Abstract
The lipophilic toxin, cereulide, emitted by emetic food poisoning causing strains of Bacillus cereus, is a powerful mitochondria toxin. It is highly lipophilic and rapidly absorbed from the gut into the bloodstream. We tested how this toxin influences natural killer (NK) cells, which are important effectors in defence against infections and malignancy. Cereulide inhibited cytotoxicity and cytokine production of natural killer cells, caused swelling of natural killer cell mitochondria, and eventually induced natural killer cell apoptosis. The suppressive effect on cytotoxicity was fast and toxic concentration low, 20–30 μg/l. As the emesis causing concentration of cereulide is around 10 μg/Kg of total body mass, our results suggest that emesis causing or even lower doses of cereulide may also have a systemic natural killer cell suppressive effect.
Keywords: Cereulide, NK cell, IL-15, IL-18
INTRODUCTION
Bacillus cereus is a gram-positive, spore-forming rod that grows both aerobically and anaerobically [1]. It is a normal soil inhabitant and frequently found in food. B. cereus is a growing problem especially in heat-treated foods, such as convenience foods and foods used in catering because of its resistance towards pasteurization and antimicrobial agents. It causes emetic and diarrheic types of food poisoning. The emetic toxin, cereulide, is not inactivated by food processing such as sterilizing by heat. It is a ring-structured dodecadepsipeptide, with three repeats of two amino and two hydroxy acid residues: (D-OLeu-D-Ala-L-OVal-L-Val)3[2]. B. cereus food poisoning is not a reportable disease and therefore its incidence is probably underestimated. B. cereus has been shown to be a causative agent in 33% of all cases of food poisonings (excluding viral aetiology) between years 1988–93 in Norway, 47% in Iceland, and 22% in Finland [3,4]. Lower values have been observed elsewhere: 0·7% in England and Wales and 0·8% in Japan [5].
Natural killer (NK) cells are lymphocytes that can lyse certain tumour cells and virus-infected cells without prior immunization [6]. In the immune system NK cells represent the first line defense. They kill abnormal cells and simultaneously secrete cytokines, such as interferon gamma (IFN-γ), tumour necrosis factor, granulocyte/macrophage colony-stimulating factor and macrophage colony-stimulating factor, to recruit other arms of the immune system [6].
In this paper we show that cereulide is toxic to NK cells. The toxicity is manifested as the inhibition of cytotoxicity and cytokine production, as well as mitochondrial swelling and eventual apoptosis of the NK cells. These results show that a bacterial toxin found in food and the environment has immunomodulating properties.
MATERIALS AND METHODS
Cell isolation and culture
Leucocyte-rich buffy coats were obtained from healthy blood donors (Finnish Red Cross Blood Transfusion Service, Helsinki, Finland). Peripheral blood lymphocytes (PBLs) were isolated by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density centrifugation. PBL were further purified by passing them through nylon wool columns in RPMI 1640 medium supplemented with glutamine, streptomycin/penicillin and 5% heat-inactivated foetal bovine serum (FBS) (Life Technologies, Germany). NK and T cells were isolated from PBL by two-step density gradient centrifugation on Percoll (Amersham Pharmacia Biotech) in 10% FBS/RPMI 1640. From the NK cell fraction, T cells were depleted by treatment with anti-CD3 antibody (Becton Dickinson, San Jose, CA, USA) and adsorption on immunomagnetic beads (Dynal, Oslo, Norway). From the T cell fraction, T cells were further enriched by depleting NK cells with anti-CD16 antibody treatment and adsorption on immunomagnetic beads. Monocytes were enriched from PBLs before nylon wool column treatment. Monocytes were isolated by Ficoll-Paque (Amersham Pharmacia Biotech) and subsequent Percoll (Amersham Pharmacia Biotech) gradient centrifugation. Human chronic myelogenic leukaemia K562 cells (American Type Culture Collection, Manassas, VA) and murine mastocytoma P-815 cells (American Type Culture Collection) were cultured in 10% FBS/RPMI 1640 at 37°C in humidified air atmosphere of 5% CO2. Malignant non-Hodgkin's lymphoma NK-92 cells (American Type Culture Collection) was cultured in MEM-α-medium (Life Technologies, Paisley, UK) supplemented with glutamine, streptomycin/penicillin, 12% horse serum (Life Technologies LTD., Auckland, New Zealand), 12% FBS, 100 IU/ml IL-2 (Chiron B.V. Amsterdam, the Netherlands) and 10−4 M β-mercaptoethanol.
Activation of T cells and monocytes
Purified T cells were incubated with 10% monocytes and activated by PHA, 1 μg/ml (Sigma, St. Louis, MO, USA) and IL-2 (Chiron) 500 IU/ml for 72 h, in 10% FBS/RPMI 1640 at 37°C in humidified air atmosphere of 5% CO2. Monocytes were activated in similar conditions for 48h by LPS 5 μg/ml (E. coli serotype 026:B6) (Sigma).
