Abstract
It is believed that chemokines and their receptors are involved in trafficking of T-cells to the central nervous system (CNS). The aim of the current study was to define the expression on cerebrospinal fluid (CSF) T-cells of six chemokine receptors associated with trafficking to sites of inflammation. Flow cytometry was used to detect chemokine receptor expression. We observed that CD3+T-cells in the CSF express a restricted array of inflammatory chemokine receptors, specifically CXCR3, CCR5 and CCR6, but little CCR1-3. This repertoire was independent of the presence of CNS inflammation, since comparable findings were obtained in patients with multiple sclerosis (MS) and individuals with non-inflammatory neurological diseases. The enrichment of CCR5+T-cells in the CSF could largely be explained by higher frequency of CD4+/CD45RO+T-cells in this compartment. In contrast, CD4+/CD45RO+T-cells expressing CXCR3 were significantly enriched in CSF as compared with blood. Similar levels of CCR6+/CD3+T-cells were observed in blood and CSF, while levels of CCR2+/CD3+T-cells were lower in CSF than in blood. The CSF was virtually devoid of CCR5+/CXCR3- T-cells, suggesting that the expression of CCR5 alone is not sufficient for the trafficking of CD3+T-cells to the CSF. We hypothesize that CXCR3 is the principal inflammatory chemokine receptor involved in intrathecal accumulation of T-cells in MS. Through interactions with its ligands, CXCR3 is proposed to mediate retention of T-cells in the inflamed CNS.
Keywords: cerebrospinal fluid, T-cells, multiple sclerosis, chemokine receptors
INTRODUCTION
Infiltration of haematogenous inflammatory cells into brain tissue is a crucial step in the process leading to tissue damage in multiple sclerosis (MS). Emerging evidence indicates that focal destruction of both myelin and axons, as well as oligodendrocyte cell-death, are directly related to numbers of activated inflam-matory cells in the lesions [1–4]. Chemokines are chemotactic molecules that govern leucocyte accumulation in tissue during inflammation, acting through high-affinity G-protein coupled receptors (CXCR and CCR) on leucocytes [5–7]. Selective chemokine receptor expression is also associated with T-cell polarization to the T helper 1 (Th1) or Th2 phenotype both in vitro and in vivo[8,9].
Co-localization of specific chemokines and cognate receptors in MS lesions suggested a pathogenic role for these elements in intrathecal accumulation of T-cells in MS. In particular, infiltrating T-cells expressed CCR5 and CXCR3 within MS plaques, and the corresponding chemokine ligands were detected in the lesional microenvironment [10–13]. Increased expression of CXCR3 and CCR5 on CD4+T-cells in cerebrospinal fluid (CSF) compared to peripheral blood mononuclear cells (PBMCs) has also been reported in patients with MS [11,14].
The aim of the current study was to define the pattern of expression of six chemokine receptors (CCR1-3, CCR5, CCR6, and CXCR3) on T-cells in paired blood and CSF samples. The receptors were chosen based on their known ability to bind to inflammatory chemokines, and their association with trafficking of T-cells to sites of inflammation in vivo. CCR5 and CXCR3 are both expressed by activated memory T-cells, predominantly of a Th1 phenotype, and have been detected on T-cells isolated from diverse inflammatory tissues [8,9,15–17]. CCR1 and CCR2 are highly expressed by monocytes/macrophages, but also by a subset of activated memory T-cells [7]. CCR2 is detected on activated CD26+T-cells, which, at least in vitro are highly competent at transendothelial migration [18]. The expression of CCR2 is associated with chronic activation of human T-cells and also with Th2 phenotypic commitment in the setting of autoimmunity, but Th1 responses to pathogen challenge [19–21]. In one study, CCR2 expression was described on T-cells in MS lesions [22]. CCR3 has been associated with T-cells of a Th2 phenotype, which together with the pattern of CCR3 expression on eosinophils, basophils and mast cells, suggests a role in the recruitment of inflammatory cells in allergic inflammation [23–27]. In contrast, little is known about the function of CCR6 on T-cells. Because of CCR6 expression on non-activated T-cells, there has been speculation that CCR6 is involved in the first stage of inflammation by recruiting resting memory T-cells to sites where activation can occur [28].
It is well known that the cellular composition of both normal and pathological CSF differs profoundly from peripheral blood. Of importance for comparative studies of expression of T-cell markers in blood and CSF is an increased CD4/CD8 ratio in CSF as compared to blood [29]. In addition, it has been demonstrated that the majority of both CD4+ and CD8+T-cells in the CSF are of a memory phenotype compared to approximately 40–50% in peripheral blood [30]. Since the expression of chemokine receptors on T-cells is dependent on the activation state of the cell, with higher expression of most inflammatory chemokine receptors on activated effector or memory T-cells compared to naïve T-cells [16,31], we elected to study chemokine receptor expression both on the total population of CD3+T-cells, as well as in the subpopulation of CD4+/CD45RO+ memory T-cells.
Our results showed that CD3+T-cells in the CSF express a restricted array of inflammatory chemokine receptors, specifically CXCR3, CCR5 and CCR6, but little CCR1-3. Interestingly, this repertoire was independent of central nervous system (CNS) inflammation, since similar findings were observed in patients with MS and controls with non-inflammatory neurological diseases. Whereas the expression of both CCR5 and CXCR3 was increased on CD3+T-cells in the CSF, the increase in CCR5 expression in the CSF compared to blood could largely be explained by an accumulation of CD4+/CD45RO+T-cells in the CSF. In contrast, CD4+/CD45RO+T-cells expressing CXCR3 were significantly enriched in CSF as compared to blood. We hypothesize that CXCR3 is the principal inflammatory chemokine receptor involved in intrathecal accumulation of T-cells in MS.
