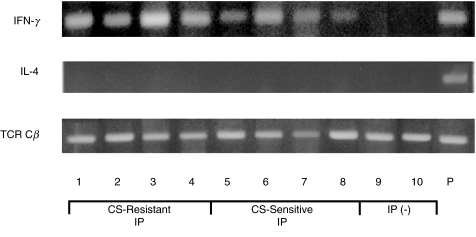
We then analysed Th1 and Th2 cytokine profiles of BALF T cells from the two IP groups by PCR for IFN-γ and IL-4. IFN-γ mRNA was more frequently detected in BALF T cells in corticosteroid-resistant IP (6 of 6 cases) than those in corticosteroid-sensitive IP (11 of 16 cases) (P < 0·01), whereas IFN-γ mRNA was virtually undetected in BALF T cells from controls without IP (n = 12) (Fig. 5). In contrast, IL-4 mRNA (Fig. 5) and IL-5 mRNA (data not shown) were virtually undetected in BALF T cells in both the two IP groups and controls without IP. These results suggest that the deviation towards Th1 immune states is importantly involved in both corticosteroid-resistant and corticosteroid-sensitive IP in DM/PM.
Fig. 5.
IFN-γ and IL-4 mRNA expression in BALF T cells from DM/PM patients with corticosteroid-resistant and corticosteroid- sensitive IP.IFN-γ and IL-4 mRNA expression was detected by PCR amplification of cDNA from BALF T cells in DM/PM patients with corticosteroid-resistant IP (n = 6) and corticosteroid-sensitive IP (n = 16) and controls without IP (n = 12). TCR Cβ mRNA expression was used as an internal control of PCR amplification of cDNA. The PCR products were run on 1·5% agarose gel, and visualized using ethidium bromide. The data shown are representative of corticosteroid-resistant IP (lanes 1–4), corticosteroid-sensitive IP (lanes 5–8), and controls without IP (lanes 9 and 10). P, activated peripheral blood T cells as a positive control.
DISCUSSION
In this study, we show that activated Th1-type pulmonary T cells are involved in the development of IP in DM/PM. We found that CD25+ CD4+ T cells and HLA-DR+ CD4+ and CD8+ T cells in BALF were significantly increased in IP in DM/PM compared with those in controls without IP including DM patients without IP (Fig. 3). We also found that IFN-γ mRNA was expressed in BALF T cells in IP in DM/PM, but not in controls without IP, whereas IL-4 mRNA was virtually undetected in BALF T cells in IP and control groups (Fig. 5). Second, we demonstrate that more pulmonary T cells are activated in corticosteroid-resistant IP than in corticosteroid-sensitive IP. We found that CD25+ CD4+ T cells in BALF were significantly more increased in corticosteroid- resistant IP in DM/PM than those in corticosteroid-sensitive IP in DM/PM. Furthermore, we found that CD25+ CD8+ T cells in BALF were significantly increased only in corticosteroid-resistant IP, but that CD25+ CD8+ T cells in BALF were not significantly different between corticosteroid-sensitive IP and controls without IP (Fig. 3). However, there were no significant differences in CD4/CD8 ratio of BALF T cells, HLA-DR+ BALF T cells or CD25+ and HLA-DR+ peripheral blood T cells between the two IP groups (Figs 2 and 4). In addition, we confirmed the effectiveness of cyclosporin in corticosteroid-resistant IP in DM/PM as shown in previous reports including ourselves [8–10], since 5 of 6 patients with corticosteroid-resistant IP in this study were also saved by cyclosporin for more than one year (Table 1). Taken together, these results indicate that the increase in CD25+ CD8+ T cells among pulmonary T cells is a useful indicator for corticosteroid-resistant IP in DM/PM and hence may be an indicator for early use of cyclosporin.
Our results indicate that more pulmonary T cells in corticosteroid-resistant IP are activated than in corticosteroid-sensitive IP. It has been shown that T cells play a critical role in the development of IP [16,17] and that both CD4+ T cells and CD8+ T cells contribute to IP probably depending on the expression of antigens on various pulmonary cells such as alveolar epithelial cells and alveolar macrophages [16,17]. The difference in the expression of T cell activation molecules CD25 and HLA-DR on pulmonary T cells between corticosteroid-resistant and corticosteroid-sensitive IP is possibly due to the different kinetics of the expression of these molecules after T cell activation [15]. CD25 is induced rapidly within 12 h after in vivo antigen administration and disappears by 24 h, whereas HLA-DR is induced within 24 h and expressed for 2 weeks after antigen administration [15]. Therefore, it is suggested that CD25 expression reflects T cell activation states more sensitively than that of HLA-DR and suggests the presence of continuous T cell activation.