Treatment of cells with cereulides
NK cells were treated by cereulides for 1 min-2h (0–100 ng/ml). Monocytes were treated by 50 ng/ml F4810/72- cereulide for 48h, 24h and 2h. Both cells were incubated in 10% FBS/RPMI 1640 at 37°C in humidified air atmosphere of 5% CO2. In similar conditions T cells were treated by 50ng/ml F4810/72- cereulide for 72h, 24h and 2h. Control cells were incubated without the toxin.
Cytotoxicity assay
K562 target cells 0·5 × 106/ml were first labelled with 50 μCi of sodium 51Cr (Radiochemical Centre, Amersham, UK). After labelling, 100 μl of target cells (diluted to 0·5 × 105/ml) were added to 100 μl effector cell suspension (PBL or monocytes) (2·5 × 106 cell/ml), to produce the effector/target ratio 50:1. After 3h incubation at 37°C, 100 μl aliquots of supernatants from each well were counted in a gamma counter (Wallac, Turku, Finland). The percentage 51Cr-release was determined according to the formula:
as previously described [7].
T cells were tested similarly with P-815 target cells in an anti-CD3 redirected (Becton Dickinson, San Jose, CA, USA) killing assays as previously described [8]
JC-1 staining and epifluorecence microscopy
Toxin induced mitochondrial damage (24 h/37°C) of viable enriched NK cells was investigated by staining the cells with JC-1 Mitochondrial Potential Sensor (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanineiodide) (Molecular Probes Inc., Eugene, Or, USA) as described by Suominen et al.[9].
Transmission electron microscopy (TEM) of cells
The density gradient enriched NK cells (2–3 × 106/ml) were incubated for 3h at 37°C in a humidified atmosphere of 5% CO2 in the presence or absence of 100–1000 ng/ml of toxins. For fixation two protocols were employed: (i) conventional ambient-temperature chemical fixation (glutaraldehyde-osmium) and (ii) ultra-rapid cryofixation (slam-freezing) followed by freeze- substitution [10]. All samples were embedded in Epon. Then 80 nm-thin sections were poststained with aqueous uranyl-acetate and lead citrate (30 and 3 min at room temperature, respectively) and examined with a JEM 1200EX (Jeol, Tokyo, Japan) electron microscope at 60 kV. The yield of adequately preserved cells varied substantially after conventional ambient-temperature fixation.
Bacterial toxins
Cereulide was purified by HPLC from methanol extracts of B. cereus strains F-5881 and F4810/72. Because of its poor water solubility the purified cereulide was dispensed straight to 96-well plates or test tubes as a methanol solution. After the methanol had evaporated the cultured cells were dispensed into the wells. A blank assay was performed with methanol alone.
Purification and analysis of the emetic toxin from B. cereus strains F4810/72 and F-5881
The methanol extracts of B. cereus 4810/72 and F-5881 were diluted to 90% of methanol with water, injected to the Sep–pak C18 cartridge (Waters Co, Milford, MA, USA), washed with 90/10 methanol/water and finally eluted with methanol.
These methanol solutions were analysed by RP-HPLC (Smart, Pharmacia Biotech, Sweden). The column was Sephasil C8 SC 2·1 mm i.d. ×100 mm, 5 m, eluent A: water with 0·1% trifluoroacetic acid (TFA); eluent B: acetonitrile with 0·075% TFA. Gradient from 10% A and 90% B to 100% B in 5 min, flow rate 100 μl/min, detection at 215 nm. For injections methanol solutions were evaporated to dryness and dissolved in 90/10 acetonitrile/water with 0·075% TFA. Valinomycin (Sigma, St. Louis, MO, USA) was used as the standard compound for quantification of cereulide.
Mass spectrometry
Electrospray mass spectra (ESI-MS) were collected using an API300 triple quadrupole mass spectrometer (Perkin-Elmer Sciex Instruments, Thornhill, Ontario, Canada). The HPLC fractions were dissolved in 50% methanol containing 5 mm ammonium acetate, and injected into the mass spectrometer with a nanoelectrospray ion source (Protana A/S, Odense, Denmark) at a flow rate of about 30 nl/min. MS/MS spectra were acquired by colliding selected precursor ions to nitrogen collision gas with acceleration voltages of 45–55 V.
Apoptosis assay
Apoptosis was measured using an ApoAlert ™ Annexin V apoptosis kit (Clontech, CA, USA) with FITC-labelled annexin V. The results were analysed using a FACScan flow cytometer (Becton Dickinson).
