Abstract
We studied immune reconstitution against the parasite T. gondii in HIV-infected patients treated for 1 years with highly active antiretroviral therapy (HAART). We used SCID mice, humanized with peripheral blood mononuclear cells (PBMC) from patients, which were then infected with T. gondii cysts. Mice humanized with PBMC from patients before the start of HAART were highly susceptible to infection. In contrast, mice humanized with PBMC from patients who had received HAART for 6 months displayed higher survival rates, correlating with lower intracerebral parasite loads. However, this resistance was lost during follow up because mice humanized with PBMC from patients treated with HAART for 12 months survived for no longer than mice that had not been humanized. Specific lymphocyte proliferation assays showed that the increase in proliferative response depended on treatment duration and that HAART induced changes in IFN-γ secretion in the presence of Toxoplasma antigens. Thus, our results indicate partial immune reconstitution against T. gondii in HIV-infected patients following HAART, possibly due to changes in the patterns of specific IFN-γ production and redistribution of functional memory CD4+ cells
Keywords: HIV, HAART, SCID, immune reconstitution, Toxoplasma gondii
INTRODUCTION
HIV infection involves a high rate of viral replication in CD4+ T cells, resulting in the progressive loss of and functional defects in these T cells [1]. CD4 depletion, the functional impairment of this subset and the subsequent breakdown of cellular immunity contribute to the emergence of opportunistic infections by intracellular pathogens, which are the main cause of morbidity and mortality in AIDS [2,3]. HAART results in the strong and sustained inhibition of HIV replication [4], which is associated with an increase in the number of CD4+ T cells, even in patients with advanced disease [5]. However, there has been intense debate concerning the functional integrity of the reconstituted T cells. Some data suggest that HAART restores lymphocyte function because reconstituted CD4+ T cells respond to opportunistic pathogens [6,7], whereas others suggest that the immune system is only partially reconstituted [5]. Most of the published studies have been based on in vitro tests such as lymphoproliferative response (LPR) [8], cytokine production [9] and specific cytotoxicity [10,11] assays. However, conflicting results have been obtained regarding the immunological response in vitro to specific antigens. Thus, in vivo assays are required to increase our understanding of HAART-induced restoration of the immune response in HIV-infected patients. Very few studies have investigated the delayed-type hypersensitivity (DTH) response [12,13] or assessed the agreement between the detection of a specific anti-antigen response in vitro and the epidemiological data on the incidence of opportunistic infections [14,15]
Toxoplasma gondii is a protozoan obligate intracellular parasite that infects humans. The specific immune response activated during infection in immunocompetent individuals results in the partial clearance of the parasite. It remains in the body, in the central nervous system in particular, encysted in the form of long-lived bradyzoites, resulting in asymptomatic chronic infection. In immunocompromised individuals, such as HIV+ patients, the loss of CD4+ T cells correlates with the reactivation of T. gondii infection, leading to severe tissue damage, such as Toxoplasma encephalitis (TE), which is often fatal, even with appropriate therapy [16]. Although the mechanisms responsible for reactivation of the disease in these immunocompromised subjects is unclear, various studies have demonstrated that CD4+ T cells play an important role in controlling reactivation [17,18]. The use of experimental models has also provided important evidence that IFN-γ production by CD8+ T cells induces immunity to Toxoplasma infection [19,20]. These cells probably act additively or synergistically with CD4+ T cells, to prevent the reactivation of chronic T. gondii infection [20,21].
The aim of this study was to use an appropriate animal model to investigate immune reconstitution against the parasite T. gondii in HIV-infected patients treated for 1 years with HAART. We used the severe combined immune deficiency (SCID) mouse model for this study. SCID mice are unable to reject xenografts. They are therefore a suitable model for reconstitution with human B and T cells. We demonstrate that human PBMC partially protect mice against Toxoplasma gondii challenge. We found that HIV+ patients displayed both a time-dependent increase in specific lymphoproliferative responses and changes in antigen-specific secretion of IFN-γ during HAART, which may account for these survival results
PATIENTS AND METHODS
Patients and study design
We enrolled 10 HIV-1-positive patients from three participating centres (CIRBS, The Pasteur Institute Hospital and Broussais Hospital, Paris, France). Patients were protease inhibitor-naïve, seropositive for T. gondii and had pretreatment CD4+ T cell counts ≤350/mm3. Treatment with a protease inhibitor-containing HAART regimen was then initiated. The study was approved by the local ethical review boards and all subjects gave written informed consent. CD4+ T cell counts, HIV RNA concentration, CD4+ T-cell responses to mitogens and antigens (Ags) were measured at baseline, and then 6 and 12 months after HAART initiation. We also carried out assays to evaluate reconstitution of the immune response to T. gondii in PBMC-SCID mice, after 0, 6 and 12 months of HAART. Four HIV-negative individuals (two T. gondii-seropositive and two T. gondii-seronegative volunteers) were used as controls. Plasma HIV-1 RNA levels were measured with a commercial quantitative reverse transcriptase (RT) polymerase chain reaction kit (Amplicor HIV Monitor Test; Roche Molecular Systems Branchburg, NJ, USA) with a mean detection limit of 2·3 log10 copies/ml.
