Abstract
Oral pemphigoid (OP) is a chronic autoimmune disease, involving the oral cavity, characterized by a homogenous linear deposition of immunoglobulins, complement, or both along the basement membrane zone (BMZ) and a subepithelial blister formation. The α6/β4 heterodimer is an integrin family of adhesion receptors, which mediates basal cell to matrix interactions. Recent evidence suggests a pathophysiologic role for antibodies against human α6 integrin in blister formation in OP, in organ culture studies. Fifty percent of OP patients have been reported to experience disease progression to involve other mucosal tissues, including the eye and larynx. To prevent this extension of disease, systemic therapy with systemic corticosteroids, dapsone, and immunosuppressive agents has been recommended. The use of intravenous immunoglobulin (IVIg) in the treatment of pemphigoid has been recently described. In this study, we present the use of IVIg, in a group of seven patients, with severe OP, in whom systemic conventional treatment was contraindicated. To determine the influence of treatment on antibodies to human α6 integrin in OP, seven patients with OP treated with IVIg therapy and a comparable control group of seven patients with OP, treated with conventional therapy, were evaluated at monthly intervals, for a 12 consecutive month treatment period. An effective clinical response was observed in all seven patients treated with IVIg therapy, after a mean treatment period of 4·5 months. IVIg therapy induced a prolonged and sustained clinical remission in all seven patients after a mean treatment period of 26·9 months. A statistically significant difference was observed in the quality of life pre- and post-IVIg therapy (P < 0·001). Both the study and the control groups had a very similar initial serological response to treatment. A statistically significant reduction in the antibody titres was observed after four months of treatment, in both groups (P = 0·015). Thereafter, patients treated with IVIg therapy had a faster rate of decline in the antibody titres, and the difference in the rate of decline between the study and control groups became statistically significant after six months of treatment (P = 0·03). The use of IVIg therapy resulted in reduction of antiα6 antibody titres and in inducing and maintaining both a sustained, clinical and serological remission.
Keywords: oral pemphigoid, intravenous immunoglobulin therapy, human alpha 6 integrin, antibody titres, immunoblot assay
INTRODUCTION
Oral pemphigoid (OP) is a rare and chronic autoimmune disease. Patients with OP present with vesicles, bullae, or erosions limited to the oral cavity, and/or desquamative gingivitis [1,2]. The clinical diagnosis is made on the basis of clinical presentation, established by histology, and confirmed by immunpathological studies [1–5].
Biopsy of an oral lesion demonstrates a subepithelial vesicle with mixed inflammatory cell infiltrate in the submucosa. A homogenous smooth linear deposition of immunoglobulins, complement, or both, are present along the basement membrane zone (BMZ) on direct immunofluorescence (DIF) examination of perilesional tissue [1–4].
The adherence of the epithelium to the basement membrane occurs through the interaction of various adhesion molecules [5–8]. Alteration of one of these molecules, can result in the loss of adhesion between the basal epithelial cell and the basement membrane, and result in the formation of a subepithelial blister [6–8]. One of the molecules that mediates this adhesion is the α6/β4 heterodimer, within the hemidesmosomes [7]. Recent studies have demonstrated, that sera from OP patients, and monoclonal and polyclonal antibodies to human α6 integrin, bind to a 120-kD protein, which has been characterized as human α6 integrin, present in normal human gingiva and bovine gingiva [9,10]. Histologic changes characteristic of OP have been observed when normal human buccal mucosa is incubated with sera of patients with OP containing antibodies to human α6-integrin [10].
In mucous membrane pemphigoid, which involves multiple mucosae, several target antigens have been identified. Some of these include BP Ag2 (180 kD), laminin 5, and human B4 integrin [2,11]. The treatment of choice for localized lesions is topical corticosteroids [12–14]. Approximately, 50percent of patients with oral pemphigoid, have been reported, to progress to involve extraoral sites, such as the eye, larynx, pharynx, or oesophagus [15]. Systemic therapy with systemic corticosteroids, dapsone, and immunosuppressive agents is considered conventional therapy or standard of care, for patients whose disease is progressive and nonresponsive to topical care [1,2,16–18]. In some patients, the use of these treatments is contraindicated for several reasons including anaemia, severe diabetes mellitus, osteoporosis, drug induced hypersensitivity reactions, renal insufficiency, steri-lity, and psychological side-effects [19,20]. Such patients require an alternative treatment modality. The use of intravenous immunoglobulin (IVIg) has been recently reported to be successful in treating oral pemphigoid (OP) and ocular cicatricial pemphigoid (OCP) patients, in whom conventional therapy had failed or produced significant and disabling side-effects [21,22].
In this study, we have presented the use of IVIg in seven patients with severe oral pemphigoid, in whom the use of systemic corticosteroids and immunosuppressive agents was contraindicated. After the initiation of IVIg therapy, antibody titres to human α6 integrin, were determined at monthly intervals, over a 12-month period, in these seven patients. The patients and the data presented in this study, has not been described in any of our earlier publications.