MATERIALS AND METHODS
Patients
Blood and CSF were obtained from 74 consecutive patients referred for diagnostic lumbar puncture at the Department of Neurology, Cleveland Clinic Foundation (CCF). The study was approved by the Institutional Review Board of the CCF and informed consent was obtained from all patients.
Twenty-nine patients (21 women) had evidence of inflam-matory CNS demyelination [32], manifested either as MS (18 patients), or as a clinically isolated syndrome suggestive of MS in combination with abnormal magnetic resonance imaging (MRI) and/or CSF studies (10 patients). One patient had recurrent multicentric myelitis. Their ages ranged from 21 to 64 years (mean 39). Twenty-two patients (76%) had evidence of intrathecal IgG synthesis in the form of CSF oligoclonal bands and/or increased IgG index. The median CSF cell count was 2·2 leucocytes/μl (range 0–14). None of the patients were examined during acute exacerbation, or treated with immunomodulatory drugs.
Forty-five patients (31 women) had non-inflammatory neurological diseases (NIND). Their diagnoses were: paresthesias (11 patients), headaches (9), CSF circulation disturbance (6), vertigo (6), psychiatric disease (3), ischaemic disease (3), cranial nerve palsy (2), CNS tumour (1), seizures (1), autonomic dysfunction (1), amyotrophic lateral sclerosis (1), and nutritional polyneuropathy (1). Their ages were between 26 and 78 years (mean 47). One patient (2%) had evidence of intrathecal IgG synthesis. The median CSF cell count was 0·8 leucocytes/μl (range 0–5).
In addition, blood and CSF were obtained from three patients (2 women) with known MS for the study of CCR5 expression on CD4+/CD45RO+T-cells. These patients were being treated with intrathecal baclofen for intractable spasticity. Their ages were 32–44 years (mean 37). Routine CSF analysis was not performed in these patients. Two of the patients were receiving imm-unomodulatory drugs (one patient each with methotrexate and the combination of methotrexate and interferon-β1a).
Immunostaining for chemokine receptor analysis
CSF
10 ml aliquots of CSF were collected, immediately chilled, centrifuged at 250 g for 7 min at +4°C, and resuspended to a volume of 100 μl. Due to low numbers of CSF cells, most samples were not divided into several stainings, but all cells were used for the analysis of one or two chemokine receptors.
Double- or triple-labelling experiments using directly conjugated antibodies Table 1, all monoclonal antibodies (moAbs) except CCR2 clones DOC-3 and DOC-4] were performed in one step. Non-specific FcR-binding was blocked with 0·2 mg/ml normal mouse IgG (Caltag Laboratories, Burlingame, CA, USA) for 15 min at +4°C. Cells were incubated with PE- or FITC-labelled antichemokine receptor moAbs in combination with anti-CD3 PerCP (SK7, BD Biosciences, San Jose, CA, USA), anti-CD4 PerCP (SK3, BD Biosciences), and/or anti-CD45RO FITC (UCHL1, PharMingen, San Diego, CA, USA) for 30 min at +4°C, after which cells were washed twice with ice cold FACS-buffer (PBS containing 1% fetal calf serum and 0·1% sodium azide), and fixed with 1% paraformaldehyde (PFA). Control experiments with CSF cells incubated for 15 min at room temperature (RT) were performed to detect differences in CCR5 expression under different staining conditions.
Table 1.
Chemokine receptors, moAbs used, and their ligand and leucocyte specificity [7]
| Chemokine Receptor | Clone | Human ligands | Cell types |
|---|---|---|---|
| CCR1 | 53504·111 (PE, R & D) | CCL3, CCL5, CCL7 | T-cells, NK-cells, macrophages, granulocytes, DC |
| CCR2 | 48607·211 (PE, R & D) | CCL2, CCL7, CCL8 | T-cells, B-cells, NK-cells, macrophages |
| DOC-3, DOC-4 (Unlabelled, MM) | |||
| CCR3 | 61828·111 (PE, R & D) | CCL5, CCL7, CCL8, CCL11 | T-cells, granulocytes |
| CCR5 | 2D7 (PE, PharMingen) | CCL3, CCL4, CCL5, CCL8 | T-cells, B-cells, macrophages, DC |
| 45531·111 (PE, R & D) | |||
| CCR6 | 11A9 (PE, PharMingen) | CCL20 | T-cells, B-cells, DC |
| CXCR3 | 49801·111 (FITC, R & D) | CXCL9, CXCL10, CXCL11 | T-cells, B-cells |
| 1C6 (PE, PharMingen) |
R & D, R & D Systems Inc., Minneapolis, MN; MM, generated by M. Mack; PharMingen, PharMingen, San Diego, CA; DC, dendritic cells.
Experiments using the non-conjugated CCR2 moAbs DOC-3 and DOC-4 were performed in three steps. After blockade of non-specific FcR-binding with 0·2 mg/ml normal goat IgG (Caltag Laboratories), cells were incubated with CCR2 moAbs DOC-3 or DOC-4, followed by incubation with PE-conjugated goat anti-mouse IgG1 or IgG2b (Southern Biotechnology Associates Inc., Birmingham, AL, USA), and subsequent incubation with anti-CD3 PerCP, anti-CD4 PerCP and/or anti-CD45RO FITC. Each step was incubated for 30 min at +4°C (DOC-3) or 15 min at RT (DOC-4) and separated by two washes with ice-cold FACS-buffer.
Lysed whole blood
One hundred μl of heparinized venous blood was blocked with 0·2 mg/ml normal mouse IgG for 15 min at RT for double- or triple labelling experiments using directly conjugated antibodies. Staining was performed for 15 min at RT using identical concentrations of moAbs as for CSF. After staining, cells were incubated with 2 ml of FACS lysing solution (BD Biosciences) for 10 min at RT to lyse erythrocytes. Cells were centrifuged, washed twice with ice cold FACS-buffer and subsequently fixed with 1% PFA. Control experiments with cells incubated for 30 min at +4°C were performed to detect differences in chemokine receptor expression under different staining conditions.