Mice
We obtained 6 to 10-week-old-female SCID/beige mice from the Central Experimental Institute for Animals, Japan. Mice were kept in sterile conditions. All manipulations were performed and samples taken in aseptic conditions. All mice were ‘nonleaky’ SCID, as shown by enzyme-linked immunosorbent assay (ELISA) and mice with Ig levels >1 μg/ml were excluded from the experiments
In vitro lymphocyte proliferation response (LPR) assays
PBMCs isolated from heparin-treated blood samples by density gradient separation were cultured in 96-well culture plates at a density of 1 × 105 cells per well in 0·2 ml of RPMI-1640 supplemented with antibiotics and 10% human AB serum (Valbiotech, France) in the presence or absence of 5 μg/ml phytohaemagglutinin (PHA, Sigma, France), 0·25 μg/ml staphylococcal enterotoxin B (SEB, Sigma, France) and 5 μg/ml soluble tachyzoite antigens from the T. gondii parasite (Chemicon International, Inc. CA, USA). LPRs were quantified by monitoring the incorporation of tritium-labelled thymidine (0·037 MBq, 1 μCi per well; Amershan, UK) for 18 h, in 5-day triplicates cultures; the radioactivity incorporated was counted in a β-scintillation counter. Results are expressed as net cpm (the difference between median cpm in the Ag-containing cultures and median cpm in cultures in medium alone), and as stimulation index (SI, the ratio between median cpm in the Ag-containing cultures and median cpm in cultures in medium alone). SI values > 3 were considered positive
Cytokine production
The production of IL-2, IL-4, IL-5, IL-10, IFN-γ and TNF-α was quantified in supernatants from stimulated mononuclear cells over a period of 72 h, using PHA, SEB or soluble tachyzoite antigens (Chemicon International, Inc. CA, USA), or medium alone. Cytokine production was determined in triplicate, with the CBA Kit, according to the manufacturer's instructions (Becton Dickinson, San Diego, CA, USA)
Flow cytometry analysis
The numbers of circulating T cells and their subsets was determined by standard four-colour fluorescence staining and flow cytometry, using fluorescent beads as an internal standard (Beckman-Coulter Immunotech, France), monoclonal antibodies and whole blood samples. The cells were identified by staining with the following monoclonal antibodies (moAb): anti- CD45-FITC, anti-CD4-RD1, anti-CD8-ECD, anti-CD3-PC5, anti-CD45RA-FITC, anti-CD45RO-FITC; anti-CD38-RD1 and anti-HLA DR-FITC (Coulter Corp, USA). Blood samples (100 μl) were incubated with these conjugated moAb for 30 min, in darkness. The red blood cells were lysed and the samples were washed with PBS. They were then immediately analysed in a Coulter EPICS-XL-MCL (Beckman-Coulter) flow cytometer. Samples were gated on live leucocytes’, forward and side light scatter. We acquired 10 000 events per sample with this gate. The normal values obtained in our laboratories are (mean ± SD in cells/μl): CD3: 1835 ± 302; CD4: 935 ± 335; CD8: 684 ± 162
Protocol for SCID mouse humanization
Mice were injected intraperitoneally (i.p.), three days before reconstitution, with a moAb directed against NK cells (1 mg/mice), TMβ-1 (Dr Toshiyuki Tanaka, Japan), which is an anti-interleukin 2 receptor β-chain moAb [22]. The animals were simultaneously injected i.p. with 200 μl of a liposome stock solution to which di-chloromethylene di-phosphonate (Cl2MDP) (Roche Diagnostics, Mannheim, Germany) was added to deplete macrophages [23]. Mice were also injected i.p. with an antipolymorphonuclear neutrophil moAb (0·3 mg/mouse), NIMP-R14 [24], one day before reconstitution (kindly provided by Dr M. Strath, National Institute for Medical Research, London, UK). Mice were reconstituted by i.p. injection of 1 × 107 live cells in 0·5 ml of serum-free RMPI medium
Measurement of human immunoglobulins in sera
We determined the levels of human IgG and IgM in the plasma of reconstituted SCID mice every 10 days by ELISA. Briefly, 96-well immunosorbent plates (Nunc, Denmark) coated with 5 μg/ml of mouse antihuman Ig (H + l) (Jackson ImmunoResearch Laboratory Inc. West Grove, PA, USA) were incubated with mice sera. Human and normal mouse sera were used as controls and purified human IgG and IgM were used as the calibration standard (Jackson ImmunoResearch Laboratory Inc. West Grove, PA). The reaction was detected by incubation with specific peroxidase-conjugated mouse antihuman IgG and antihuman IgM (Jackson ImmunoResearch Laboratory Inc.) followed by O-phenylenediamine reaction (SIGMA Chemical Company, St. Louis, MO, USA). We used an automatic microplate reader to measure absorbance at 490 nm
Toxoplasma gondii infection