MATERIALS AND METHODS
Study group
This study presents seven patients with severe (3+) pemphigoid disease limited to the oral cavity. The severity of disease was determined by the Ciarrocca and Greenberg scale [16], described as follows: 1 = mild, 2 = desquamative gingivitis, 3 = generalized severe disease. Two patients were male and five were female. The age of onset ranged from 36 to 64 years (mean 55·5). Since the use of systemic corticosteroids, dapsone, and immunosuppressive agents was contraindicated for various reasons (Table 1), IVIg was the only systemic treatment used, in all seven patients, to treat their pemphigoid disease.
Table 1.
Clinical data, contraindications to the use of conventional therapy, treatment outcomes, and long-term follow-up in 7 patients with severe oral pemphigoid treated with IVIg Rx
| Contraindications to the use of conventional therapy | Quality of life | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient no. | Age at onset | Sex | Duration of disease Pre-IVIg Rx (in months) | Systemic corticosteroids | Dapsone | Immuno-suppressive agents | Time to achieve an effective clinical response (in months) | Total duration of IVIg Rx (in months) | Total no. of IVIg cycles | Total duration of clinical remission (in months) | Total observation period (in months) | Pre- IVIg Rx | Post IVIg Rx |
| 1 | 64 | M | 11 | Osteoporosis Rib fracture | Unexplained anaemia | Unexplained chronic anaemia | 3·7 | 17 | 10 | 22 | 50 | 1·50 | 4·50 |
| 2 | 58 | F | 9 | 1) Peptic ulcer with GI* bleed | Allergy to sulpha medication/urticaria | Multiple family members with history of cancer | 4·5 | 24 | 12 | 15 | 48 | 2·25 | 4·25 |
| 2) Labile hyper-tension requiringmultiple drug Rx | |||||||||||||
| 3 | 36 | F | 7 | 1) Peptic ulcer with GI bleed | Severe vomiting and diarrhea from sulpha medication | Concerns about sterility and teratogenicity | 4·8 | 28 | 16 | 18 | 51 | 2·25 | 4·50 |
| 2) Labile hyper-tension requiring multiple drug Rx | |||||||||||||
| 4 | 51 | F | 13 | Hx of severe psychosis with earlier usefor other medical reasons | G-6-PD below normal levels | Multiple small skin cancers requiring surgery, malignant polyp in the colon, & suspicious breast nodule | 5·2 | 31 | 18 | 36 | 80 | 1·75 | 4·25 |
| 5 | 63 | F | 15 | Severe diabetes mellitus | Allergic to sulfa medication | Multiple family members with history of cancer | 4·6 | 33 | 15 | 17 | 65 | 2·50 | 4·75 |
| 6 | 57 | F | 10 | Glaucoma | G-6-PD below normal limits | History of breast cancer (4 years ago) | 3·2 | 21 | 14 | 28 | 59 | 2·50 | 4·75 |
| 7 | 60 | M | 9 | Severe diabetes mellitus and other resultant medical complications (Renal insufficiency, peripheral neuropathy) | Allergic to sulpha medication | Severe renal insufficiency | 5·5 | 34 | 18 | 16 | 59 | 2·50 | 4·75 |
GI, Gastrointestinal
Diagnostic and inclusion criteria
The following criteria needed to be fulfilled for inclusion in this study.
The presence of pemphigoid disease manifesting as vesicles, bullae, or erosions, limited to the oral cavity and/or desquamative gingivitis;
Patients in both the study and control groups had severe (3+) oral disease;
A detailed physical examination to exclude involvement of other mucosae or the skin and an endoscopic examination by an ear, nose, and throat (ENT) surgeon, to exclude any upper aerodigestive pathway involvement. Detailed examination by an ophthalmologist, including a slit lamp examination and schrimer's test, and documentation of lack of conjunctival involvement and normal tear gland function;
Routine histology of oral biopsy demonstrating a submucosal vesicle with mixed inflammatory cell infiltrate in the submucosa [1,2];
Direct immunofluorescence examination of perilesional oral tissue, demonstrating deposition of IgG and/or C3 on the basement membrane zone [3];
Indirect immunofluorescence on salt split skin (SSS) demonstrating binding of the patient's sera to the epidermal side of the split [4];
Loss of weight of 15 pounds or more during six to 12-month period prior to the initiation of IVIg therapy. This weight loss was due to inability to eat from severe oral disease.
Contraindications to the use of systemic corticosteroids, dapsone, and immunosuppressive agents [19,20];
IVIg treatment protocol
The IVIg treatment protocol used has been recently described [21,22].