Cytokines and cytokine ELISA
NK cells purified by a two-step density gradient were treated with toxins (100 ng/ml) for 2 h, followed by a 24-h incubation with 5 ng/ml of IL-12 (R & D Systems, Minneapolis, MN, USA), 5 ng/ml of IL-15 (R & D Systems), 20 ng/ml of IL-18 [11], or with their combinations. T cells were purified like NK cells and activated by PHA and IL-2. IFN-γ concentrations were measured from culture supernatants by ELISA using paired antibodies for IFN-γ (Diaclone, Besancone, France). IL-1-β concentrations from monocyte culture supernatants were measured by ELISA (R & D Systems, Abingdon, UK). Statistical differences were determined by the paired t-test using the Statview 512 program (Brainpower Inc., Calabasa, CA, USA).
RESULTS
Source and identification of cereulide
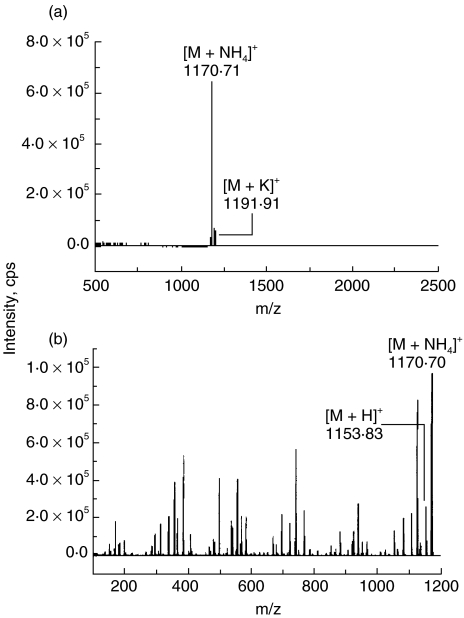
Cereulide was isolated from methanol extracts of both B. cereus strains F-5881 and F4810/72. It was purified by HPLC to a single peak and identified as the cereulide by ESI-MS and ESI-MS/MS (m/z 1170·70 and 1191·91) (Fig. 1). These purified toxins were used in this study.
Fig. 1.
(a) ESI-MS and (b) ESI-MS/MS spectra of the toxin from B. cereus F-5881. M + NH4]+ is ammonium adduct ion and M + H]+ is protonated molecular ion of cereulide. Two peaks were obtained m/z 1170·70 and 1191·91 by ESI-MS, representing the ammonium and potassium adducts of cereulide, respectively. The ESI-MS/MS spectrum of the precursor ion m/z 1170·70 shows that first fragment lost is ammonia and the result is a protonated molecular ion of m/Z 1153·83.
Inhibition of NK cell activity by cereulide
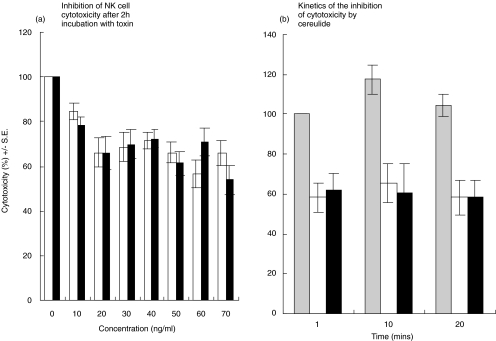
In Fig. 2, the effect of cereulide on NK cell activity is shown. Clear inhibition of NK cell cytotoxicity is seen with cereulide concentrations from 20 ng/ml (Fig. 2a). The kinetics of the inhibition was fast, the maximum effect being detectable already after one minute of exposure of effector cells to cereulide (Fig. 2b). The control with only methanol did not affect NK cell activity nor lymphocyte morphology (data not shown).
Fig. 2.
Inhibition of NK cell activity by cereulides purified from B. cereus strains F-5881 (□) and F4810/72 (▪). (a) The inhibitory effect is seen from concentrations of 20–30 ng/ml. (b) The kinetics of the inhibition. The inhibition is seen as early as 1 min at this concentration. The initial cytotoxicity in both figures is expressed as 100%, and the relative inhibition of lytic activation is calculated from individual cytotoxicity figures. (░) Control.