A strain of T. gondii (BOU) originally obtained from the brain of an HIV-infected patient was kindly provided by Dr Darde (Hôpital Universitarie Dupuytren, France). This strain was used for the experimental infection of mice. Parasites were maintained by serial passage in Swiss-Webster female mice. Animals were infected by i.p. inoculation with 50 cysts. Six to eight weeks later, mice were killed and their brains were removed, placed in 4 ml PBS and homogenized. In each experiment, we used the following groups of mice: (i) mice humanized with PBMC from T. gondii-seropositive HIV+ patients; (ii) mice humanized with PBMC from T. gondii-seronegative HIV− donors (PBMC toxo−); (iii) mice humanized with PBMC from T. gondii-seropositive HIV− donors (PBMC toxo+); (iv) nonhumanized mice (control). For each group, PBMC from one patient (D0, M6 and M12) were included in the same set of experiments. The mice in all groups were injected i.p. with 50 cysts 10 days after humanization. The survival of the animals was evaluated and the plasma levels of human anti-Toxoplasma IgG and IgM were determined by ELISA, 10 days after infection, according to the manufacturer's instructions (Sigma kit, Poole, Dorset, UK)
Histological studies
Brains were removed from animals that had died due to T. gondii infection and were fixed in 10% formalin and embedded in paraffin. Serial 5-μm sections were cut and stained with haematoxylin and eosin, and then with immunohistochemical reagents, to detect cysts and tachyzoites in all sections of the brain. For immunohistochemistry, we used rabbit polyclonal antibody against T. gondii and an indirect immunoperoxidase method (StrepABComplex/ horseradish peroxidase, HRP, Dako). All sections were counterstained with haematoxylin. For each series, positive and negative controls were used
Statistical analysis
Differences in the results were evaluated by the Student's two-tailed t-test for independent means. All P-values < 0·05 were considered significant. Kaplan-Meier survival curves were constructed for experimental groups, and differences in survival were assessed by Wilcoxon's rank sum test
RESULTS
Changes in plasma viral load and T cell subsets following HAART
Ten Toxoplasma-seropositive, HIV-1-infected patients were enrolled in this study. Median viral load on entry into the study was log 4·69 copies/ml (range, log 3·52 – log 6·05 copies/ml), decreasing to below the level of detection (<log 2·3 copies/ml) by week 8 of HAART in all subjects, and remaining undetectable over the one-year treatment period (data not shown)
The mean numbers of cells for each lymphocyte subset during treatment are shown in Table 1. Neither AIDS nor a TE event was detected among these patients during this 1-year follow-up period. Changes in CD4+ and CD8+ T cell subsets were monitored during treatment. The initiation of HAART was associated with a progressive increase in mean CD4+ T cell count. By month 12 of therapy, CD4+ T cell levels were higher than at baseline. HAART was associated with a substantial decrease over time in CD8 T cell counts in blood
Table 1.
Median values for lymphocyte subpopulation counts from HIV-infected patients before and after 6 and 12 months of HAART
| CD3CD4+ | CD3CD8+ | CD4CD45RA+ | CD4CD45RO+ | CD8CD45RA+ | CD8CD45RO+ | CD4CD38DR+ | CD8CD38DR+ | |
|---|---|---|---|---|---|---|---|---|
| D0* | 298 ± 34† | 816 ± 97 | 83 ± 20 | 215 ± 24 | 142 ± 26 | 680 ± 93 | 84 ± 18 | 313 ± 61 |
| M6 | 345 ± 39 | 778 ± 104 | 115 ± 17 | 231 ± 30 | 154 ± 11 | 631 ± 112 | 25 ± 7 | 109 ± 24 |
| M12 | 403 ± 33 | 423 ± 175 | 201 ± 99 | 263 ± 19 | 107 ± 50 | 139 ± 86 | 15 ± 8 | 21 ± 12 |
Duration of follow-up (months).
The absolute mean is expressed as cells/μl ± SD.
Both naïve and memory CD4+ T cells increased in number during therapy. In contrast, major differences were observed between naïve and memory CD8+ T cells: naïve CD8 cell levels did not change during treatment whereas the number of memory CD8+ T cells gradually decreased
A progressive decrease in the expression of activation markers on CD4+ and CD8+ T cells was also observed. Activated CD4+ T cell levels after 6 and 12 months of HAART were significantly different (P < 0·05) from those at HAART initiation. The population of activated CD8+ T cells was significantly smaller (P < 0·05) after 6 and 12 months of HAART than upon entry into the study
Increase in parasite-specific lymphocyte proliferation and changes in IFN-γ production during HAART
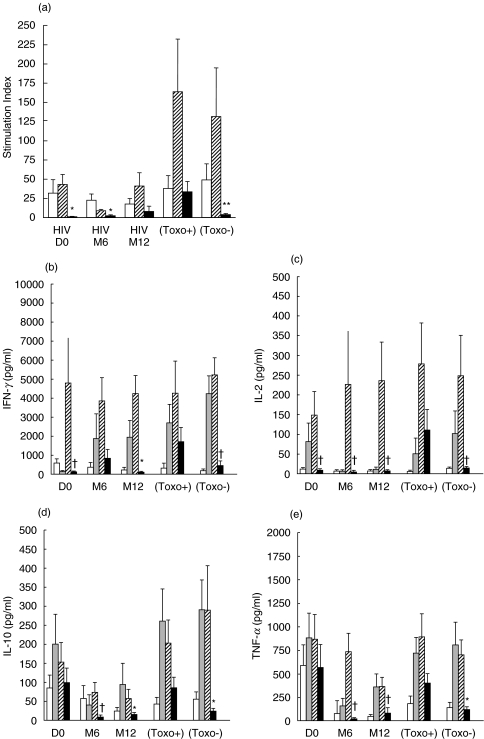