Experiments using the non-conjugated CCR2 moAbs DOC-3 and DOC-4 were performed in three steps as described for the CSF. Each step was incubated for 30 min at +4°C (DOC-3) or 15 min at RT (DOC-4) and separated by two washes with ice-cold FACS-buffer. Erythrocytes were lysed after the last staining step using FACS lysing solution as described above. Experiments using non-conjugated isotype matched control moAbs did not show any non-specific binding of the secondary goat anti-mouse IgG1 and IgG2b Abs.
PBMCs
To obtain PBMCs, equal volumes of heparinized venous blood and ice cold PBS containing 0·3% human serum albumin (HSA, Sigma-Aldrich, St. Louis, MO, USA) were mixed and centrifuged through Lymphocyte Separation Media (Mediatech Inc., Herndon, VA, USA). Cells at the interface were harvested, washed twice with PBS/HSA, and resuspended to 106 cells/ml in ice cold FACS-buffer. 100 μl of cell suspension was blocked with 0·2 mg/ml normal mouse IgG for 15 min at RT. Staining was performed for 15 min at RT using identical concentrations of moAbs as for lysed whole blood stainings. After staining, cells were washed twice with ice cold FACS-buffer and subsequently fixed with 1% PFA.
Selection of antibodies for flow cytometry
Preliminary studies showed that CCR2 clones 48607·211, DOC-3 and DOC-4, and CXCR3 clones 49801·111 and 1C6 resulted in comparable stainings. The CCR5 clone 2D7 stained a higher percentage of cells than clone 45531·111 and was used based on its documented ability to completely block the binding of the natural ligands of CCR5 to CCR5 transfectants [33].
Effects of staining conditions on chemokine receptor expression
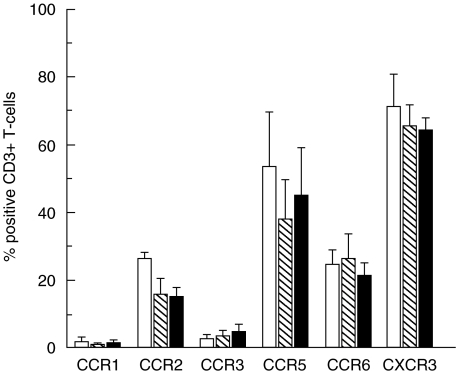
The most common procedure to stain chemokine receptors on T-cells from PB for flow cytometry has been to isolate PBMCs using density centrifugation on Ficoll cushions. It has, however, been suggested that this protocol may induce selective down-regulation of chemokine receptor surface expression [34,35]. When comparing the expression on CD3+T-cells of the six chemokine receptors under study in three healthy donors, it was observed that the expression of CCR2 and CCR5 was consistently reduced in stainings of PBMCs as compared to stainings of whole blood performed at RT, with subsequent selective erythrocyte lysis (Fig. 1). Since the principal object of the current study was to compare chemokine receptor expression in blood and CSF, we stained chemokine receptors in whole blood, with subsequent selective erythrocyte lysis. This method reduced ex vivo handling of blood samples and was directly comparable to the protocol used for CSF.
Fig. 1.
Chemokine receptor expression on CD3+T-cells was compared in stainings of whole blood, followed by selective erythrocyte lysis, performed at RT (□) and +4°C ( ), and in stainings of PBMCs performed at RT (▪). Figure shows mean +SD of three healthy donors.
), and in stainings of PBMCs performed at RT (▪). Figure shows mean +SD of three healthy donors.
It was also observed that the expression of CCR2 and CCR5 on CD3+T-cells was reduced in stainings performed at +4°C compared to RT. This led us to perform stainings of peripheral blood at RT. Since CSF usually contains few cells that decay rapidly [36], the majority of CSF samples were stained at +4°C in order to minimize cell losses. Additional experiments were performed to exclude the possibility that differential receptor detection at RT (for blood cells) as compared with +4°C (for CSF cells) could explain observed differences in the expression of CCR2 and CCR5 between PB and CSF.
Flow cytometric analysis and statistical methods
Cells were acquired on a FACScan flow cytometer (BD Biosciences) and analysis performed using WinList software (Verity Software House Inc., Topsham, ME, USA). Cells were gated according to forward- and side light-scattering properties, and were positively selected for CD3 or CD4 expression. Isotype matched control moAbs (purchased from BD Biosciences and Southern Biotechnology Associates Inc.) were used for defining background fluorescence. Since the results were not normally distributed, the nonparametric Mann–Whitney U-test and Wilcoxon signed rank test were used for statistical analysis. Reported P-values are two-tailed and considered statistically significant at P < 0·05.
RESULTS
Expression of inflammatory chemokine receptors on CD3+T-cells in PB and CSF
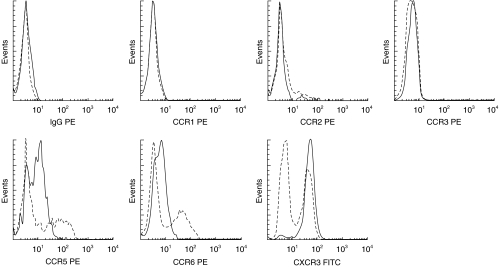
In initial studies, the expression of chemokine receptors on CD3+T-cells was compared in paired blood and CSF samples from an unselected group of neurological patients referred for diagnostic lumbar puncture. Characteristic flow plots are shown in Fig. 2.
Fig. 2.
Cells from peripheral blood (PB) and CSF were gated according to forward- and side light scattering properties, and were positively selected for CD3 expression. Histograms for each chemokine receptor on CSF T-cells (——) are shown overlaid on the paired staining of PB T-cells (–––––) from the same donor. Isotype matched control moAbs were used for each chemokine receptor.