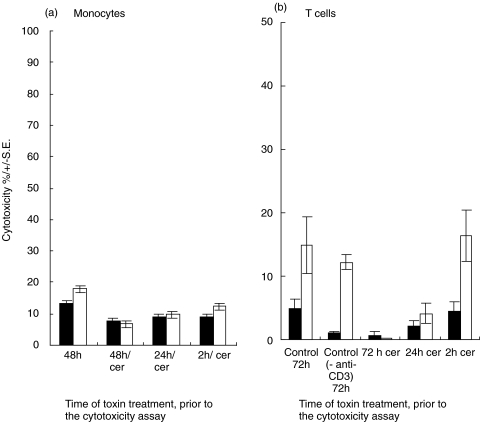
Inhibition of monocyte and T cell activity by cereulide
In Fig. 3a and b the effect of cereulide, the cytotoxicity of monocytes and T cells is shown. The lytic activity of LPS-stimulated monocytes was slightly inhibited by the toxin, whereas the cytotoxicity of unstimulated monocytes and PHA and IL-2 activated T cells were unaffected. The measurement of T cell-mediated cytotoxicity was performed with anti-CD3 treated P815 cells (antibody redirected killing assay) to avoid NK cell activity. However, if the toxin was added to the T cell cultures at the beginning, no stimulation of cytotoxic activity PHA and IL-2 (72 h) was seen, indicating the sensitivity of resting T cells to the toxin.
Fig. 3.
(a) The influence of cereulide on monocyte cytotoxicity. Monocytes were incubated on plastic plates for 48h and tested for cytotoxicity against K-562 cells. Cereulide (50 ng/ml) was added to the cultures at the beginning of the assay ( = 48 h) as well as at 24h and 2h prior to the cytotoxicity assay. Both unstimulated (▪) and LPS-stimulated (5 g/ml)(□) monocytes were tested. There is only a slight inhibition of cytotoxicity of LPS-stimulated monocytes by cereulide. (b) Effect of cereulide on the cytotoxicity of T cells incubated in medium only (▪) or with PHA and IL-2 (□) for 72 h. The toxin was added to the cells 72 h, 24h or 2h prior to the cytotoxicity assay. Target cells were treated with anti-CD3 antibody. Anti-CD3, control cytotoxicity without anti-CD3 antibody.
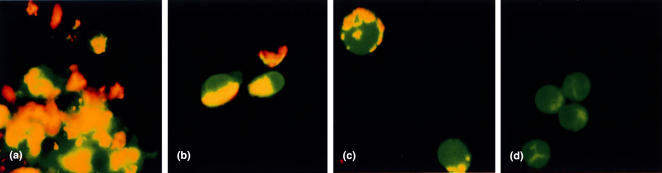
Effect of cereulide shown by JC-1 staining
Epifluorescence microscopy of live NK cells stained with JC-1 showed that exposure to 10–500 ng/ml of cereulide quenched the yellow-orange fluorescence of NK cells in a dose dependent manner. This indicates that cereulide caused dissipation of the mitochondrial inner membrane potential (Fig. 4).
Fig. 4.
The JC-1 stain of NK cell mitochondria. Normal mitochondria show homogenous yellow/orange fluorescence inside NK cells (a, b) indicating that the mitochondria are powered. With the increasing cereulide dose, the emitted yellow fluorescence becomes first spotted (c) and disappears with high concentrations, and only the green fluorescence remains (d). The diameter of the NK cells varies from 8 to 20m depending of the state activation (original magnification ×400). (a) control, (b) 0.1 ng/ml, (c) 10ng/ml, (d) 500 ng/ml.
Effect on NK cell morphology and apoptosis
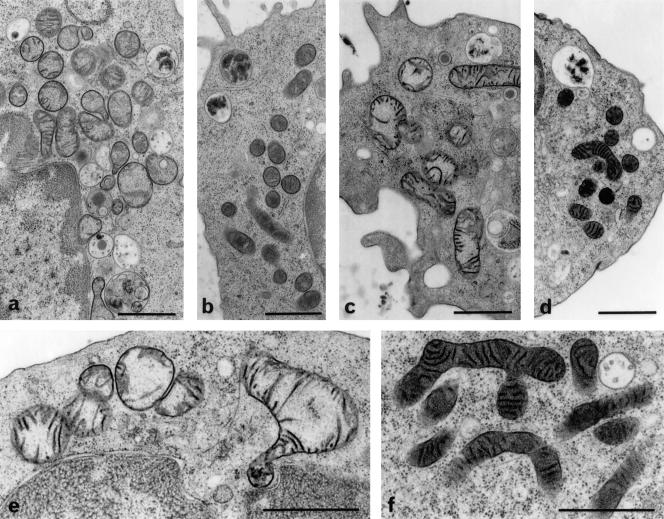
Enriched fresh NK cells and NK-92 cells treated for 3h with 100–1000 ng/ml of cereulide showed a swelling of mitochondria (Fig. 5). The enlarged mitochondria exhibited rounded outlines and a transparent to moderately stained matrix. Other subcellular constituents appeared not to be affected. The result was obtained with the cryofixation protocol (Fig. 5) and glutaraldehyde fixation (not shown), ruling out possible cumulative and/or synergistic effects of cereulide with fixatives. The patterns observed should therefore be genuine toxin effects.