The immune status of HIV-infected subjects and donors was assessed by in vitro LPR and cytokine production assays in the presence of soluble tachyzoite antigens from T. gondii. Although the patients were seropositive for T. gondii, no parasite-specific response (Fig. 1a) or specific production of either IFN-γ or IL-2 was detected (Fig. 1b) before the initiation of HAART. However a small increase in the specific LPR was observed following treatment; this response was greatest after 12 months of HAART but was still weaker than the Toxo+ LPR (8·1 ± 6·7 versus 33·8 ± 13·1; P = 0·08), as shown in Fig. 1a. As expected, normal seronegative donors (Toxo−) displayed a weak proliferative response to Toxoplasma antigens (4·0 ± 1·3). The specific production of IFN-γ had increased by the sixth month of treatment, to levels similar to those seen in parasite-seropositive, HIV-negative donors (P = 0·1). However, this high level of IFN-γ production was not sustained over time, as the levels of secretion of this cytokine after 12 months of HAART were significantly lower than those in Toxoplasma-seropositive, HIV-negative individuals (P = 0·009). IL-2 production was not increased by HAART (D0: P = 0·02; M6: P = 0·01 and M12: P = 0·01), whereas the levels of specific production of IL-10 and TNF-α in PBMC from treated patients after 6 and 12 months of treatment were lower than those in PBMC from Toxoplasma-seropositive donors: IL-10 (P = 0·04 and P = 0·004, respectively) and TNF-α (P = 0·02 and p = 0·01, respectively). Levels of IL-4 production did not differ significantly between HIV patients (before or after HAART) and Toxoplasma-seropositive donors (data not shown). Finally, IL-5 production increased during the first six months to values similar to those in PBMC from Toxoplasma-seropositive donors, but were not significantly higher at the end of the 12-month period than at the beginning (data not shown). The Toxoplasma-seronegative donors displayed a weak response to Toxoplasma antigens, as reflected by lower levels of production of IL-2 (P = 0·01), IL-10 (P = 0·009), TNF-α (P = 0·006) and IFN-γ (P = 0·04) than were observed in parasite-immune donors. These results suggest that 1 years of therapy results in a gradual, small increase in the parasite-specific LPR, with a signifi-cant increase in the specific production of IFN-γ after six months. However, levels of IFN-γ production decreased by the end of the 12-month treatment period. No IL-2 production was detected following HAART
Fig. 1.
In vitro quantification of the reconstitution of the Toxoplasma-specific response during HAART. (a) Proliferation of lymphocytes specific for T. gondii Ag, PHA and SEB is shown as stimulation index. □ PHA,  SEB, ▪ Ag toxo. Differences were compared by the Student's t-test. *P < 0·005 versus Toxo+; **P < 0·05 versus Toxo+. Bars indicate SEM. The production of (b) IL-2, (c) INF-γ, (d) IL-10 and (e) TNF-α was quantified in mononuclear cell supernatants stimulated for 72 h with PHA, SEB and soluble T. gondii tachyzoite antigens. □ Cells,
SEB, ▪ Ag toxo. Differences were compared by the Student's t-test. *P < 0·005 versus Toxo+; **P < 0·05 versus Toxo+. Bars indicate SEM. The production of (b) IL-2, (c) INF-γ, (d) IL-10 and (e) TNF-α was quantified in mononuclear cell supernatants stimulated for 72 h with PHA, SEB and soluble T. gondii tachyzoite antigens. □ Cells,  PHA,
PHA,  SEB, ▪ Toxo. Differences in the means between the controls, donors and HIV-infected patients during treatment were compared by Student's t-test. *P < 0·01 versus Toxo+; †P < 0·05 versus Toxo+. Bars indicate SEM.
SEB, ▪ Toxo. Differences in the means between the controls, donors and HIV-infected patients during treatment were compared by Student's t-test. *P < 0·01 versus Toxo+; †P < 0·05 versus Toxo+. Bars indicate SEM.
Humanization of SCID mice
The PBMC obtained from the individuals included in the study were used to humanize SCID mice, as described in the patients and methods section. We verified that the SCID/beige mice were correctly humanized by assessing serum levels of human IgM and IgG 10 and 20 days after humanization (Table 2). On day 10, the plasma concentrations of both human IgG and human IgM exceeded 1 μg/ml in all humanized mice. Similar results have previously been reported for reconstituted SCID mice [25,26]. On day 20, plasma human IgG levels were found to have increased in all reconstituted animals, except the PBMC toxo- mice. Thus, the SCID environment provides all the immune factors required for B-cell function and antibody production
Table 2.
Plasma levels of human IgM and IgG and human Toxoplasma-specific immunoglobulin production in humanized SCID mice
| Days posthumanization in SCID mice | ||||||||
|---|---|---|---|---|---|---|---|---|
| D10 | D20 | |||||||
| Groups | HIgM (μg/ml) | HIgG (μg/ml) | α-T. gondii HIgM* | α-T. gondii HIgG* | HIgM (μg/ml) | HIgG (μg/ml) | α-T. gondii HIgM* | α-T. gondii HIgG* |
| Not humanized | 0·3 ± 0·1 | 0·2 ± 0·1 | <0·1 | <0·1 | 0·5 ± 0·2 | 0·4 ± 0·3 | <0·1 | <0·1 |
| HIV D0 | 4·8 ± 1·9 | 2·0 ± 0·7 | <0·1 | <0·1 | 2·4 ± 0·2 | 3·6 ± 1·8 | 0·83 ± 0·25 | 0·91 ± 0·35 |
| HIV M6 | 2·9 ± 1·6 | 1·7 ± 0·8 | <0·1 | <0·1 | 2·2 ± 0·9 | 2·2 ± 0·9 | 0·59 ± 0·10 | 0·59 ± 0·15 |
| HIV M12 | 6·7 ± 3·1 | 4·1 ± 2·3 | <0·1 | <0·1 | 6·5 ± 2·9 | 10·4 ± 8·5 | 0·66 ± 0·10 | 0·58 ± 0·14 |
| PBMC (toxo+) | 0·9 ± 0·5 | 1·7 ± 0·8 | <0·1 | <0·1 | 2·8 ± 1·3 | 9·4 ± 7·2 | 1·03 ± 0·12 | 1·03 ± 0·27 |
| PBMC (toxo−) | 1·3 ± 0·7 | 1·9 ± 1·1 | <0·1 | <0·1 | 1·2 ± 0·5 | 1·6 ± 0·6 | 0·60 ±0·15 | 0·46 ± 0·10† |
absorbance at 490 nm.