Local treatments
Adjuvant local therapy was used to facilitate the healing process [25]. If lesions persisted, a similar regime of sublesional injection with triamcinolone acetonide was given to patients in both the study group and control group [25]. Steroid serum cortisol levels were measured in the IVIg treated group, to determine that sublesional injections of triamcinolone were not systemically absorbed in any substantial degree.
Clinical response to IVIg therapy
The following objective parameters were used to determine the clinical outcome of IVIg therapy [25].
Duration to achieve an effective clinical response.
Duration of maintenance therapy. This was defined as the total time period in months, between the time of effective clinical response and the end point of therapy.
Total number of IVIg cycles.
Total duration of IVIg therapy.
Total observation period. This was the time interval between the initial diagnosis and the last office visit of the patient.
Quality of life. All patients evaluated their quality of life on a basic numeric scoring system. The scoring system they used is as follows 1 = poor, 2 = unsatisfactory, 3 = livable, 4 = reasonably good, and 5 = high quality of life [21,22]. This evaluation was done both, at the initiation of IVIg therapy and at the patient's last office visit.
Control group
Seven random patients with severe (3+) oral pemphigoid were studied simultaneously. The were two males and five females, with a mean age onset of 55 (range 35–65) years. The disease severity was identical to the study group. All 7 patients received a mean dose of 45 (40–65) mg of prednisone. In addition three patients received dapsone and one patient received each azathioprine, methotrexate, tacrolimus, and cyclosporin. The treatment details of the patients are not the focus of this paper. The purpose of studying these patients was to determine the monthly titres of antibody to human α6-integrin over 12 consecutive months period after the initiation of therapy. These titres were compared to the group treated with IVIg therapy.
Serological assay
Immunoblot assay
An immunoblot assay (IBA) was performed using bovine gingival lysate (BGL) as substrate. BGL has been used as an appropriate substrate, to identify the antigens involved in the pathogenesis of a autoimmune mucocutaneous blistering diseases [9]. BGL was prepared according to the protocol described earlier [9].
Serum samples were serially diluted. The highest titres which distinctly demonstrated binding to a 120-kD protein, was considered as the titre of the anti α6 antibody, in the test sample. If no binding to the 120 kD was observed, the test sample was considered negative, for the presence of an antibody to human α6-integrin [10].
During the treatment period, serum samples from 14 patients with OP, were collected monthly and antibody titres to human α6-integrin were determined in these samples over a 12-month period. In the seven patients who received IVIg therapy, if the date of drawing the serum sample coincided with the first day of the IVIg cycle, the sera to determine the antibody titre to human α6-integrin was drawn before beginning the IVIg infusion.
Specificity of immunoblot assay
To study the binding specificity, sera of all 14 study patients with OP, five patients with pemphigus vulgaris (PV), three with bullous pemphigoid (BP), 15 with mucous membrane pemphigoid (MMP), seven with OCP, two with epidermolysis bullosa acquisita (EBA), one with linear IgA bullous disease (LABD), and 25 from normal healthy individuals were used. The positive control for the assay was antibody to α6-integrin (BQ16). To determine if antibody to human α6-integrin was present in the IVIg preparation used to treat patients, samples from six batches of IVIg, from different manufacturers, were used in the IBA.
Blocking experiments
Total IgG fraction from OP patients or antibody to human α6 integrin (BQ16) was coupled to cyanogen bromide activated Sepharose 4B, as previously described [26]. BGL was repeatedly passed through these columns. These absorbed lysates were used in the immunoblot assay and reacted with sera from patients with OP or antibody to human α6 integrin.
Serum levels of antitetanus toxoid
Serum levels of antibodies to tetanus toxoid were determined by an ELISA, in the same samples that were tested for the presence of human α6 integrin [27]. The purified tetanus toxoid antigen (lot#TP-1001) was obtained from the Biology Laboratory of the University of Massachusetts. All 14 patients did not receive a tetanus toxoid injection during the two year period, prior to the initiation of the study or during the 12 month study period.
Correlation between disease severity and serological titre of antibody to human α6 integrin
We studied 39 patients with OP. In all 39 patients, the disease was limited to the oral cavity during a 36–50 month (range 44·2) period. The diagnosis was based on clinical features, established by the presence of a submucosal blister and confirmed by deposition of IgG and/or C3 on the basement membrane zone (BMZ) of perilesional mucosa. The severity of clinical disease was classified as 1+, 2+, or 3+ based on the scoring system of Ciarrocca and Greenberg [16]. The sera was collected during the active stage of the disease, prior to the institution of therapy and frozen at −70°C. in 27 patients. The sera was compared with 12 patients with OP now in a prolonged remission. Prolonged remission was defined as the absence of disease for a minimum of 3 years and no systemic or topical therapy. Sera of 25 patients normal human volunteers and 15 patients with MMP involving multiple mucosae were studied and used as controls. The titre of the antibody to human α6-integrin was determined by a modified immunoblot using bovine gingival lysate. The 7 patients treated with IVIg and 7 patients treated with conventional therapy, reported in this study, were not included amongst the 39 patients with OP in whom a correlation between disease severity and antibody titres was studied. The immunoblot was performed by individuals who were unaware of the severity of the disease in these patients.