We also demonstrate that pulmonary T cells produce IFN-γ in both corticosteroid-resistant and corticosteroid-sensitive IP in DM/PM. IFN-γ activates macrophages to produce proinflammatory cytokines such as IL-1 and TNF-α and superoxide that cause tissue damage [18–20]. It has recently been shown that IFN-γ- producing Th1 cells activate alveolar macrophages to induce IP through TNF-α production in mice [17]. IFN-γ also induces the expression of adhesion molecules intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on vascular endothelial cells which allows migration of inflammatory cells into alveolar spaces [21,22]. Therefore, it is suggested that IFN-γ production by pulmonary T cells may be importantly involved in IP in DM/PM.
Corticosteroid suppresses T cells by inhibiting cytokine production [23] and inducing apoptosis [24]. On the other hand, it has also been shown that some activated T cells are resistant to corticosteroid-induced inhibition [24–26]. Corticosteroid could not block the proliferation and IFN-γ production of activated CD45RO+ CD4+ memory T cells, but cyclosporin prevented it [26]. In addition, activated T cells were rescued from apoptosis by corticosteroid through IL-2 secretion [24,25]. Thus, strongly activated pulmonary T cells that produce IFN-γ could be resistant to corticosteroid and could contribute importantly to the development of corticosteroid-resistant IP in DM/PM.
Our findings indicate that analysis of activation states of BALF T cells from patients with IP in DM/PM is thus a useful method for evaluating their corticosteroid responsiveness. This procedure is less invasive than open lung biopsy and repeated analysis is easily performed. In addition, we and others have reported that corticosteroid-resistant IP develops mostly in DM/PM patients without CPK elevation at the onset of IP [6,8]. We found that IP without CPK elevation was frequently resistant to corticosteroid therapy (82% of 17 cases) as compared with IP with CPK elevation (11% of 19 cases) [8]. Therefore, analysis of BALF T cells and measurement of CPK levels at the onset of IP are simple methods for the determination of corticosteroid therapy for IP in DM/PM.
It has been suggested that histopathological classification of IP in DM/PM using open lung biopsies would be useful for evaluating its corticosteroid responsiveness. Tazelaar et al. [7] showed that patients with diffuse alveolar damage (DAD) in DM/PM were resistant to corticosteroid therapy and had poor prognosis, whereas DM/PM patients with bronchiolitis obliterans with organizing pneumonia (BOOP) responded well to corticosteroid therapy. Katzenstein & Fiorelli [27] reported that some patients with IP in DM/PM represented nonspecific interstitial pneumonia (NSIP) that responded well to corticosteroid therapy. Therefore, it seems to be important future projects to elucidate the relationship between the activation states of BALF T cells including the expression of activation molecules and cytokine production and the histopathological classification of IP in DM/PM.
In summary, we have shown that activated Th1-type pulmonary T cells play an important role in the development of corticosteroid-resistant IP in DM/PM and that the increase in CD25+ CD8+ T cells among pulmonary T cells is a useful indicator for corticosteroid-resistant IP in DM/PM and hence may be an indicator for early use of cyclosporin.
Acknowledgments
We thank Drs S. Yoshida and H. Saito and Mr H. Takahashi for helpful discussion and technical assistance. This work was supported in part by grants from the Ministry of Education, Science and Culture and from the Ministry of Health and Welfare, Japan.
REFERENCES
- 1.Frazier AR, Miller RD. Interstitial pneumonitis in association with polymyositis and dermatomyositis. Chest. 1974;65:403–7. doi: 10.1378/chest.65.4.403. [DOI] [PubMed] [Google Scholar]
- 2.Songcharoen S, Raju SF, Pennebaker JB. Interstitial lung disease in polymyositis and dermatomyositis. J Rheumatol. 1980;7:353–60. [PubMed] [Google Scholar]
- 3.Salmeron G, Greenberg SD, Lidsky MD. Polymyositis and diffuse interstitial lung disease. Arch Intern Med. 1981;141:1005–10. [PubMed] [Google Scholar]
- 4.Dickey BF, Myers AR. Pulmonary disease in polymyositis/ dermatomyositis. Semin Arthritis Rheum. 1984;14:60–76. doi: 10.1016/0049-0172(84)90010-6. [DOI] [PubMed] [Google Scholar]
- 5.Lakhanpal S, Lie JT, Conn DL, Martin WJ., 2nd Pulmonary disease in polymyositis/dermatomyositis. a clinicopathological analysis of 65 autopsy cases. Ann Rheum Dis. 1987;46:23–9. doi: 10.1136/ard.46.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takizawa H, Shiga J, Moroi Y, Miyachi S, Nishiwaki M, Miyamoto Y. Interstitial lung disease in dermatomyositis: clinicopathological study. J Rheumatol. 1987;14:102–7. [PubMed] [Google Scholar]