P < 0·005 versus PBMC (Toxo+).
Humanized SCID mice present a specific humoral response against T. gondii
The levels of human antigen-specific immunoglobulin production after infection with Toxoplasma are summarized for all SCID mice in Table 2. We found that SCID mice humanized with PBMC from different groups of individuals produced different levels of IgG or IgM antibodies 10 days after infection. Parasite–specific IgM were present at significantly higher levels (P < 0·05) in the PBMC toxo+ group than in the HIV M6 and HIV M12 groups. In contrast, although the PBMC toxo+ group presented higher levels of specific IgG (P < 0·05) than the PBMC toxo− group, specific IgG levels were similar in all the other groups
Increase in survival rates for humanized SCID mice infected with T. gondii
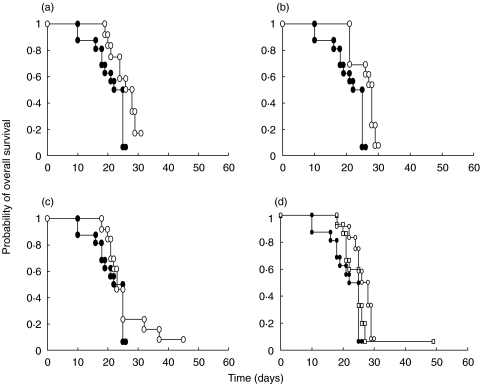
The overall results for survival are shown in Fig. 2. Unreconstituted SCID mice were highly susceptible to Toxoplasma infection: all animals died within 10–20 days of infection. SCID mice humanized with PBMC from Toxoplasma-seropositive donors displayed significantly higher levels of survival than the control unreconstituted mice (P = 0·002) following infection with the parasite. In contrast, mice of the Toxo− group were more susceptible to infection, with survival not differing significantly from the controls (P = 0·07) (Fig. 2d). Similar results were obtained for mice of the HIV D0 group, which were highly susceptible to infection and displayed levels of survival close to those of the control group (P = 0·06) (Fig. 2a). The mice of the HIV M6 group were resistant to infection and their survival rates were significantly higher than those of both the controls (P = 0·0007; Fig. 2b) and Toxo− group mice (P = 0·03). In contrast, the mice of the HIV M12 group were less resistant to infection and the survival rates of this group were similar to those of the control group (P = 0·2; Fig. 2c). Although, we did not observe a significant difference in the survival data between the M6 and M12 groups of mice (P = 0·4), it tended to differ between the M6 and D0 groups (P = 0·08). Thus, we observed partial reconstitution of the T. gondii-specific response in these patients during HAART, as reflected by the resistance of the reconstituted SCID mice to infection after 6 months of therapy. However, this resistance was lost after 12 months of treatment
Fig. 2.
Survival rates of T. gondii-infected SCID mice humanized with PBMC. (a) SCID mice humanized with PBMC from an HIV-infected patient at HAART initiation (○, HIV D0, n = 12, P = 0.06). (b) SCID mice humanized with PBMC from an HIV-infected patient after six months of HAART (○, HIV M6, n = 13, P = 0.0007). (c) SCID mice humanized with PBMC from an HIV-infected patient after 12 months of HAART (○, HIV M12, n = 13, P = 0.2). (d) SCID mice humanized with PBMC from HIV-negative subjects with (○, PBMC Toxo+, n = 12, P = 0.002) or without (□, PBMC Toxo−, n = 15, P = 0.07) T. gondii-specific immunoglobulins. • Control (n = 16 a–d). The figure shows data pooled from four independent experiments. Values of P < 0·05 were defined as statistically significant. Kaplan-Meier survival curves were constructed for experimental groups, and differences in survival were assessed by Wilcoxon's rank sum test
Differences in T. gondii parasite load
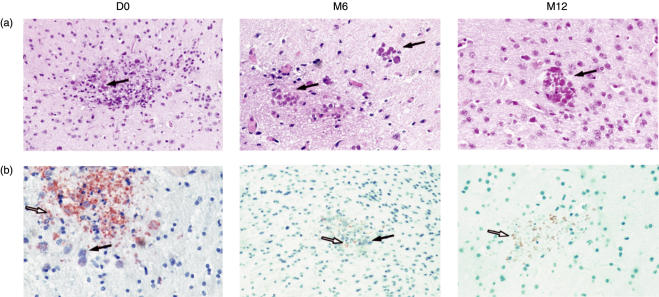
We aimed to investigate whether the different patterns of survival in infected SCID mice correlated with intracerebral parasite load. Quantification of the intracerebral parasite load showed that the numbers of cysts and tachyzoites varied with the duration of HAART in HIV-positive patients (Table 3). Numbers of Toxoplasma cysts and tachyzoites were significantly higher in the brains of animals humanized with PBMC from HIV-positive patients than in the brains of mice from the other groups at the beginning of HAART, whereas the mice of the HIV M6 group appeared to be more resistant to infection because they have fewer cysts and tachyzoites. Finally, the HIV M12 group presented moderate, intermediate infections, with the presence of small numbers of cysts and numerous tachyzoites (Fig. 3a,b and Table 3). These findings demonstrate that the small increase in survival, following infection with T. gondii, of SCID mice reconstituted with PBMC from patients treated with HAART for 6 months was correlated with a decrease in intracerebral parasite load, whereas the increase in susceptibility to infection observed in the HIV D0 and M12 mice groups was associated with an increase in parasite load
Table 3.