Statistical analysis
The pre-IVIg and post-IVIg values for the quality of life were statistically analysed using the SAS UNIVARIATE software and running the two sided Wilcoxon sign test. Antibody titres to human α6-integrin were statistically analysed for both the study and control groups, using the SAS UNIVARIATE software running the 1-sample student t and signed rank tests, and SAS GLM running 2-sample student t-tests and anova[28,29].
RESULTS
Parameters to assess clinical outcome
Response to IVIg Therapy was assessed using the following parameters.
Before the initiation of IVIg therapy, the duration of disease ranged from 7 to 15 months;
The total number of IVIg cycles ranged from 10 to 18 cycles (mean 15);
The time period for patients to achieve an effective clinical response ranged from 3·2 to 5·5 months (mean 4·5);
The duration of maintenance therapy ranged from 13·3 to 25·8 (mean 22·4) months. None of the patients deteriorated while receiving IVIg therapy, and the intervals between the cycles were prolonged as per the designed protocol;
All seven patients went into a sustained remission after receiving a total duration of IVIg therapy which ranged from 17 to 34 months (mean 26·9);
The total observation period ranged from 48 to 80 months (mean 58·9);
All patients described an improvement in the quality of life after IVIg therapy. The quality of life on the numerical scale, pre-IVIg therapy ranged from 1·5 to 2·5 (mean 2·2) and post-IVIg therapy, 4·2–4·8 (mean 4·5). This difference was statistically significant (P < 0·001).
Immunoblot assay
Specificity of assay
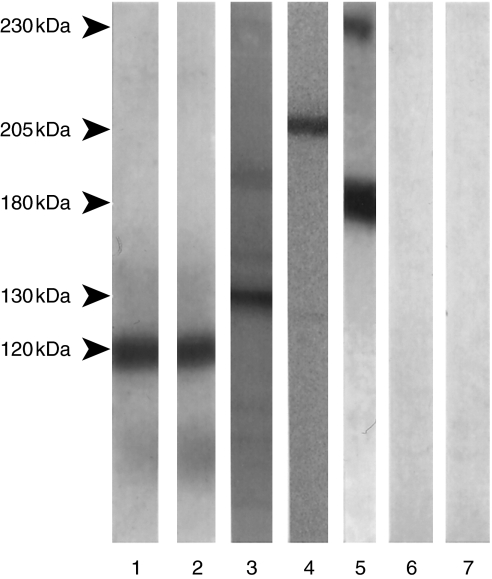
The serum of all 14 tested patients with oral pemphigoid demonstrated binding to a 120-kD protein in bovine gingival lysate. The antibody to human α6 integrin bound to a 120-kD protein in the BGL [10]. PV serum bound to a 130-kD protein, BP serum bound to 230 and 180 kD proteins, and sera of 15 patients with MMP bound to a 205-kD protein (Fig. 1) [30–32]. EBA sera bound to a 290-kD protein and LABD sera bound to a 97-kD protein (data not shown) [33,34]. No binding to BGL was observed in the six batches of IVIg preparations and in the sera of 25 normal human controls.
Fig. 1.
Specificity of immunoblot assay; binding pattern of test sera on an immunoblot assay, using bovine gingival lysate as substrate. Lane 1: Immunoblot of sera from a patient with oral pemphigoid. Note binding to a 120-kD protein. Lane 2: Immunoblot performed with a monoclonal antibody to human α6 integrin. Note binding to a 120-kD protein. Lane 3: Immunoblot of serum from a patient with pemphigus vulgaris. Note binding to a 130-kD protein. Lane 4: Immunoblot of serum from a patient with mucous membrane pemphigoid. Note binding to a 205-kD protein. Lane 5: Immunoblot of serum from a patient with bullous pemphigoid. Note binding to both 230 kD (BP Ag1) and 180 kD (BP Ag2) proteins. Lanes 6 & 7: Immunoblots with serum from a normal healthy individual and a patient with oral erosive lichen planus, used as negative controls. Note absence of binding.
Absorption study
When BGL absorbed with OP sera and immunoblotted with antibody to α6 integrin (BQ16), binding to a 120-kD was not observed. Similarly when BGL was absorbed with anti-α6 antibody and immunoblotted with OP sera, binding to the 120 kD was not observed (data not shown).