- 7.Tazelaar HD, Viggiano RW, Pickersgill J, Colby TV. Interstitial lung disease in polymyositis and dermatomyositis. Clinical features and prognosis as correlated with histologic findings. Am Rev Respir Dis. 1990;141:727–33. doi: 10.1164/ajrccm/141.3.727. [DOI] [PubMed] [Google Scholar]
- 8.Nawata Y, Kurasawa K, Takabayashi K, et al. Corticosteroid resistant interstitial pneumonitis in dermatomyositis/polymyositis. prediction and treatment with cyclosporine. J Rheumatol. 1999;26:1527–33. [PubMed] [Google Scholar]
- 9.Gruhn WB, Diaz-Buxo JA. Cyclosporine treatment of steroid-resistant interstitial pneumonitis associated with dermatomyositis/polymyositis. J Rheumatol. 1987;14:1045–7. [PubMed] [Google Scholar]
- 10.Maeda K, Kimura R, Komuta K, Igarashi T. Cyclosporine treatment for polymyositis/ dermatomyositis: is it possible to rescue the deteriorating cases with interstitial pneumonitis? Scand J Rheumatol. 1997;26:24–9. doi: 10.3109/03009749709065660. [DOI] [PubMed] [Google Scholar]
- 11.Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344–7. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 12.Crystal RG, Gadek JE, Ferrans VJ, Fulmer JD, Line BR, Hunninghake GW. Interstitial lung disease. Current concepts of pathogenesis, staging and therapy. Am J Med. 1981;70:543–68. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- 13.Feutren G, Mihatsch MJ. Risk factors for cyclosporine-induced nephropathy in patients with autoimmune diseases. N Engl J Med. 1992;326:1654–60. doi: 10.1056/NEJM199206183262502. [DOI] [PubMed] [Google Scholar]
- 14.Workshop summary and guidelines. Investigative use of bronchoscopy, lavage, and bronchial biopsies in asthma and other airway diseases. J Allergy Clin Immunol. 88:808–14. doi: 10.1016/0091-6749(91)90189-u. [DOI] [PubMed] [Google Scholar]
- 15.Yachie A, Miyawaki T, Uwadana N, Ohzeki S, Taniguchi N. Sequential expression of T cell activation (Tac) antigen and Ia determinants on circulating human T cells after immunization with tetanus toxoid. J Immunol. 1983;131:731–5. [PubMed] [Google Scholar]
- 16.Enelow RI, Mohammed AZ, Stoler MH, Liu AN, Young JS, Lou YH, Braciale TJ. Structural and functional consequences of alveolar cell recognition by CD8+ T lymphocyte in experimental lung disease. J Clin Invest. 1998;102:1653–61. doi: 10.1172/JCI4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark JG, Madtes DK, Hackman RC, Chen W, Cheever MA, Martin PJ. Lung injury induced by alloreactive Th1 cells is characterized by host-derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol. 1998;161:1913–20. [PubMed] [Google Scholar]
- 18.Gifford GE, Lohmann-Matthes ML. Gamma interferon priming of mouse and human macrophages for induction of tumor necrosis factor production by bacterial lipopolysaccharide. J Natl Cancer Inst. 1987;78:121–4. doi: 10.1093/jnci/78.1.121. [DOI] [PubMed] [Google Scholar]
- 19.Nathan CF, Murray HW, Wiebe ME, Rubin BY. Identification of interferon-γ as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983;158:670–89. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams JG, Jurkovich GJ, Hahnel GB, Marie RV. Macrophage priming by interferon-γ: a selective process with potentially harmful effects. J Leukoc Biol. 1992;52:579–84. doi: 10.1002/jlb.52.6.579. [DOI] [PubMed] [Google Scholar]
- 21.Pober JS, Gimbrone MA, Jr, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893–6. [PubMed] [Google Scholar]
- 22.Thornhill MH, Wellicome SM, Mahiouz DL, Lanchbury JS, Kyan-Aung U, Haskard DO. Tumor necrosis factor combines with IL-4 or IFN-γ to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991;146:592–8. [PubMed] [Google Scholar]
- 23.Gillis S, Crabtree GR, Smith KA. Glucocorticoid-induced inhibition of T cell growth factor production. I. The effect on mitogen-induced lymphocyte proliferation. J Immunol. 1979;123:1624–31. [PubMed] [Google Scholar]
- 24.Brunetti M, Martelli N, Colasante A, Piantelli M, Musiani P, Aiello FB. Spontaneous and glucocorticoid-induced apoptosis in human mature T lymphocytes. Blood. 1995;86:4199–205. [PubMed] [Google Scholar]
- 25.Iwata M, Hanaoka S, Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol. 1991;21:643–8. doi: 10.1002/eji.1830210316. [DOI] [PubMed] [Google Scholar]
- 26.Brinkmann V, Kristofic C. Regulation by corticosteroids of Th1 and Th2 cytokine production in human CD4+ effector T cells generated from CD45RO− and CD45RO+ subsets. J Immunol. 1995;155:3322–8. [PubMed] [Google Scholar]
- 27.Katzenstein AL, Fiorelli RF. Nonspecific interstitial pneumonia/ fibrosis. Histologic features and clinical significance. Am J Surg Pathol. 1994;18:136–47. [PubMed] [Google Scholar]