Intracerebral parasite load in SCID mice humanized with PBMC from H IV-infected patients during HAART
| Animal group | Number of cysts* | Number of tachyzoite foci (mean ± SEM)† |
|---|---|---|
| D0 | + + + + | 105 ± 15 |
| M6 | + | 5 ± 2* |
| M12 | + + to + + + | 77 ± 11** |
| PBMC Toxo + | + + | 8 ± 1* |
| PBMC Toxo − | + + + | 100 ± 21** |
The number of Toxoplasma cysts was determined on 400 high-power fields/section. A semiquantitative grading system was used to evaluate the number of cysts (+ 1–5 cysts; + + 5–10 cysts; + + + 10–20 cysts; + + + + more than 20). A total of 10–15 sections from various regions of the brain were counted, and data are expressed as the mean number of cysts/section for four mice per group.
The number of foci of Toxoplasma tachyzoites was determined on 400 high-power fields/section. A total of 10–15 sections from various regions of the brain were counted, and data are expressed as the mean (± SEM) number of tachyzoites/section for four mice per group. Differences between groups of animals in the mean number of tachyzoites were compared by Wilcoxon's test.
P < 0·05 versus D0
P < 0·05 versus M6
Fig. 3.
Representative sections of the brains of SCID mice, humanized with PBMC from HIV-infected patients after treatment with HAART for various periods of time, infected with T. gondii. (a) haematoxylin-eosin staining for cyst detection and (b) antiperoxidase moAb staining for tachyzoite detection. Brain parenchyma from a mouse humanized with PBMC from an HIV-infected patient before HAART (D0) shows necrotic foci, a large number of cysts (+ + + +) and numerous tachyzoites. Brain parenchyma from a mouse humanized with PBMC from an HIV-infected patient after 6 months of HAART (M6) shows necrotic foci, with a few cysts (+ to + +) and a few tachyzoites. Brain parenchyma from a mouse humanized with PBMC from an HIV-infected individual after 12 months of HAART (M12). The section shows moderate necrotic foci with a few cysts (+ + to + + +) and numerous tachyzoites. Original magnification: ×400
DISCUSSION
In this study, we demonstrated that HAART partially restored the immune response to T. gondii, as attested by the higher survival rates of SCID mice humanized with PBMC from treated HIV-infected patients, subsequently challenged with parasite cysts, compared with other non-humanized mice. Our findings also indicate that there is a time-dependent relationship between treatment and the immune response to Toxoplasma. Moreover, survival was associated with a detectable difference in intracerebral parasite load. The poor survival of mice in the HIV D0 group mice may be due to there being too few parasite-specific CD4+ or CD8+ T cell clones to induce a protective response to the parasite. This hypothesis is supported by the weak parasite–specific lymphoproliferative response and the low levels of specific IL-2 and IFN-γ production observed in the PBMC of untreated patients. IFN-γ has been shown to be essential to control parasite proliferation and prevent death due to TE in mice [20,27–30].
The pattern of cytokine secretion found in our cohort before therapy consisted of spontaneously high levels of production of IL-10 and TNF-α, with low levels of specific IL-2 and IFN-γ secretion. These results may be accounted for by the immune activation state in our patients, as attested the by high plasma viral load and increased expression of activation markers on CD4+ and CD8+ T cells. Our results are consistent with those of previous studies, showing an increase in the concentrations of cytokines such as IL-10 and TNF-α and a decrease in the secretion of IL-2 and IFN-γ throughout the course of HIV disease [31]
We observed an increase in the survival of SCID mice reconstituted with PBMC from patients, obtained after 6 months of therapy. This increase in survival was correlated with a decrease in intracerebral parasite load and an increase in IFN-γ production in the presence of parasite antigens. Our results may be accounted for by the redistribution and reactivation of functionally specific T cells after a decrease in viral replication in the lymphoid organs. Specific LPR assays showed only a slight increase in proliferative response to Toxoplasma antigens in HIV-infected patients after six months of treatment. Most of the patients studied had CD4+ T cell counts close to 250 cells/μl (Table 1), the critical cut-off point separating responders from nonresponders [13]. The significant increase in IFN-γ production seen at 6 months and in mouse survival does not directly correlate with CD4 count or CD4- specific proliferation, as shown by the results after 12 months of treatment
The reconstitution kinetics of CD4+ T cells may have important implications for the analysis of immune response reconstitution in HIV patients receiving HAART. In our cytometric analysis of the various cell subsets in HIV+ patients following HAART for one year, we observed an increase in the absolute number of CD4+ T cells (Table 1). Consistent with previous results [5,9,32], an increase in the number of memory CD4+ cells (CD4+CD45RO+) preceded the increase in the number of naïve CD4+ cells (CD4+CD45RA+) following HAART (Table 1). The first wave of memory CD4+ T cells resulted from either redistribution or the peripheral expansion of pre-existing cell populations and is unlikely to correspond to new immune recovery via de novo production of T cells [32]. In contrast, the increase in naïve CD4+ cell numbers probably corresponds to gradual repair and the slow reconstitution of a neo-immune system [33], though the extent of this recovery remains unclear
However, after 12 months of HAART, the humanized SCID mice were no longer resistant to T. gondii infection. It is possible that the memory T-cell clones that increase during treatment have a short half life and are therefore subjected to apoptosis. Moreover, naïve T cells may be unable (i) to exert adequate effector functions (low levels of production of IFN-γ and IL-2), or (ii) to expand their populations sufficiently in humanized SCID mice during treatment due to anergy (defect in APC priming or IL2 production). The results of Hellerstein et al.[34] demonstrated that, following the inhibition of viral replication after 12 weeks of HAART, the rates of production of circulating CD4+ and CD8+ T cells were high, but that the half-lives of these cells did not increase. Instead, the level of fractional replacement was higher and the half-life lower than those for CD4+ and CD8+ cells from untreated patients. The authors suggested that these results might reflect complex underlying population dynamics, with activated memory and naïve CD4+ T cells possibly presenting differences in survival after HAART
Finally, we also observed the Toxoplasma-specific secretion of human IgG or IgM following infection in SCID mice. However, this secretion was observed in all groups of humanized SCID mice, including those with low survival rates. These findings suggest that the specific humoral immune response may not play an important role in our model, consistent with previous reports [35,36].
