Abstract
To examine the influence of genetics on the OVA-induced allergic inflammatory response in lungs we compared rats that are genetically Th2-predisposed (Brown Norway, inbred) or not genetically predisposed (Sprague Dawley, outbred). Rats were sensitized with ovalbumin (OVA) and challenged four weeks later with OVA aerosol. Eighteen hours after challenge, lung tissue was studied for evaluation of numbers of eosinophils, neutrophils, macrophages and mast cells, as well as for expression of P-selectin, E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells. From a separate portion of the pulmonary tissue, leucocytes were isolated to analyse numbers of IFNγ and IL-4 producing cells (ELISPOT assay) and frequencies of T-cell subsets and B cells. We found increased numbers of eosinophils and neutrophils in the lung, an increased number of IL-4 producing cells in lung cell isolates and increased levels of serum (OVA- specific)-IgE in both rat strains. In addition, expression of E-selectin and ICAM-1 was up regulated in both rat strains whereas expression of VCAM-1 was only up regulated in the BN rat. Although the ‘allergic’ Th2 response to OVA was detectable in both rat strains, it was more pronounced in the BN rat than in the SD rat. However, the SD rat, which is not predisposed to respond in either a Th2 or Th1-like way, appeared capable of mounting an allergic response to OVA. This suggests that other factors than genetic contribute to allergic disease.
Keywords: asthma, inflammation, airways, cytokines, rat
INTRODUCTION
Allergic asthma is characterized by chronic airway inflammation associated with goblet cell hyperplasia and mucus plugging of airways, sub epithelial fibrosis and airway smooth muscle cell hypertrophy [1]. Th2-type inflammatory cells are thought to contribute to this process by producing a variety of inflammatory mediators, oxygen radicals and Th2-type cytokines such as IL-4, IL-5 and IL-13 [2,3]. Acute exacerbations of asthma appear to be related to environmental allergen exposures [4,5], or viral respiratory infections [6]. However, there is a significant genetic predisposition to the development of asthma in humans [7]. Twin studies have revealed that monozygotic twins of individuals with asthma have a four-fold likelihood of having asthma as compared with the general population while dizygotic twins have a two-fold likelihood of having the disease [8]. It has proven difficult to localize and ultimately identify the genes coding for this predisposition in genetically heterogeneous human populations. Large study populations are required in order to obtain meaningful results.
The use of animal models for genetic studies of the genetic predisposition for asthma-like pulmonary changes have the advantage of genetic homogeneity of the study subjects as well as ready availability. Rat models are becoming more useful as many immunological reagents including monoclonal antibodies to cell adhesion molecules and cytokines have become available.
The aim of the study was to compare the OVA-induced allergic inflammatory response in lungs of rats, which are genetically Th2-predisposed (Brown Norway, inbred) to a strain not so predisposed (Sprague Dawley, outbred). The BN rat model mimics human allergic asthma in several respects. BN rats produce high levels of IgE in response to active immunization and develop both early and late airway constriction responses after inhalation of antigen [9,10]. These two different rat strains were used to study the relative contributions of genetic factors to the development of this experimental asthma-like condition.
MATERIALS AND METHODS
Animals
Male, 6–8 weeks old BN/RIJ HSD (RT1n) and SD/HSD (outbred) rats were obtained from Harlan (Zeist, the Netherlands) and maintained under specific pathogen-free conditions in the Central Animal Facility of the University of Groningen.
Antibodies
Antibodies used in this study were B41-1 (anti-IgE), B41-3 (biotinylated anti-IgE), ED1 (anti-CD68), G4·18 (anti-CD3), G53-238 (anti-IgM), HIS52 (antiendothelium), OX8 (anti-CD8), OX35 (anti-CD4), OX81 (anti-IL-4), R73 (anti-TCRαβ), V65 (anti-TCRγδ), CD54 (inter adhesion molecule-1, ICAM-1), polyclonal antibody against CD62P (P-selectin). These antibodies were obtained from PharMingen (San Diego, CA, USA). DB1 (anti-IFN-γ, Biosource, Etten Leur, the Netherlands). Polyclonal antibodies against CD62E (E-selectin) and vascular cell adhesion molecule-1 (VCAM-1) were obtained from Sanvertech, Heerhugowaard, the Netherlands.
The polyclonal rabbit antirat antibodies directed to IFN-γ and IL-4 were a kind gift of Dr P. van der Meide (BPRC, Rijswijk, the Netherlands).