A restricted repertoire of chemokine receptors was detected on CD3+T-cells in the CSF. More than 90% of CD3+T-cells in the CSF expressed CXCR3, and more than 65% expressed CCR5 (Table 2). A smaller fraction (30%) of CD3+T-cells in the CSF expressed CCR6. Few CD3+T-cells in the CSF expressed CCR1, CCR2, or CCR3.
Table 2.
Chemokine receptor expression on CD3+ and CD45RO+/CD4+† T-cells in peripheral blood and CSF
| CCR1 Median (range) | CCR2 Median (range) | CCR3 Median (range) | CCR5 Median (range) | CCR6 Median (range) | CXCR3 Median (range) | ||
|---|---|---|---|---|---|---|---|
| CD3+ | |||||||
| MS | Blood (%) | 0·9 (0·5–1·1) | 13·7 (7·3–27·9)*† | 7·4 (7·1–18·4) | 34·5 (20·8–49·6)*† | 29·6 (15·8–49·0) | 44·4 (32·3–58·6)*†† |
| CSF (%) | 0·7 (0·5–1·1) | 3·0 (1·1–4·8) | 0·8 (0·8–2·5) | 55·9 (48·4–62·8) | 26·4 (17·3–51·2) | 91·2 (88·2–93·1) | |
| No· exam· | 4 | 5 | 3 | 5 | 9 | 5 | |
| NIND | Blood (%) | 1·1 (0·5–2·1) | 17·2 (10·7–26·0)*† | 2·8 (0–18·8) | 36·6 (29·3–53·4)*† | 29·5 (22·8–49·1) | 51·2 (34·1–62·6)*†† |
| CSF (%) | 2·3 (1·2–4·5) | 2·7 (1·1–3·7) | 1·2 (0–4·2) | 63·6 (26·5–78·7) | 31·1 (17·7–50·2) | 91·9 (86·0–97·1) | |
| No· exam· | 7 | 6 | 4 | 14 | 14 | 11 | |
| CD45RO+/CD4+ | |||||||
| MS | Blood (%) | n·d· | n·d· | n·d· | 43·8 (26·6–69·00) | n·d· | 66·4 (61·4–71·8)**†† |
| CSF (%) | 38·1 (24·0–68·3) | 89·8 (86·2–94·7) | |||||
| No· exam· | 9 | 3 | |||||
| NIND | Blood (%) | n·d· | 31·4 (22·5–47·4)** | n·d· | 51·8 (30·7–70·5) | 56·9 (43·7–67·1)** | 61·0 (44·8–72·1)**†† |
| CSF (%) | 16·4 (8·2–30·3) | 52·2 (26·0–74·4) | 39·0 (32·2–54·5) | 90·3 (80·4–95·4) | |||
| No. exam. | 8 | 12 | 8 | 6 | |||
Since preliminary studies showed that >95% of all CD4+ T-cells in the CSF were CD45RO+, this marker was omitted in CSF stainings in order to optimally use the few cells available
P-values calculated on all patients as one group
P < 0·001
P < 0·01, blood versus CSF; MS, patients with inflammatory CNS demyelination {manifested as MS (21 patients), a clinically isolated syndrome suggestive of MS (10 patients), or recurrent multicentric myelitis (one patient); NIND, non-inflammatory neurological diseases.
Compared to PB, there was a clear accumulation in the CSF of CD3+T-cells expressing CXCR3 and CCR5 (P < 0·001 for both comparisons; Table 2). In contrast, the expression of CCR6 was similar on CD3+T-cells in PB and CSF, while the expression of CCR2 was significantly reduced on CD3+T-cells in the CSF (P < 0·001). To exclude the possibility that differential receptor detection at RT (for blood cells) as compared with +4°C (for CSF cells) could explain reduced percentages of CCR2+*CD3+T-cells in CSF, we took advantage of the availability of two additional moAbs, one of which (CCR2 clone DOC-3) induced weak receptor endocytosis at RT and was used at +4°C, while the other (CCR2 clone DOC-4) did not induce receptor endocytosis at RT and was used at this temperature (MM, unpublished results). With CCR2 clone DOC-3, we detected a median of 8% (range 3–12%; n = 6, all NIND) CCR2+*CD3+T-cells in CSF compared to 23% (range 18–38%; P = 0·05) in PB. CCR2 clone DOC-4 detected 12% (range 9–26%; n = 7, all NIND) CCR2+*CD3+T-cells in CSF compared to 21% (range 17–31%; P = 0·01) in PB. Thus, it was confirmed that the expression of CCR2 on CD3+T-cells was reduced in CSF, compared to PB.
Chemokine receptor expression on CD3+T-cells was comparable, regardless of patient diagnosis
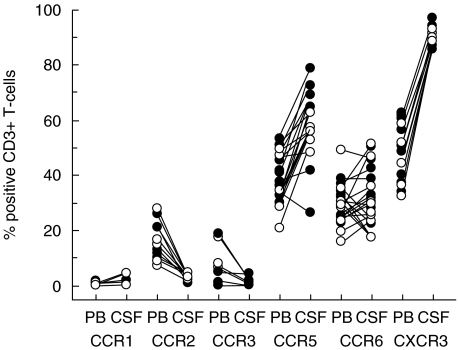
To determine whether accumulation of CXCR3+and CCR5+T-cells in the CSF only occurs during CNS inflammation, the patients were retrospectively subgrouped as having inflammatory CNS demyelination (MS) or non-inflammatory neurological disorders (NIND), according to underlying diagnoses. From this analysis, it was clear that the repertoire of chemokine receptor expression on CD3+T-cells was similar in patients with CNS inflammation and individuals with NIND, although the small number of patients analysed precludes detection of minor quantitative differences. In particular, CXCR3+*CD3+and CCR5+*CD3+T-cells were enriched equally in the CSF of MS patients with inflammatory CNS demyelination, and patients with NIND lacking CNS inflammation (Fig. 3,Table 2).