In conclusion, this study of humanized SCID mice demonstrated that a 12-month antiretroviral regimen led to partial restoration of the specific immune response to T. gondii in HIV-infected patients. The observed protection against Toxoplasma may be due to a T cell-mediated response and is probably related to a change in the pattern of IFN- γ secretion and the redistribution of long-lasting memory CD4+ cells. Finally, the SCID mouse model described here has the potential to be a powerful and versatile tool for investigating the role of HAART in the restoration of the human immune response to pathogens, particularly when such experiments, for ethical or practical reasons, cannot be undertaken in humans
Acknowledgments
We thank Dr G. Dighiero for helpful discussions. We thank Dr Laure Dardé for providing the T. gondii strain, Dr Toshiyuki Tanaka for providing the TMβ-1 moAb, Dr Masayuki Miyasaka for providing SCID mice and M. Huot Khun for histological studies. We would also like to thank the patients who participated in this study.
REFERENCES
- 1.Marriott D, McMurchie M, Managing HIV. Part 2. Phases of disease. 2.4 HIV and advanced immune deficiency. Med J Aust. 1996;164:111–2. [PubMed] [Google Scholar]
- 2.Jaffe HW, Bregman DJ, Selik RM. Acquired immune deficiency syndrome in the United States: the first 1,000 cases. J Infect Dis. 1983;148:339–45. doi: 10.1093/infdis/148.2.339. [DOI] [PubMed] [Google Scholar]
- 3.Kovacs JA, Masur H. Opportunistic infections. In: De Vita TJ, Hellman S, Rosenberg S, editors. AIDS, Etiology, Diagnosis Treatment and Prevention. philadelphia: J.B. Lippincott and Co; 1992. pp. 199–225. [Google Scholar]
- 4.Collier AC, Coombs RW, Schoenfeld DA, et al. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. AIDS Clin Trials Group N Engl J Med. 1996;334:1011–7. doi: 10.1056/NEJM199604183341602. [DOI] [PubMed] [Google Scholar]
- 5.Autran B, Carcelain G, Li TS, et al. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–6. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 6.Carr A, Cooper DA. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet. 1997;350:589. doi: 10.1016/S0140-6736(05)63175-3. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson MA, Zegans M, Pavan P, O'Donnell JJ, Sattler F, Rao N, Owens S, Pollard R. Cytomegalovirus retinitis after initiation of highly active antiretroviral therapy. Lancet. 1997;349:1443–5. doi: 10.1016/S0140-6736(96)11431-8. [DOI] [PubMed] [Google Scholar]
- 8.Pontesilli O, Kerkhof-Garde S, Pakker NG, et al. Antigen-specific T-lymphocyte proliferative responses during highly active antiretroviral therapy [HAART] of HIV-1 infection. Immunol. Lett. 1999;66:213–7. doi: 10.1016/s0165-2478(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 9.Sondergaard SR, Aladdin H, Ullum H, Gerstoft J, Skinhoj P, Pedersen BK. Immune function and phenotype before and after highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 1999;21:376–83. [PubMed] [Google Scholar]
- 10.Greenough TC, Brettler DB, Somasundaran M, Panicali DL, Sullivan JL. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes [CTL], virus load, and CD4 T cell loss: evidence supporting a protective role for CTL in vivo. J Infect Dis. 1997;176:118–25. doi: 10.1086/514013. [DOI] [PubMed] [Google Scholar]
- 11.Pontesilli O, Klein MR, Kerkhof-Garde SR, Pakker NG, de Wolf F, Schuitemaker H, Miedema F. Kinetics of immune functions and virus replication during HIV-1 infection. Immunol Lett. 1997;57:125–30. doi: 10.1016/s0165-2478(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 12.Dolan MJ, Clerici M, Blatt SP, et al. In vitro T cell function, delayed-type hypersensitivity skin testing, and CD4+ T cell subset phenotyping independently predict survival time in patients infected with human immunodeficiency virus. J Infect Dis. 1995;172:79–87. doi: 10.1093/infdis/172.1.79. [DOI] [PubMed] [Google Scholar]
- 13.Wendland T, Furrer H, Vernazza PL, Frutig K, Christen A, Matter L, Malinverni R, Pichler WJ. HAART in HIV-infected patients. restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viremia. AIDS. 1999;13:1857–62. doi: 10.1097/00002030-199910010-00007. [DOI] [PubMed] [Google Scholar]