Fig. 5.
Ultrastructure of mitochondria in 3h cereulide-treated NK cells (a, NK-92; c, e, fresh NK cells) and controls (b, NK-92; d, f, fresh NK cells). Samples were treated with/without 1000 ng/ml cereulide, followed by cryofixation and freeze-substitution. Swollen mitochondria with moderately stained matrix and translocated cristae after toxin-treatment (a, c, e) versus intact mitochondria with an intensely stained matrix and regularly arranged cristae in control cells (b, d and f); scale bars = 1μm.
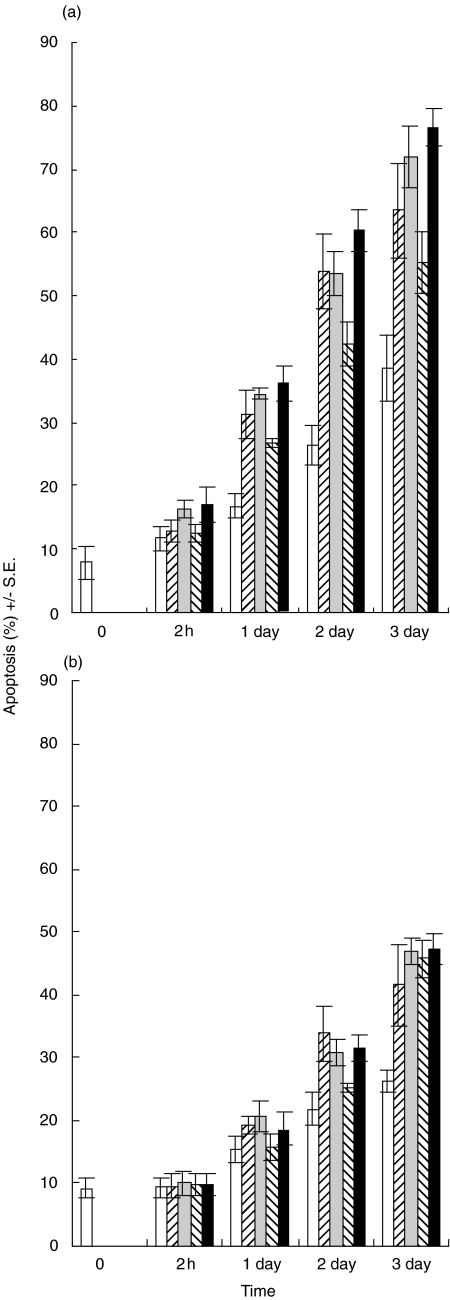
Cereulide induced the apoptosis of NK cells after 1 day of incubation (Fig. 6a). T cells were more resistant to the apoptosis induction by cereulide (Fig. 6b).
Fig. 6.
Apoptosis of NK cells (a) and T cells (b). Cereulides cause more apoptosis in NK cells. □ Control, untreated cells;  F-5881 (100ng/ml);
F-5881 (100ng/ml);  F-5881 (500ng/ml);
F-5881 (500ng/ml);  F4810/72 (100ng/ml); ▪ F4810/72 (500ng/ml). The annexin V shows clear apoptosis after 1 day of toxin treatment.
F4810/72 (100ng/ml); ▪ F4810/72 (500ng/ml). The annexin V shows clear apoptosis after 1 day of toxin treatment.
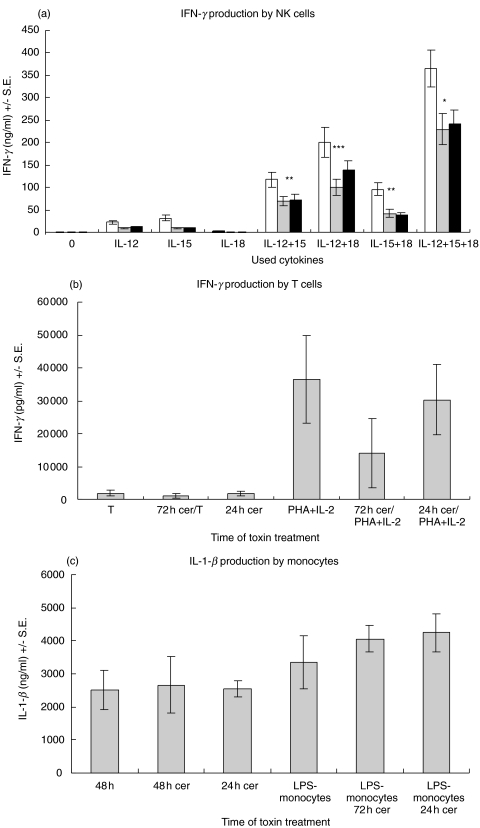
Effect of cereulide on IL-12,IL-15,IL-18-induced IFN-γ production by NK cells
Cereulide inhibited the modest IFN-γ production of NK cells induced by IL-12 or IL-15 (Fig. 7a). IL-18 stimulated NK cells did not produce IFN-γ. The combination of IL-12 and IL-15, IL-12 and IL-18, IL-15 and IL-18 or especially of IL-12, IL-15 and IL-18 synergistically enhanced the IFN-γ production of NK cells (Fig. 7a). The IFN-γ production was partially inhibited by cereulide.