Fig. 3.
Chemokine receptor expression was determined on CD3+T-cells in peripheral blood (PB) and CSF from patients with inflammatory CNS demyelination (manifested as MS (21 patients), a clinically isolated syndrome suggestive of MS (10 patients), or recurrent multicentric myelitis (1 patient); (○) and control individuals with non-inflammatory neurological disorders (NIND; •) using flow cytometry. Lines connect paired blood and CSF samples from individual patients.
CCR5 and CXCR3 coexpression on individual CD3+T-cells in PB and CSF
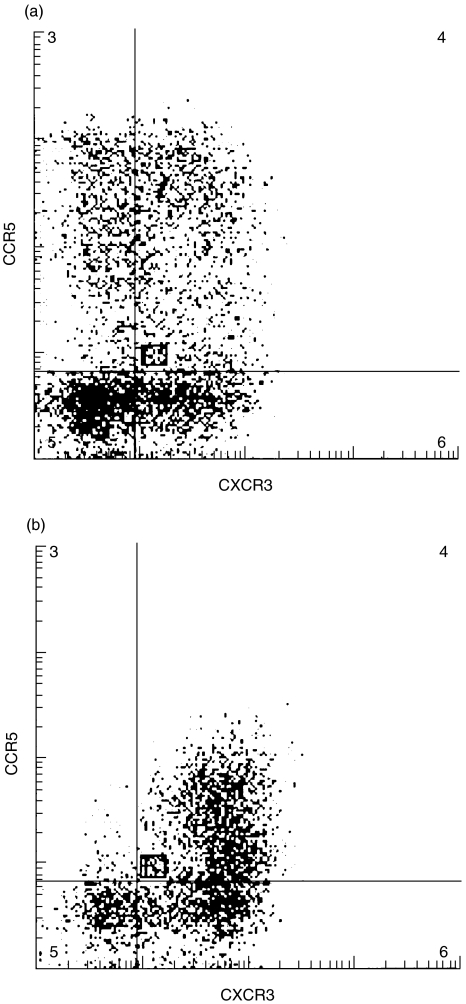
Since > 90% of all CD3+T-cells in the CSF were CXCR3+, a significant overlap between the expression of CXCR3 and CCR5 could be anticipated. Upon directly analysing coexpression of CXCR3 and CCR5 on individual T-cells, we found that essentially all CCR5+/CD3+T-cells in the CSF (median 96%, range 93–99%, n = 4 MS/6 NIND) expressed CXCR3. Interestingly, while 9·9% (range 4·8–18·7%) of all CD3+T-cells in PB were CCR5+*CXCR3-, only 2·3% (range 0·5–3·9%; P < 0·005) of all CD3+T-cells in the CSF had this phenotype (Fig. 4).
Fig. 4.
Co-expression of CXCR3 and CCR5 on individual T-cells in (a) peripheral blood and (b) CSF was compared using three-colour flow cytometry. Cells were gated according to forward- and side-light scattering properties, and were positively selected for CD3 expression.
Expression of inflammatory chemokine receptors on CD45RO+/CD4+ T-cells in PB and CSF
Since CSF contains higher percentages of CD4+/CD45RO+T-cells compared to PB [30], experiments were performed to compensate for this difference in cellular composition between blood and CSF. Preliminary studies showed that >95% (median 98%, range 96–100, n = 7 NIND) of all CD4+T-cells in the CSF were CD45RO+, permitting us to analyse chemokine receptor expression on CD4+T-cell in the CSF, using the assumption that these cells constituted a comparable population to CD4+/CD45RO+T-cells in blood. This approach allowed us to use optimally the few CSF cells available.
These experiments showed that the enrichment of CCR5+T-cells in the CSF could be explained by accumulation of CD4+/CD45RO + T-cells in the CSF. Approximately 50% of all CD4 + T-cells in the CSF expressed CCR5, a level that was comparable to the expression of CCR5 on CD4+*CD45RO+T-cells in PB (P = 0·7; Table 2). No differences in CCR5 expression on CD4+*CD45RO+T-cells were observed between patients with MS and NIND (blood, P = 0·4; CSF, P = 0·6; Table 2).
In preliminary analyses, we found moderate reduction in CCR5 expression in cells analysed at +4°C as compared with RT (Fig. 1). This observation raised the possibility that comparison of T-cell CCR5 in blood (stained at RT) and CSF (stained at +4°C) might underestimate CCR5 detection on CSF T-cells. To address this possibility, we stained paired blood and CSF samples from five consecutive patients with NIND at RT. In these experiments, CD4+T-cells in the CSF showed significant enrichment for CCR5 (61%, range 58–77%), as compared to CD4+* CD45RO+T-cells in PB (44%, range 37–69%; P < 0·05). However, even under these optimal conditions for detecting CCR5 expression, CSF enrichment for CCR5+T-cells remained modest, and a substantial proportion of CD4+T-cells in the CSF remained CCR5 negative.
The CSF population of CD4+T-cells was significantly enriched for CXCR3 both in patients with MS and NIND, even after compensating for the accumulation of CD4+*CD45RO+ cells in the CSF (Table 2). Strikingly, 90% of CSF CD4+T-cells expressed CXCR3, as compared with 60% of CD4+*CD45RO+T-cells in PB (P < 0·01). Enrichment for CXCR3+T-cells in CSF was relatively selective, as expression of both CCR2 and CCR6 was lower on CSF CD4+T-cells, as compared to CD4+*CD45RO+T-cells in PB (Table 2, P < 0·01 for both comparisons).