- 14.Egger M, Hirschel B, Francioli P, et al. Impact of new antiretroviral combination therapies in HIV-infected patients in Switzerland: prospective multicentre study. Swiss HIV Cohort Study BMJ. 1997;315:1194–9. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michelet C, Arvieux C, Francois C, et al. Opportunistic infections occurring during highly active antiretroviral treatment. AIDS. 1998;12:1815–22. doi: 10.1097/00002030-199814000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Luft BJ, Remington JS. AIDS commentary: toxoplasmic encephalitis. J Infect Dis. 1998;157:1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Oksenhendler E, Charreau I, Tournerie C, Azihary M, Carbon C, Aboulker JP. Toxoplasma gondii infection in advanced HIV infection. AIDS. 1994;8:483–7. doi: 10.1097/00002030-199404000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Pueyo S, Salmi LR, Chene G, et al. Survival after AIDS-defining events in patients with < 200 lymphocytes CD4+ × 106/l who are toxoplasmosis antibody-positive. ANRS 005/ACTG 154 Trial Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:459–464. doi: 10.1097/00042560-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 19.Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol. 1996;157:2103–018. [PubMed] [Google Scholar]
- 20.Suzuki Y, Orellana MA, Schreiber RD, et al. Interferon-gamma. the major mediator of resistance against Toxoplasma gondii. Science. 1998;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 21.Gazzinelli RT, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–80. [PubMed] [Google Scholar]
- 22.Tanaka T, Kitamura F, Nagasaka K, Urabe T, Orita K. Selective long-term elimination of natural killer cells in vivo by an anti-interleukin 2-receptor beta chain monoclonal antibody in mice. J Exp Med. 1993;178:1103–7. doi: 10.1084/jem.178.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Rooijen N, Sanders A. Liposome-mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Meth. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 24.Lopez AF, Strath M, Sanderson CJ. Differentiation antigens on mouse eosinophils and neutrophils identified by monoclonal antibodies. Br J Haematol. 1984;57:489–94. doi: 10.1111/j.1365-2141.1984.tb02923.x. [DOI] [PubMed] [Google Scholar]
- 25.Olive C, Cheung C, Falk M. T cell engraftment in lymphoid tissues of human peripheral blood lymphocyte reconstituted SCID mice with or without prior activation of cells. Immunol Cell Biol. 1998;76:520–5. doi: 10.1046/j.1440-1711.1998.00786.x. [DOI] [PubMed] [Google Scholar]
- 26.Carballido JM, Namikawa R, Carballido-Perrig N, Antonenko S, Roncarolo MG, de Vries JE. Generation of primary antigen-specific human T- and B-cell responses in immunocompetent SCID-hu mice. Nat Med. 2000;6:103–6. doi: 10.1038/71434. [DOI] [PubMed] [Google Scholar]
- 27.Alexander J, Hunter CA. Immunoregulation during toxoplasmosis. Chem Immunol. 1998;70:81–102. doi: 10.1159/000058701. [DOI] [PubMed] [Google Scholar]
- 28.Hunter CA, Abrams JS, Beaman MH, Remington JS. Cytokine mRNA in the central nervous system of SCID mice infected with Toxoplasma gondii: importance of T-cell-independent regulation of resistance to T. gondii. Infect Immun. 1993;61:4038–44. doi: 10.1128/iai.61.10.4038-4044.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson LJ. SCID mouse models of acute and relapsing chronic Toxoplasma gondii infections. Infect Immun. 1992;60:3719–24. doi: 10.1128/iai.60.9.3719-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schluter D, Deckert-Schluter M, Schwendemann G, Brunner H. Expression of major histocompatibility complex class II antigens and levels of interferon-gamma, tumour necrosis factor, and interleukin-6 in cerebrospinal fluid and serum in Toxoplasma gondii-infected SCID and immunocompetent C.B-17 mice. Immunology. 1993;78:430–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Pantaleo G, Fauci AS. Immunopathogenesis of HIV infection. Annu Rev Microbiol. 1996;50:825–54. doi: 10.1146/annurev.micro.50.1.825. [DOI] [PubMed] [Google Scholar]
- 32.Carcelain G, Blanc C, Leibowitch J, et al. T cell changes after combined nucleoside analogue therapy in HIV primary infection. AIDS. 1999;13:1077–81. doi: 10.1097/00002030-199906180-00011. [DOI] [PubMed] [Google Scholar]
- 33.Connors M, Kovacs JA, Krevat S, et al. HIV infection induces changes in CD4+ T-cell phenotype and depletions within the CD4+ T-cell repertoire that are not immediately restored by antiviral or immune-based therapies. Nat Med. 1997;3:533–40. doi: 10.1038/nm0597-533. [DOI] [PubMed] [Google Scholar]
- 34.Hellerstein M, Hanley MB, Cesar D, et al. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–9. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- 35.Chinchilla M, Frenkel JK. Specific mediation of celular immunity to Toxoplasma gondii in somatic cells of mice. Infect Immunol. 1984;46:862–6. doi: 10.1128/iai.46.3.862-866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vollmer TL, Waldor MK, Steinman L, Conley FK. Depletion of T-4 + lymphocytes with monoclonal antibody reactivates toxoplasmosis in the central nervous system: a model of superinfection in AIDS. J Immunol. 1987;138:3737–41. [PubMed] [Google Scholar]