Fig. 7.
(a) IFN-γ production by NK cells. □ control; ░ F-5882; ▪ F4810/72. The cereulide toxins partially inhibit the IFN-γ production in IL-12, IL-15 and IL-18 stimulated NK cells. (*P ≤ 0·01, **P ≤ 0·02, ***P ≤ 0·05) (b) IFN-γ production by T cells. Non-activated T cells do not produce IFN-γ and activated T cells produce IFN-γ to the same extent with or without cereulide. (c) Cereulide does not affect of the IL-1-β production of monocytes.
Effect of cereulide on IFN-γ and IL-1-β production by T cells and monocytes
Cereulide did not affect the IFN-γ production of activated T cells, and nonactivated T cells did not produce IFN-γ (Fig. 7b). The IL-1-β production of monocytes and LPS-stimulated monocytes was not affected by cereulide (Fig. 7c).
DISCUSSION
Bacillus cereus is ubiquitous in nature. It has been isolated from environments where it may cause serious health hazard, such as infant food and hospital linen, and it contaminates vegetables, meat, eggs and dairy products (5). B. cereus strains produce at least seven different types of toxins, of which the emetic toxin cereulide is probably the most dangerous one [12]. The emetic syndrome has been described in Britain in 234 outbreaks, where more than 1200 persons were affected [13]. The documented biological effects of cereulide include emesis in primates and musk shrew, swelling of mitochondria in HEP-2 cells and in boar spermatozoa, as well as in the hepatocytes in a fatally food-poisoned patient [2,14–19]. This may be direct consequence of the ionophoric action of cereulide, as the mitochondrial K+ channels are known to play a dominant role in mitochondrial volume control [20].
The ESI-MS/MS spectrum of the toxin from B. cereus F-5881 (Fig. 1), shows that it was identical to the cyclic dodecadepsipeptide, previously described as cereulide from B. cereus strain F4810/72 [18]. The present work is the first to show that human cells suffered serious damage from exposure to this nonprotein toxin. We have earlier shown that cereulide is a K+ specific ionophore [21], similar to and as potent as the well-known K+ ionophore valinomycin [22]. The structural and biochemical similarities of cereulide with valinomycin explain why the effects of cereulide on the cytotoxicity and cytokine production by human NK cells were closely similar to those earlier observed for valinomycin [23].
The peptide antibiotics acting as potassium ionophores, emitted by B. cereus (cereulide) and Streptomyces griseus (valinomycin) [24] may be targeted against other microorganisms, but due to biochemical similarities between mitochondria and bacterial cells they also affect human cells. The nonvoltage gated K+ channels created by such peptides will result in an efflux of K+ and rapid but transient hyperpolarization of the membrane [25]. This could explain why the toxic effect on NK cells was fast, being detectable in one minute of treatment. It could also explain the other distinct feature in the suppressive effect of both valinomycin [23] and cereulide (this paper): the inhibition of cytotoxicity and cytokine production was only partial. Alternatively, the incomplete toxicity may be due to the varying sensitivity of different NK cell subsets [26] to the compounds.
Cereulide quenched the fluorescence of JC-1 stained NK cells, indicating that the mitochondrial inner membrane potential was dissipated [20,27]. Dissipation of mitochondrial inner membrane potential is probably the first point of no return directing the damaged cell towards apoptosis [20,28,29], preceding the activation of apoptosis specific caspases. Maybe the cytotoxicity test can be a good method to investigate apoptosis. Thus the induction of apoptosis in NK cells is likely linked to the K+ ionophoric activity of cereulide.
Work on the Suncus murinus animal model [15] has shown that the emesis seen on exposure to cereulide is mediated by its action on the afferent vagus. Vagotomy or administering 5-HT3, serotonin receptor antagonist, abolished the emetic effect. The cereulide induced emesis occurred in minutes after oral administration, indicating rapid absorption of cereulide from the oesophagus into the neuronal serotonine receptors. Valinomycin had similar effects but 100 fold higher doses were required [15]. The threshold concentration on NK cells was also lower for cereulide than for valinomycin [23]. The low threshold may relate to the higher lipophilicity of cereulide (log Kow > 6, the same level as that of dietary lipids), and thus better penetration into cells, as compared to valinomycin [21].