Sensitization procedure
Ovalbumin (OVA grade V, Sigma-Aldrich Inc., Zwijndrecht, the Netherlands) was prepared at 2 mg/ml in pyrogen-free PBS and precipitated at a 1:1 ratio with Al(OH)3 (45 mg/ml, Imject™ Alum; Pierce, Rockford, IL, USA), following the instructions of the manufacturer. Rats (n = 6) were sensitized with 1 mg of OVA (1 ml of OVA- Al(OH)3 suspension) given intradermally to the back. Sham immunizations were done with PBS (n = 6).
Airway allergen challenge
Four weeks after sensitization, rats were placed in a perspex exposure chamber (9 l) and challenged for 30 min on 2 consecutive days with an aerosol of 1% OVA in saline. The aerosol was delivered by a De Vilbiss nebulizer (type 646, De Vilbiss, Somerset, PA, USA) driven by an airflow of 8 l/min providing aerosol with an output of 0·33 ml/min.
Experimental procedure
Six weeks after birth, animals were sensitized with either OVA + alum or PBS, followed by a challenge with either OVA or PBS at 10 weeks. Eighteen hours after challenge, sacrifice and lung cell isolation took place.
Cell preparation and lung digest
Single-cell suspensions from lungs were obtained as described [11]. Briefly, rats were sacrificed and the lung vascular bed was flushed in situ via the right cardiac ventricle with 20 ml of cold PBS to remove any blood and intravasculair leucocytes. Minced lungs were incubated for 90 min at 37°C on a rocker, in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (FCS), DNAse I (100 U/ml; Boehringer, Mannheim, Germany), and collagenase I (250 U/ml; C9891; Sigma–Aldrich Inc., Zwijndrecht, the Netherlands). Purified vital lung cells were obtained by passing the digested lung tissue through a stainless steel mesh and subsequently performing discontinuous Percoll gradient (Pharmacia, Uppsala, Sweden) centrifugation (20–55%). Cells were counted using a Coulter Counter Z1 (Coulter, Hialeah, USA).
Flow cytometry
Three colour flow cytometry was performed to determine frequencies of T and B-cell subsets. Our reagents included commonly used fluorochromes such as fluorescein isothiocyanate (FITC), phycoerythrin (Pe) and allophycocyanin (APC). Frequencies of T and B-cell subsets were based on the following label combinations (FITC-PE-APC): CD4-CD8-TCRαβ; CD3-TCRγδ-TCRαβ; IgM-CD8-TCRαβ. Cells were stained on ice for 20– 30 min at a concentration of 106 cells/25 μl with the various combinations of fluorochrome- or biotin-conjugated antibodies diluted in cold FACS buffer (PBS containing 0·5% Dulbecco B, 5% normal calf serum and 0·03% natriumazide). As a second step reagent, APC conjugated to streptavidin was used. All antibodies were used in separately determined optimal concentrations. Before staining, cells were blocked for 15 min on ice with Fc-block (normal rat serum, 50 μg/ml).
Cell populations (4 × 104 events) were analysed using an Epics Elite flow cytometer (Coulter Epics, Hialeah, USA) and analysis was performed using FlowJo (ThreeStar, San Carlos, CA, USA).
Determination of cytokines by ELISPOT assay
IFN-γ
For the detection of numbers of IFN-γ producing cells the ELISPOT assay was used as described previously [12]. The mAb DB-1 (anti-IFN-γ) was used as a capture antibody and a polyclonal rabbit antirat IFNγ as a detection Ab. For detection of IFN-γ producers 4 × 104 cells in 100 μl were tested. Cells were stimulated with 4β-phorbol 12β-myristate 13α-acetate (PMA, 20 ng/ml, Sigma) and ionomycin (1 μm, Sigma) for 18 h at 37°C in a humidified atmosphere with 5% CO2. Spots could be counted using an inverted microscope. As a negative control, unstimulated cells were used. No spots were found in control wells.
IL-4
For the detection of numbers of IL-4 producing cells, the mAb OX81 was used as capture antibody and a polyclonal rabbit anti-IL-4 as detection Ab. For detection of numbers of IL-4 producing cells, 4 × 105 cells were tested. Ratios of IFN-γ/IL-4 were calculated by dividing numbers of IFN-γ producing cells by numbers of IL-4 producing cells.
Total IgE sandwich ELISA
Serum was collected just before sensitization (day 0) and 7, 14, 21 and 28 days after sensitization. Samples were stored at −20°C until analysed. Total serum IgE was determined using a sandwich ELISA (PharMingen, San Diego, CA, USA) according to instructions of the manufacturer. Serum samples, diluted 1: 25, were added to the plates and were two-fold titrated. On each plate serial dilutions of a hyper immune rat serum sample as well as a normal serum sample were run as positive and negative controls, respectively, to correct for interassay variation.