Influence of systemic therapy on the antibody to human α6-integrin
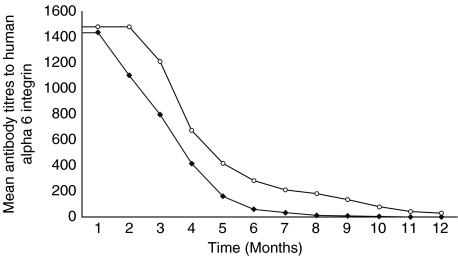
Compared to the initial titre, before the institution of systemic therapy or IVIg, a statistically significant (P = 0·015) reduction in the antibody titre observed after four months of treatment in both groups. After 4 months of treatment, a larger decrease in the mean antibody titres was observed in patients treated with IVIg therapy, and hence a faster rate of decline in the antibody titres in patients treated with IVIg therapy. The difference in the mean rate of decline between the two groups became statistically significant at month six of therapy (P = 0·03). All seven patients treated with IVIg therapy, achieved nondetectable antibody titre, after a mean treatment period of 7·2 months (range 5–10). Antibody titres were not detected in the sera of six of seven patients in the control group after a mean treatment period of 10·7 months (range 9–12). The difference between the mean antibody titres in the two groups was not statistically significant at the end point of the study. The mean of the titres of the antibody to human α6 integrin, in the two groups is graphically presented in Fig. 2.
Fig. 2.
Comparison of antibody titres to human α6 integrin in OP patients treated with IVIg (✦) and conventional therapy (○).
Serum levels of antitetanus toxoid antibody during IVIg therapy
There was no statistically significant difference in the serum levels of antitetanus toxoid antibodies during the 12-month period of this study, in both the study and control groups.
Correlation between disease severity and serological titre of antibody to human α6 integrin
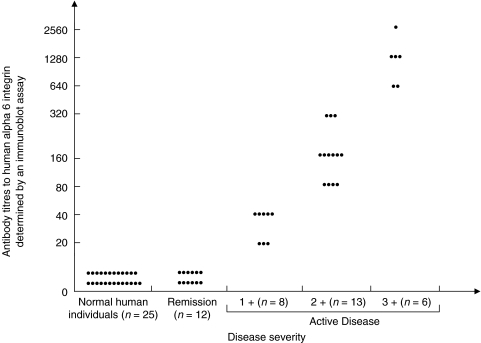
No detectable levels of antibody to human α6 integrin were observed in the sera of 12 patients in remission, sera of 15 patients with MMP who had involvement of multiple mucosae, or in normal human volunteers. The antibody titres in 8 patients with 1+ disease were 1:20–1:40. In 13 patients with 2+ disease, the titres ranged between 1:80 and 1:320. Titres greater than or equal to 1:640 dilution of patients was found in 6 patients with 3+ disease. This data is presented in Fig. 3.
Fig. 3.
Correlation between disease activity and serum levels ofantibodies to human α6 integrin. A direct relationship between antibody titres to human α6 integrin and disease activity is observed in 27 patients with active disease. Antibody titres to human α6 integrin are nondetectable in patients in 12 patients who are in remission and 25 normal healthy individuals.
DISCUSSION
In this study we have described the successful use of IVIg in seven patients with severe oral pemphigoid, in whom the use of dapsone, systemic corticosteroids, and immunosuppressive therapy was contraindicated.
Prior to the initiation of IVIg therapy, these patients had a minimum weight loss of 15 pounds. This was due to decreased oral intake, secondary to pain and discomfort from eating. Since conventional agents were contraindicated, it was medically necessary to treat these patients with an alternative therapy because severe recurrent nonresponsive disease, could lead to blindness from ocular involvement and death from sudden asphyxiation secondary to fibrosis from recurrent laryngeal disease [2,15]. The successful use of IVIg in treating OCP and OP was considered as evidence that IVIg therapy may be a reasonable alternative [21,22].
In a recent study, it has been demonstrated that IVIg therapy is superior to conventional therapy in treating patients with severe oral pemphigoid [21]. Patients treated with conventional therapy had multiple side-effects to systemic corticosteroids and immunosuppressive agents, continued to have a recurrence of their disease, and over 50% demonstrated extra-oral disease accompanied by a poor quality of life. In contrast, patients treated with IVIg therapy on the described protocol, achieved a sustained and prolonged clinical remission, had minimal side-effects, did not have extra-oral disease, and a high quality of life [21].
After the initiation of IVIg therapy, patients had an effective clinical response after a mean time period of 4·5 months. During the maintenance therapy phase, the patients were clinically disease free, and the IVIg was gradually withdrawn until the end point of therapy was achieved. Earlier studies had demonstrated that abrupt cessation of IVIg therapy, after effective clinical response, frequently resulted in clinical recurrence [21,25]. Therefore, maintenance therapy was considered an integral component of the treatment protocol, in order to maintain a sustained and prolonged clinical remission. Hence, all seven patients continued to receive IVIg maintenance therapy over a mean time interval of 22·4 months. During maintenance therapy, a gradual withdrawal of the IVIg therapy was made while maintaining the clinical response.