The emesis inducing dose of cereulide in human is not known, but in Suncus murinus it was 8 μg/kg [15] and in rhesus monkeys a very similar value of 10 μg/kg was reported [14]. The efficacy of different B. cereus strains in cereulide production varies, overproducers may emit >5 μg/ml [30]. Consuming 100–200 g (fresh wt) of food heavily contaminated by such strains of B. cereus may thus lead to body burdens of 10–20 μg/kg. The inhibition of NK cell occurred at a cereulide concentration 20–30 μg/l. Therefore, systemic inhibition of NK cell activity can be expected with the cereulide concentrations found in food poisonings. Concentrations of this lipophilic substance (similar to dietary lipids) may be even significantly higher in blood than elsewhere in the body early after ingestion.
The monocytes were the least sensitive to the toxicity of cereulide, T cells being somewhere in between the NK cells and monocytes. Clearly, the resting T cells showed sensitivity to the toxin, whereas PHA + IL-2 activated T cells were resistant. Furthermore, the toxic effect of cereulide on monocytes and T cells was detectable after considerable longer incubation times than for NK cells. It could be speculated that due to their relative resistance to cereulide, monocytes might continue antigen presentation in the presence of the toxin. With the simultaneous compromised NK functions, for example diminished IFN-γ production, the immune response may deviate to the Th2 type. Therefore, it will be of interest to study whether cereulide affects the cytokine production profile of helper T cells, perhaps facilitating allergic reactions.
The food poisoning caused by cereulide is believed to be self-limited and the acute phase lasts for maximum of one day. Therefore, the actual food poisoning does not necessarily cause long-lasting immune deviations. However, since there is no test for B. cereus contamination in routine use and cereulide is heat-stabile and may be accumulated from many foods. It is possible that subclinical quantities of cereulide may cause frequent compromised NK cell activity, thus weakening the immune defence. Although it is too early to say, NK cell assay – because of its simplicity and sensitivity – may be the method of choice for testing for the toxic effects of potentially hazardous environmental compounds.
Acknowledgments
This work was financially supported by the Sigrid Juselius Foundation, the Helsinki University Central Hospital and the Academy of Finland (Grant 50733). We also thank Leena Saikko, Douwe Hoornstra, Pirkko Leikas-Lazanyi and Tuire Koro for their excellent technical assistance.
REFERENCES
- 1.Drobniewski F. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6:324–38. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agata N, Mori M, Ohta M, Suwan S, Ohtani I, Isobe M. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol Lett. 1994;121:31–4. doi: 10.1111/j.1574-6968.1994.tb07071.x. [DOI] [PubMed] [Google Scholar]
- 3.Granum PE. Bacillus cereus and its toxins. Soc Appl Bacteriol Symp Series. 1994;23:61S–66S. [PubMed] [Google Scholar]
- 4.Schmidt K, editor. Sixth Report 1990–1992. Berlin: Federal Institute for Health Protection of Consumers and Veterinary Medicine; 1995. WHO surveillance programme for control of foodborne infections and intoxications in Europe. [Google Scholar]
- 5.Kramer JM, Gilbert RJ. Bacillus cereus and other Bacillus species. In: Doyle MP, editor. Foodborne Bacterial Pathogens. New York: Marcel Dekker; 1989. pp. 21–70. [Google Scholar]
- 6.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense. function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 7.Somersalo K, Tarkkanen J, Patarroyo M, Saksela E. Involvement of beta 2-integrins in the migration of human natural killer cells. J Immunol. 1992;149:590–8. [PubMed] [Google Scholar]
- 8.Mingari MC, Poggi A, Biassoni R, et al. In vitro proliferation and cloning of CD3- CD16+ cells from human thymocyte precursors. J Exp Med. 1991;174:21–6. doi: 10.1084/jem.174.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suominen I, Andersson M, Andersson MC, Hallaksela AM, Kämpfer P, Rainey FA, Salkinoja-Salonen MS. Toxic Bacillus pumilus from indoor air, Norway spruce, recycled pulp, food poisoning outbreaks and clinical samples. Syst Appl Microbiol. 2001;24:267–76. doi: 10.1078/0723-2020-00025. [DOI] [PubMed] [Google Scholar]