DISCUSSION
Using flow cytometry, we demonstrated that CD3+T-cells in the CSF express a restricted repertoire of inflammatory chemokine receptors, specifically CXCR3, CCR5 and CCR6, but little CCR1-3. The expression of CXCR3 and CCR5 was increased on CD3+T-cells in the CSF compared to PB, whereas levels of CCR6 expression were comparable in these two compartments. Accumulation of CXCR3+ and CCR5+T-cells has previously been demonstrated in CSF compared to PBMCs [11,14]. A potential explanation for increased expression of CXCR3 and CCR5 in the CSF could, however, be that CSF contains more activated memory T-cells compared to PB. We observed, in agreement with earlier studies [30], that the majority of CD4+T-cells in the CSF express the CD45RO isoform, compared to only 30–40% of PB T-cells. CD45RO has been shown to correlate with the ability of the cell to proliferate to recall antigens and provide help for antibody production [37], and can be used as a marker for a memory phenotype. As part of this phenotype, CD4+*CD45RO+T-cells express high levels of adhesion- and activation-related molecules promoting cell trafficking, including chemokine receptors such as CXCR3 and CCR5 [16,30,31].
We found that most of the accumulation of CCR5+T-cells in the CSF could be explained by a selective enrichment of CD4+*CD45RO+T-cells in the CSF. By contrast, CXCR3 expression was clearly increased on T-cells in the CSF even after compensating for the enrichment of CD4+*CD45RO+T-cells in this compartment. A less important role for CCR5 in the accumulation of T-cells in the CSF was also suggested by the fact that almost no CCR5+*CXCR3- T-cells could be detected in the CSF, and that CCR5+/CXCR3- T-cells were more abundant in PB than CSF. Interestingly, it has been reported that CCR5+/CXCR3- T-cells in PB produce less interferon-γ and more interleukin-4 compared to CCR5+/CXCR3+T-cells, and constitute a subset of CCR5+ cells which are not associated with a Th1 phenotype [9]. Taken together, these data indicate that the expression of CCR5 alone seems not to be sufficient for the accumulation of CD3+T-cells in the CSF, but that there might be a requirement for CXCR3 expression as well. The large number of CCR5+T-cells observed in the CSF may plausibly reflect the association of CCR5 with previous cell activation and coexpression with CXCR3 on individual T-cells rather than a specific attraction of T-cells through CCR5.
An unexpected finding was that the expression of CCR2 on CD3+T-cells and CD4+/CD45RO+T-cells was reduced in the CSF compared to PB regardless of the presence of CNS inflammation. This is in contrast to an earlier study, which showed (nonstatistically) increased levels of CCR2+/CD4+T-cells in the CSF, compared to PBMCs from patients with MS [14]. In that study, levels of CCR2+/CD4+T-cells in PB from patients with MS were around 3%, compared to 14% of CCR2+/CD3+T-cells in the present study. Even though a moAb from the same clone was used in both studies, the group of Misu et al. used a biotinylated form, which in our hands gives a dim staining with considerably fewer stained cells compared to the directly conjugated form (PK, RMR: unpublished observation).
CCR2 binds to multiple inflammatory chemokines such as CCL2, 7, 8, and 13 [7,38]. Of these, CCL2 has been detected in the CSF from patients with a wide range of inflammatory and non-inflammatory neurological diseases including MS, as well as orthopedic patients without inflammatory disease who under- went epidural anaesthesia [11,39–41]. CCL2 is dramatically up-regulated in the CSF of patients with bacterial meningitis or HIV-associated dementia, but significantly reduced in MS patients during relapses, as compared with remission [11,39–43]. In patients with arthritis the percentage of CCR2+T-cells was considerably higher in the synovial fluid than in PB suggesting that CCR2 plays a different role in CNS and joint inflammation [44,45].
CXCR3 is highly expressed on T-cells isolated from diverse inflamed tissues, as reported in rheumatoid arthritis, ulcerative colitis, hepatitis C, sarcoidosis, and inflammation of the skin [16,46–49], and is proposed to mark a subset of lymphocytes with enhanced ability to accumulate in inflammatory sites. Several investigators have demonstrated increased levels of CXCL10, one of three ligands for CXCR3, in the CSF from patients with MS compared to control individuals [39,41]. CXCL10 immunoreactivity has been detected in MS brain lesions colocalizing with CXCR3 positive cells in individual perivascular infiltrates, suggesting biological significance for the observation of increased frequencies of CXCR3+T-cells in the CSF [13]. Administration of neutralizing antibodies against CXCL10 ameliorated further disease activity and reduced accumulation of mononuclear cells in experimental autoimmune encephalomyelitis, an animal model of MS [50].
A major finding of the current study was that the pattern of chemokine receptor expression on CD3+T-cells in the CSF was not different in MS patients compared to patients with NIND, but seemed to be independent of underlying CNS pathology. In animal models, activated and memory T-cells enter the CNS compartment across an intact blood–brain barrier regardless of the presence of inflammation, presumably in the process of physiological immune surveillance of the CNS [51,52]. Aside from a requirement for P-selectin binding activity, little is known about the molecular determinants by which these cells enter the intrathecal compartment [53]. Emerging evidence suggests that the expression of certain chemokine receptors on PB T-cells helps to define discrete, well-defined tissue-committed subpopulations, such as skin-homing (expressing CCR4) and gut-homing (expressing CCR9) memory T-cells [54]. Whether specific chemokines and their receptors could account for physiologic trafficking of T-cells through the brain remains uncertain. The wide range of tissues in which CXCR3 has been detected argues against a role for CXCR3 in CNS-specific homing of T-cells.