Extraoral involvement was not observed in these seven patients during a mean total observation period of 58·9 months. Hence, it appears that IVIg may have the capacity to arrest disease progression. All seven patients described an improvement in the quality of their lives. This difference in the quality of life pre- and post-IVIg therapy was statistically significant.
The long-term follow-up was essential to determine the efficacy of the treatment and its impact on the clinical course of the disease, since OP is a chronic disease. After the discontinuation of IVIg therapy, patients were observed to be in a prolonged remission over a mean period 26·9 months. Since IVIg was used as monotherapy, this sustained remission could be attributed to the effect of IVIg therapy. Therefore, it would appear that IVIg had the potential to influence the natural or clinical course of the disease.
Side-effects from IVIg therapy have been previously reported [23,24]. However, the incidence of side-effects of IVIg therapy is relatively low compared to other alternative treatments. None of our seven patients experienced any significant side-effects.
It has been proposed that there are five potential mechanisms for the action of IVIg that may facilitate or enhance the achievement of its clinically beneficial effects [35]. In any disease process, one or more of these mechanisms may be involved. Different mechanisms may operate during different stages of the disease. The precise mechanism of action of IVIg in OP or MMP is presently not known.
Epidermal Langerhans cells and cytokines have been reported to be involved in inflammation [35]. It has been reported that antialpha antibody completely prevents the spontaneous migration of Langerhans cells [36]. This would suggest an important role for human alpha 6 integrin in the migration of Langerhans cells from the epidermis to the draining lymph node [36]. Although the role of Langerhans cells is not clearly understood in OP, it is possible that the antibody to alpha 6 integrin may interfere with the mobility of Langerhans cells, and thus play a role in the pathogenesis of OP [36].
In this preliminary study, a direct correlation was observed between the severity of the disease in the patients and the titres of the antibody to human α6-integrin. The antibody was absent in patients in a prolonged remission and not detected in the sera of patients with MMP involving multiple mucosal membranes and normal healthy individuals. This assay may help distinguish the OP antibody from the MMP antibody.
The influence of treatment on antibodies to human α6- integrin were compared between patients treated with IVIg therapy and conventional therapy. Both groups were comparable since they were similar in age, sex, and disease severity. A statistically significant decline was observed after four months of treatment in both groups. After four months, patients treated with IVIg therapy, had increased rate of decline in the antibody titres and the difference in the rate of decline between the study and control groups became statistically significant after six months of treatment. The titres of antibody to tetanus toxoid did not change during the study period in both groups. These results would also suggest that patients treated with IVIg therapy, achieved an earlier serological remission, compared to those treated with conventional therapy. The decline and sustained absence of antibody to α6-integrin, in OP patients treated with IVIg therapy, suggest that IVIg may play a role in the restoration of normal human immunoregulatory mechanism, perhaps through idiotypic– anti-idiotypic interactions, as recently described [35].
Serum antibody titres have also been studied and followed after the initiation of IVIg treatment in other autoimmune diseases [37–39]. Similar patterns of decline in antibody titres have been reported in both autoimmune mucocutaneous diseases and other antibody mediated diseases [36,38].
In conclusion, the data presented in this study suggests that IVIg produced a prolonged and sustained clinical remission without significant side-effects, in patients with OP in whom dapsone, systemic corticosteroids, and immunosuppressive agents were contraindicated. It appears that the use of IVIg can arrest disease progression and result in the reduction of antibody titres to human α6-integrin and maintain a sustained serological remission. This study suggests a multicentre trial is warranted, to define the role of IVIg therapy in the management of patients with severe oral pemphigoid.