Optical densities were converted to arbitrary units (ELISA units) according to the standard curve obtained from serial dilutions of the hyper immune rat serum sample.
Measurement of OVA-specific IgE
OVA-specific IgE was measured as described previously [13] with some modifications. Wells were coated overnight at 4°C with a mouse monoclonal antibody to rat IgE (B41-1, PharMingen, San Diego, CA) at 2 μg/ml, 100 μl/well. After washing the plates three times with PBS/0·05% Tween-20, plates were blocked with 1% milk (Elk, Campina, Eindhoven, the Netherlands) in PBS for 2 h and serum samples were added to the wells at a 1:5 dilution in 2% BSA/PBS. Serum samples were titrated two-fold and plates were incubated for 1 h at 37°C. After washing the plates three times with PBS/0·05% Tween-20, biotinylated OVA (10 μg/ml in 2% BSA/PBS) was added for 1 h and plates were washed again. As a second-step reagent horseradish peroxidase-conjugated streptavidin (Dako, P0397, Denmark) was added for 15 min in a 1:4000 dilution in 1% milk. Plates were developed, stopped and read as described for the total IgE ELISA. Optical densities were converted to arbitrary units (ELISA units) according to the standard curve obtained from serial dilutions of a hyper immune rat serum sample.
Analysis of numbers of eosinophils, neutrophils, macrophages and mast cells
Eosinophils were determined in frozen lung tissue, stained for endogenous peroxidase. Measurement of numbers of eosinophils in lung tissue was done by morphometric analysis and expressed as volume percentages.
The presence of neutrophils was analysed in frozen lung sections by staining for neutrophil alkaline phosphatase. The presence of macrophages was analysed in cytospins of lung cell isolates by staining with the monoclonal antibody ED1 (CD68) and the presence of mast cells was analysed by metachromatic staining of frozen lung sections with toluidine blue.
Expression of P-selectin, E-selectin, ICAM-1 and VCAM-1 on lung vessels
For these experiments, frozen tissue sections from the lung were double stained for endothelial cells (HIS52) in combination with one of the specific antibodies for P-selectin, E-selectin, ICAM-1 and VCAM-1. Staining of adhesion molecules was performed following the instructions of the manufacturer. Data are expressed as percentage of blood vessels (HIS52+) positive for the different adhesion molecules.
Statistical analysis
For statistical analysis, Mann–Whitney test was used for com-parison of different groups.
RESULTS
Increase of eosinophils in lung tissue after OVA sensitization and challenge
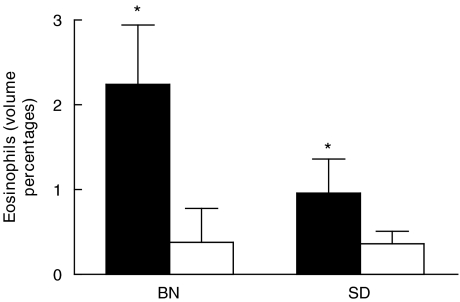
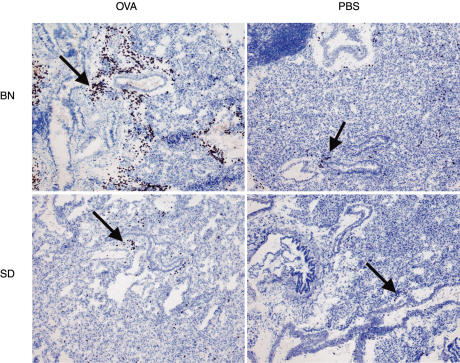
The presence of eosinophils was analysed in frozen lung sections, 18 hours after OVA challenge. As shown in Fig. 1, volume percentages of eosinophils increased significantly (P < 0·05) in both rat strains when compared to the control groups, as measured by computer assisted morphometry. However, this response was more pronounced in the BN rat (P > 0·05) than in the SD rat. Distribution of eosinophils was peribroncholar and perivascular in both rat strains as shown in Fig. 2.
Fig. 1.
Eosinophils, expressed as volume percentages in frozen lung sections of two different rat strains treated with OVA (▪, n = 6) or PBS (□, n = 6), 18 h after allergen/PBS challenge. Data are presented as the mean ± SD. *Value significantly different from rats treated with PBS (P < 0·05).
Fig. 2.
Distribution of eosinophils (as denoted by arrows) in frozen lung sections of two different rat strains after treatment with OVA or PBS, 18 h after allergen/PBS challenge. Magnification × 75.