Our working hypothesis is that CXCR3 is involved in intrathecal retention of T-cells in the presence of inflammation, rather than recruitment of T-cells into the CNS [55]. In MS, ligands for CXCR3 are present both in CSF and perivascular tissue [10–12,39,56], resulting in the activation of leukointegrins on T-cells expressing CXCR3, causing retention of these cells and the subsequent formation of perivascular T-cell infiltrates. T-cells entering the CNS in the absence of inflammation, when only negligible levels of CXCR3 ligands are present, fail to be retained and consequently recirculate back to the periphery. Indirect proof for this notion comes from studies of brain tissue that revealed abundant expression of CXCR3 on T-cells in perivascular MS lesions but only rarely in non-inflamed brain [10–12]. Presently, it is not feasible to distinguish between CSF T-cells that derive from the choroid plexus or through migration across the ependymal barrier or the perivascular spaces around parenchymal vessels in clinical materials. It is consequently not possible to differentiate factors involved in physiological from those involved in pathological trafficking in such settings, but studies in experimental model system are necessary.
In conclusion, we observed that CD3+T-cells in the CSF express a restricted repertoire of inflammatory chemokine receptors, specifically CXCR3, CCR5 and CCR6, but little CCR1-3. This repertoire was independent of the presence of CNS inflammation, since similar findings were observed in MS patients and patients with NIND. The enrichment of CCR5+T-cells in the CSF could largely be explained by higher frequency of CD4+/CD45RO+T-cells in this compartment. In contrast, CD4+/ CD45RO+T-cells expressing CXCR3 were significantly enriched in CSF as compared with blood. These results identify CXCR3 as an inflammatory chemokine receptor likely to be involved in the intrathecal accumulation of T-cells in MS. The role for CXCR3 is probably not exerted at the level of trafficking of T-cells into the CSF compartment, but the expression of CXCR3 ‘arms’ T-cells to persist in the CNS in the presence of inflammation.
REFERENCES
- 1.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–9. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 2.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–85. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 3.Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122:2279–95. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- 4.Bitsch A, Wegener C, da Costa C, et al. Lesion development in Marburg's type of acute multiple sclerosis: from inflammation to demyelination. Mult Scler. 1999;5:138–46. doi: 10.1177/135245859900500302. [DOI] [PubMed] [Google Scholar]
- 5.Luster AD. Chemokines – chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T- lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 8.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–83. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CH, Rott L, Kunkel EJ, et al. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balashov KE, Rottman JB, Weiner HL, Hancock WW. CCR5 (+) and CXCR3 (+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci USA. 1999;96:6873–8. doi: 10.1073/pnas.96.12.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sørensen TL, Tani M, Jensen J, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–15. doi: 10.1172/JCI5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol Appl Neurobiol. 2000;26:133–42. doi: 10.1046/j.1365-2990.2000.026002133.x. [DOI] [PubMed] [Google Scholar]
- 13.Sørensen TL, Trebst C, Kivisäkk P, et al. Multiple sclerosis. A study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol. 2002;127:59–68. doi: 10.1016/s0165-5728(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 14.Misu T, Onodera H, Fujihara K, et al. Chemokine receptor expression on T cells in blood and cerebrospinal fluid at relapse and remission of multiple sclerosis: imbalance of Th1/Th2-associated chemokine signaling. J Neuroimmunol. 2001;114:207–12. doi: 10.1016/s0165-5728(00)00456-2. [DOI] [PubMed] [Google Scholar]
- 15.Loetscher P, Uguccioni M, Bordoli L, et al. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–5. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 16.Qin S, Rottman JB, Myers P, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–54. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel EJ, Boisvert J, Murphy K, et al. Expression of the Chemokine Receptors CCR4, CCR5, and CXCR3 by Human Tissue-Infiltrating Lymphocytes. Am J Pathol. 2002;160:347–55. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin S, LaRosa G, Campbell JJ, et al. Expression of monocyte chemoattractant protein-1 and interleukin-8 receptors on subsets of T cells: correlation with transendothelial chemotactic potential. Eur J Immunol. 1996;26:640–7. doi: 10.1002/eji.1830260320. [DOI] [PubMed] [Google Scholar]
- 19.Nieto M, Frade JM, Sancho D, Mellado M, Martinez A, Sanchez-Madrid F. Polarization of chemokine receptors to the leading edge during lymphocyte chemotaxis. J Exp Med. 1997;186:153–8. doi: 10.1084/jem.186.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karpus WJ, Lukacs NW, Kennedy KJ, Smith WS, Hurst SD, Barrett TA. Differential CC chemokine-induced enhancement of T helper cell cytokine production. J Immunol. 1997;158:4129–36. [PubMed] [Google Scholar]
- 21.Traynor TR, Kuziel WA, Toews GB, Huffnagle GB. CCR2 expression determines T1 versus T2 polarization during pulmonary Cryptococcus neoformans infection. J Immunol. 2000;164:2021–7. doi: 10.4049/jimmunol.164.4.2021. [DOI] [PubMed] [Google Scholar]
- 22.Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue. J Neuroimmunol. 2000;108:192–200. doi: 10.1016/s0165-5728(00)00274-5. [DOI] [PubMed] [Google Scholar]
- 23.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–7. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 24.Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med. 1999;190:267–80. doi: 10.1084/jem.190.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath H, Qin S, Rao P, et al. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–84. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerber BO, Zanni MP, Uguccioni M, et al. Functional expression of the eotaxin receptor CCR3 in T lymphocytes co- localizing with eosinophils. Curr Biol. 1997;7:836–43. doi: 10.1016/s0960-9822(06)00371-x. [DOI] [PubMed] [Google Scholar]