REFERENCES
- 1.Mobini N, Nagarwalla N, Ahmed AR. Oral pemphigoid. Oral Surg Oral Med Oral Path Oral Radiol Endod. 1998;85:37–43. doi: 10.1016/s1079-2104(98)90395-x. [DOI] [PubMed] [Google Scholar]
- 2.Fleming TE, Korman NJ. Cicatricial Pemphigoid. J Am Acad Dermatol. 2000;43:571–91. doi: 10.1067/mjd.2000.107248. [DOI] [PubMed] [Google Scholar]
- 3.Laskaris G, Angelopoulos A. Cicatricial pemphigoid. direct and indirect immunofluorescence studies. Oral Surg Oral Med Oral Pathol. 1981;51:48–54. doi: 10.1016/0030-4220(81)90125-0. [DOI] [PubMed] [Google Scholar]
- 4.Gammon WR, Briggaman RA, Inman A, Queen LL, Wheeler LE. Differentiating anti-lamina lucida and anti-sub lamina densa anti-BMZ antibodies by indirect immunofluorescence on 1.0 M sodium chloride-separated skin. J Invest Dermatol. 1984;82:139–44. doi: 10.1111/1523-1747.ep12259692. [DOI] [PubMed] [Google Scholar]
- 5.Scully C, Carrozzo M, Gandolfo S, Puiatti P, Monteil R. Update on mucous membrane pemphigoid: a heterogeneous immune-mediated subepithelial entity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:56–68. doi: 10.1016/s1079-2104(99)70194-0. [DOI] [PubMed] [Google Scholar]
- 6.Barradori L, Sonnenberg A. A structure and function of hemidesmosome: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411–8. doi: 10.1046/j.1523-1747.1999.00546.x. [DOI] [PubMed] [Google Scholar]
- 7.Stepp MA, Spurr-Michaud S, Tisdale A, Elwewll J, Gipson I. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosome. Proc Natl Acad Sci U S A. 1990;87:8970–4. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones J, Downer CS, Speight PM. Changes in the expression of integrins and basement membrane proteins in benign mucous membrane pemphigoid. Oral Dis. 1995;1:159–65. doi: 10.1111/j.1601-0825.1995.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 9.Engineer L, Johnson RE, Bhol KC, Ahmed AR. Bovine gingival lysate. a novel substrate for rapid diagnosis of autoimmune vesiculo-bullous diseases. A preliminary observation. Exp Dermatol. 2000;9:271–4. doi: 10.1034/j.1600-0625.2000.009004271.x. [DOI] [PubMed] [Google Scholar]
- 10.Bhol KC, Goss L, Kumari S, Colon JE, Ahmed AR. Autoantibodies to human alpha 6 integrin in patients with oral pemphigoid. J Dent Res. 2001;80:1711–5. doi: 10.1177/00220345010800080601. [DOI] [PubMed] [Google Scholar]
- 11.Colón JE, Bhol KC, Razzaque MS, Ahmed AR. In vitro organ culture model for mucous membrane pemphigoid. Clin Immunol. 2001;98:229–34. doi: 10.1006/clim.2000.4972. [DOI] [PubMed] [Google Scholar]
- 12.Lamey PJ, Rees TD, Binnie WH, Rankin KV. Mucous membrane pemphigoid. Oral Surg Oral Med Oral Pathol. 1992;74:50–3. doi: 10.1016/0030-4220(92)90214-b. [DOI] [PubMed] [Google Scholar]
- 13.Lozada F, Siliverman S., Jr Topically applied fluocinonide in an adhesive base in the treatment of oral vesiculoerosive diseases. Arch Dermatol. 1980;116:898–901. [PubMed] [Google Scholar]
- 14.Lozada-Nur F, Huang MZ, Zhou G. Open preliminary trial of clobetasol propionate ointment in adhesive pastefor treatement of chronic oral vesiculoerosive diseases. Oral Surg Oral Med Oral Pathol. 1991;71:283–7. doi: 10.1016/0030-4220(91)90300-2. [DOI] [PubMed] [Google Scholar]
- 15.Rogers RS III, Sheridan PJ, Nightingale SH. Desquamative gingivitis. Clinical, histopathologic, immunopathologic, and therapeutic observations. J Am Acad Dermatol. 1982;7:729–35. doi: 10.1016/s0190-9622(82)70153-7. [DOI] [PubMed] [Google Scholar]
- 16.Ciarrocca KN, Greenberg MS. A retrospective study of the management of oral mucous membrane pemphigoid with dapsone. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 1999;88:159–63. doi: 10.1016/s1079-2104(99)70110-1. [DOI] [PubMed] [Google Scholar]
- 17.Dave VK, Vickers CFH. Azathioprine in the treatment of mucocutaneous pemphigoid. Br J Dermatol. 1993;90:89–93. doi: 10.1111/j.1365-2133.1974.tb06383.x. [DOI] [PubMed] [Google Scholar]
- 18.Sami N, Bhol KC, Ahmed AR. Intravneious Immunoglobulin therapy in patients with multiple mucosal involvement in mucous membrane pemphigoid. Clin Immunol. 2002;102:59–67. doi: 10.1006/clim.2001.5150. [DOI] [PubMed] [Google Scholar]
- 19.Macdonald CJ. Immunosuppressive agents for use in dermatology. J Am Acad Dermatol. 1985;12:753–75. doi: 10.1016/s0190-9622(85)70097-7. [DOI] [PubMed] [Google Scholar]