- 10.Hess MW, Schwendinger MG, Eskelinen EL, Pfaller K, Pavelka M, Dierich MP, Prodinger WM. Tracing uptake of C3dg-conjugated antigen into B-cells via complement receptor type 2 (CR2, CD21) Blood. 2000;95:2617–23. [PubMed] [Google Scholar]
- 11.Ushio S, Namba M, Okura T, et al. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–9. [PubMed] [Google Scholar]
- 12.Granum PE, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol Lett. 1997;157:223–8. doi: 10.1111/j.1574-6968.1997.tb12776.x. [DOI] [PubMed] [Google Scholar]
- 13.Hausler WJ, Sussman M. Topley and Wilson's microbiology and microbial infections. 9. Vol. 3. London: Arnold Publications; 1998. pp. 554–5. [Google Scholar]
- 14.Shinagawa K, Konuma H, Sekita H, Sugii S. Emesis of rhesus monkeys induced by intragastric administration with the HEp-2 vacuolation factor (cereulide) produced by Bacillus cereus. FEMS Microbiol Lett. 1995;130:87–90. doi: 10.1016/0378-1097(95)00188-B. [DOI] [PubMed] [Google Scholar]
- 15.Agata N, Ohta M, Mori M, Isobe M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol Lett. 1995;129:17–20. doi: 10.1016/0378-1097(95)00119-P. [DOI] [PubMed] [Google Scholar]
- 16.Shinagawa K, Ueno Y, Hu D, Ueda D, Sugii S. Mouse lethal activity of a Hep-2 vacuolation factor, cereulide, produced by Bacillus cereus isolated from vomiting-type food poisoning. J Vet Med Sci. 1996;58:1027–9. doi: 10.1292/jvms.58.10_1027. [DOI] [PubMed] [Google Scholar]
- 17.Sakurai N, Koike K, Irie Y, Hayashi H. The rice culture filtrate of Bacillus cereus isolated from emetic-type food poisoning causes mitochondrial swelling in HEp-2 cell. Microbiol Immunol. 1994;38:337–43. doi: 10.1111/j.1348-0421.1994.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 18.Andersson MA, Mikkola R, Helin J, Andersson MC, Salkinoja-Salonen M. Novel sensitive bioassay for detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl Environ Microbiol. 1998;64:1338–43. doi: 10.1128/aem.64.4.1338-1343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahler H, Pasi A, Kramer J, Schulte P, Scoging A, Bär W, Krähenbuhl S. Fuliminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med. 1997;336:1142–8. doi: 10.1056/NEJM199704173361604. [DOI] [PubMed] [Google Scholar]
- 20.Scheffler IE. A century of mitochondrial research: achievements and perspectives. Mitochondrion. 2000;1:3–31. doi: 10.1016/s1567-7249(00)00002-7. [DOI] [PubMed] [Google Scholar]
- 21.Mikkola R, Saris NE, Grigoriev P, Andersson M, Salkinoja-Salonen MS. Ionophoretic properties and mitochondrial effects of cereulide. Eur J Biochem. 1999;263:112–7. doi: 10.1046/j.1432-1327.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DL, Cox MM. Lehninger principles of Biochemistry. New York: Worth; 2000. pp. 423–41. [Google Scholar]
- 23.Paananen A, Mikkola R, Sareneva T, Matikainen S, Andersson M, Julkunen I, Salkinoja-Salonen MS, Timonen T. Inhibition of human NK cell function by valinomycin, a toxin from Streptomyces griseus in indoor air. Infect Immun. 2000;68:165–9. doi: 10.1128/iai.68.1.165-169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson MA, Mikkola R, Kroppenstedt RM, Rainey FA, Peltola J, Helin J, Sivonen K, Salkinoja-Salonen M. Mitochondrial toxin produced by Streptomyces griseus strains isolated from indoor environment is valinomycin. Appl Environ Microbiol. 1998;64:4767–73. doi: 10.1128/aem.64.12.4767-4773.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackerman MJ, Clapman DE. Ion channels – basic science and clinical disease. N Engl J Med. 1997;336:1575–86. doi: 10.1056/NEJM199705293362207. [DOI] [PubMed] [Google Scholar]
- 26.Jonges LE, Albertsson P, van Vlierberghe RL, et al. The phenotypic heterogeneity of human natural killer cells: presence of at least 48 different subsets in the peripheral blood. Scand J Immunol. 2001;53:103–10. doi: 10.1046/j.1365-3083.2001.00838.x. [DOI] [PubMed] [Google Scholar]
- 27.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 28.Alnemri ES. Hidden powers of the mitochondria. Nat Cell Biol. 1999;1:E40–E42. doi: 10.1038/10034. [DOI] [PubMed] [Google Scholar]
- 29.O'Rourke B. Apoptosis, rekindling the mitochondrial fire. Circ Res. 1999;85:880–3. doi: 10.1161/01.res.85.10.880. [DOI] [PubMed] [Google Scholar]
- 30.Agata N, Ohta M, Mori M. Production of an emetic toxin, cereulide, is associated with a specific class of Bacillus cereus. Curr Microbiol. 1996;33:67–9. doi: 10.1007/s002849900076. [DOI] [PubMed] [Google Scholar]