- 27.Uguccioni M, Mackay CR, Ochensberger B, et al. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest. 1997;100:1137–43. doi: 10.1172/JCI119624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao F, Rabin RL, Smith CS, Sharma G, Nutman TB, Farber JM. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha. J Immunol. 1999;162:186–94. [PubMed] [Google Scholar]
- 29.Svenningsson A, Andersen O, Edsbagge M, Stemme S. Lymphocyte phenotype and subset distribution in normal cerebrospinal fluid. J Neuroimmunol. 1995;63:39–46. doi: 10.1016/0165-5728(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 30.Svenningsson A, Hansson GK, Andersen O, Andersson R, Patarroyo M, Stemme S. Adhesion molecule expression on cerebrospinal fluid T lymphocytes: evidence for common recruitment mechanisms in multiple sclerosis, aseptic meningitis, and normal controls. Ann Neurol. 1993;34:155–61. doi: 10.1002/ana.410340210. [DOI] [PubMed] [Google Scholar]
- 31.Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162:3840–50. [PubMed] [Google Scholar]
- 32.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis. guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Paxton WA, Kassam N, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–91. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–20. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharron M, Pohlmann S, Price K, et al. Expression and coreceptor activity of STRL33/Bonzo on primary peripheral blood lymphocytes. Blood. 2000;96:41–9. [PubMed] [Google Scholar]
- 36.Dux R, Kindler-Rohrborn A, Annas M, Faustmann P, Lennartz K, Zimmermann CW. A standardized protocol for flow cytometric analysis of cells isolated from cerebrospinal fluid. J Neurol Sci. 1994;121:74–8. doi: 10.1016/0022-510x(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 37.Clement LT. Functional and phenotypic properties of ‘naive’ and ‘memory’ CD4+ T cells in the human. Immunol Res. 1991;10:189–95. doi: 10.1007/BF02919691. [DOI] [PubMed] [Google Scholar]
- 38.Zlotnik A, Yoshie O. Chemokines. a new classification system and their role in immunity. Immunity. 2000;12:121–7. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 39.Franciotta D, Martino G, Zardini E, et al. Serum and CSF levels of MCP-1 and IP-10 in multiple sclerosis patients with acute and stable disease and undergoing immunomodulatory therapies. J Neuroimmunol. 2001;115:192–8. doi: 10.1016/s0165-5728(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 40.Sindern E, Niederkinkhaus Y, Henschel M, Ossege LM, Patzold T, Malin JP. Differential release of beta-chemokines in serum and CSF of patients with relapsing-remitting multiple sclerosis. Acta Neurol Scand. 2001;104:88–91. doi: 10.1034/j.1600-0404.2001.104002088.x. [DOI] [PubMed] [Google Scholar]
- 41.Sørensen TL, Sellebjerg F, Jensen CV, Strieter RM, Ransohoff RM. Chemokines CXCL10 and CCL2: differential involvement in intrathecal inflammation in multiple sclerosis. Eur J Neurol. 2001;8:665–72. doi: 10.1046/j.1468-1331.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 42.Lahrtz F, Piali L, Nadal D, et al. Chemotactic activity on mononuclear cells in the cerebrospinal fluid of patients with viral meningitis is mediated by interferon-gamma inducible protein-10 and monocyte chemotactic protein-1. Eur J Immunol. 1997;27:2484–9. doi: 10.1002/eji.1830271004. [DOI] [PubMed] [Google Scholar]
- 43.Lahrtz F, Piali L, Spanaus KS, Seebach J, Fontana A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol. 1998;85:33–43. doi: 10.1016/s0165-5728(97)00267-1. [DOI] [PubMed] [Google Scholar]
- 44.Mack M, Brühl H, Gruber R, et al. Predominance of mononuclear cells expressing the chemokine receptor CCR5 in synovial effusions of patients with different forms of arthritis. Arthritis Rheum. 1999;42:981–8. doi: 10.1002/1529-0131(199905)42:5<981::AID-ANR17>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 45.Brühl H, Wagner K, Kellner H, Schattenkirchner M, Schlondorff D, Mack M. Surface expression of CC- and CXC-chemokine receptors on leucocyte subsets in inflammatory joint diseases. Clin Exp Immunol. 2001;126:551–9. doi: 10.1046/j.1365-2249.2001.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agostini C, Cassatella M, Zambello R, et al. Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol. 1998;161:6413–20. [PubMed] [Google Scholar]
- 47.Shields PL, Morland CM, Salmon M, Qin S, Hubscher SG, Adams DH. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999;163:6236–43. [PubMed] [Google Scholar]
- 48.Flier J, Boorsma DM, Bruynzeel DP, et al. The CXCR3 activating chemokines IP-10, Mig, and IP-9 are expressed in allergic but not in irritant patch test reactions. J Invest Dermatol. 1999;113:574–8. doi: 10.1046/j.1523-1747.1999.00730.x. [DOI] [PubMed] [Google Scholar]
- 49.Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 50.Fife BT, Kennedy KJ, Paniagua MC, et al. CXCL10 (IFN- gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2001;166:7617–24. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- 51.Wekerle H, Fierz W. T cell approach to demyelinating diseases. Springer Semin Immunopathol. 1985;8:97–110. doi: 10.1007/BF00197249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. J Neurosci Res. 1991;28:254–60. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- 53.Carrithers MD, Visintin I, Kang SJ, Janeway CA., Jr Differential adhesion molecule requirements for immune surveillance and inflammatory recruitment. Brain. 2000;123:1092–101. doi: 10.1093/brain/123.6.1092. [DOI] [PubMed] [Google Scholar]
- 54.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 55.Trebst C, Ransohoff RM. Investigating chemokines and chemokine receptors in patients with multiple sclerosis: opportunities and challenges. Arch Neurol. 2001;58:1975–80. doi: 10.1001/archneur.58.12.1975. [DOI] [PubMed] [Google Scholar]
- 56.Sørensen TL, Sellebjerg F. Distinct chemokine receptor and cytokine expression profile in secondary progressive MS. Neurology. 2001;57:1371–6. doi: 10.1212/wnl.57.8.1371. [DOI] [PubMed] [Google Scholar]