- 20.Williams LC, Nesbitt LT., Jr Update on systemic glucocorticoids in dermatology. Dermatol Clin. 2001;19:63–77. doi: 10.1016/s0733-8635(05)70230-8. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed AR, Colon JE. Comparison between intravenous immunoglobulin and conventional immunosuppressive therapy regimens in patients with severe oral pemphigoid: Effects of disease progression in patient nonresponsive to dapsone therapy. Arch Dermatol. 2001;137:1181–9. doi: 10.1001/archderm.137.9.1181. [DOI] [PubMed] [Google Scholar]
- 22.Foster CS, Ahmed AR. Intravenous immunoglobulin therapy for ocular cicatricial pemphigoid: a preliminary study. Opthalmology. 1999;106:2136–43. doi: 10.1016/S0161-6420(99)90496-7. [DOI] [PubMed] [Google Scholar]
- 23.Mobini N, Sarela A, Ahmed AR. Intravenous immunoglobulins the therapy of autoimmune and systemic inflammatory disorders. Ann Allergy Asthma Immuno. 1995;74:119–28. [PubMed] [Google Scholar]
- 24.Rutter A, Luger TA. High-dose intravenous immunoglobulins. an approach to treat severe immune-mediated and autoimmune diseases of the skin. J Am Acad Dermatol. 2001;44:1010–24. doi: 10.1067/mjd.2001.112325. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed AR. Intravenous immunoglobulin therapy in the treatment of patients with pemphigus vulgaris unresponsive to conventional immunosuppressive treatment. J Am Acad Dermatol. 2001;45:679–90. doi: 10.1067/mjd.2001.116339. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi S, Bhol K, Natarajan K, Livir-Rallatos C, Foster CS, Ahmed AR. Ocular cicatricial pemphigoid antigen. partial sequence and biochemical characterization. Proc Natl Acad Sci USA. 1996;93:14714–9. doi: 10.1073/pnas.93.25.14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristinsen M, Aggerback H, Heron I. Improved ELISA for determination of anti-diptheria and/or anti-tetanus antitoxin antibody in sera. APMIS. 1997;105:843–53. doi: 10.1111/j.1699-0463.1997.tb05093.x. [DOI] [PubMed] [Google Scholar]
- 28.Dixon WJ. Introduction to Statistical Analysis. 4. New York: McGraw-Hill; 1983. [Google Scholar]
- 29.Scheffe H. The Analysis of Variance. New York: John Wiley; 1959. [Google Scholar]
- 30.Hashimoto T, Amagai M, Garood DR, Nishikawa T. Immunofluorescence and immunoblot studies on the reactivity of pemphigus vulgaris and pemphigus foliaceus sera with desmoglein 3 and desmoglein 1. Epithelial Cell Biol. 1995;4:63–9. [PubMed] [Google Scholar]
- 31.Korman NJ. Bullous Pemphigoid. The latest in the diagnosis, prognosis, and therapy. Arch Dermatol. 1998;134:1137–41. doi: 10.1001/archderm.134.9.1137. [DOI] [PubMed] [Google Scholar]
- 32.Bhol KC, Dans MJ, Simmons RK, Foster CS, Giancotti FG, Ahmed AR. The autoantibodies to alpha 6 beta 4 integrin of patients affected by ocular cicatricial pemphigoid recognized predominantly epitopes within the large cytoplasmic domain of human beta4. J Immunol. 2000;165:2824–9. doi: 10.4049/jimmunol.165.5.2824. [DOI] [PubMed] [Google Scholar]
- 33.Woodley DT, O'Keefe EJ, Reese MJ, Mechanic GL, Briggaman RA, Gammon WR. Epidermolysis bullosa acquisita antigen, a new major component of cutaneous basement membrane, is a glycoprotein with collagenous domains. J Invest Dermatol. 1986;86:668–72. doi: 10.1111/1523-1747.ep12275978. [DOI] [PubMed] [Google Scholar]
- 34.Ishiko A, Shimizu H, Masunaga T, et al. 97 kDa linear IgA bullous dermatosis (LAD) antigen localizes to the lamina lucida of the epidermal basement membrane. J Invest Dermatol. 1996;106:739–43. doi: 10.1111/1523-1747.ep12345793. [DOI] [PubMed] [Google Scholar]
- 35.Mouthon L, Kaveri SV, Spalter SH, et al. Mechanisms of action of intravenous immunoglobulin in immune-mediated diseases. Clin Exp Immunol. 1996;104:3–9. [PubMed] [Google Scholar]
- 36.Price AA, Cumberbatch M, Kimber I, Ager A. Alpha 6 integrins are required for Langerhans cell migration from the epidermis. J Exp Med. 1997;186:1725–35. doi: 10.1084/jem.186.10.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahaly GJ. Effect of intravenous gammaglobulin on organ specific antibodies and lymphocyte subsets. Clin Exp Rheum. 1996;14:S37–40. [PubMed] [Google Scholar]
- 38.Levy Y, Sherer Y, Ahmed A, et al. A study of 20 SLE patients treated with intravenous immunoglobulin-clinical and serologic response. Lupus. 1999;8:705–12. doi: 10.1191/096120399678841007. [DOI] [PubMed] [Google Scholar]
- 39.Letko E, Bhol KC, Foster CS, Ahmed AR. Influence of intravenous immunoglobulin therapy on serum levels of anti-B4 antibodies in ocular cicatricial pemphigoid. Curr Eye Res. 2000;21:646–54. [PubMed] [Google Scholar]