IFNγ- and IL-4-producing cells in lung cell isolates after OVA sensitization and challenge
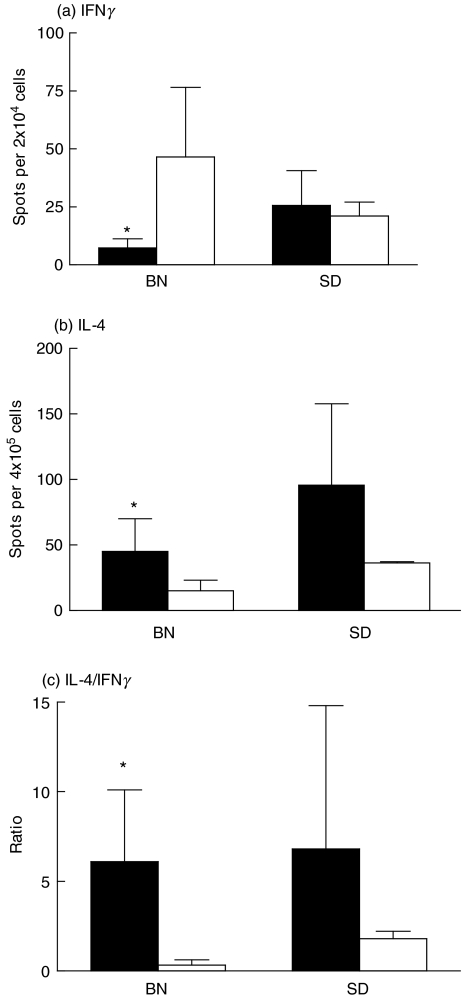
To compare Th1 and Th2 cytokine responses after OVA treatment in both rat strains, numbers of IFNγ and IL-4 producing cells were assayed in lung cell isolates by ELISPOT assay. Figure 3 shows that in the BN rat numbers of lung IFNγ producing cells were decreased and numbers of IL-4 producing cells were increased when compared to the PBS control (P < 0·05). This resulted in increased IL-4/IFNγ ratios. In the SD rat, this overall Th2-type response was less pronounced. Treatment with OVA did not down regulate numbers of IFNγ producing cells in this rat strain. However, numbers of IL-4 producing cells in the lung were increased after OVA treatment, leading to an increased IL-4/IFNγ ratio.
Fig. 3.
IFNγ (a) and IL-4 (b) producing cells in isolated lung cells of 2 different rat strains treated with OVA alone (▪, n = 6) and rats treated with PBS (□, n = 6), 18 h after allergen/PBS challenge. Numbers of cytokine producing cells were determined by ELISPOT assays after stimulation for 18 h with PMA and ionomycin. Ratios of IL-4/IFNγ producing cells (c) were calculated by dividing numbers of IL-4 producing cells by numbers of IFNγ producing cells. Means ± SD are presented for each group. *Values significantly different from rats treated with PBS (P < 0·05).
Increase of (OVA-specific)-IgE after OVA sensitization in serum of both rat strains
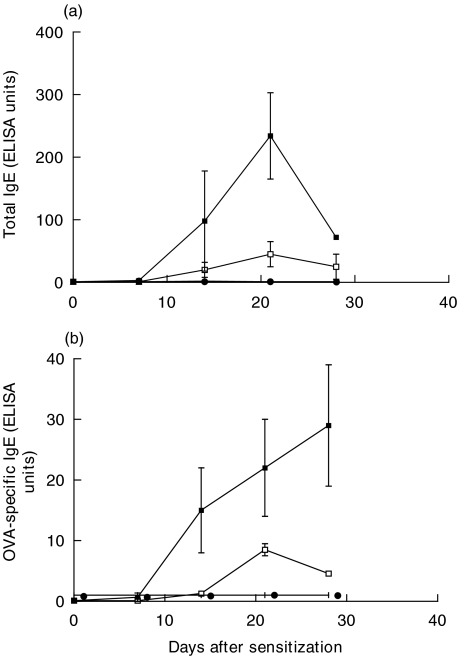
Total IgE and OVA-specific IgE was measured in serum of both strains, 0, 7, 14, 21 and 28 days after OVA sensitization by ELISA. Both rat strains produced significantly increased levels of both total IgE and OVA-specific-IgE in response to OVA sensitization. However, this response was more prominent in the BN rat (P < 0·005 at day 21, Fig. 4a,b).
Fig. 4.
Total serum IgE (a) in BN rat or SD rat or OVA-specific serum IgE, (b) in BN rat or SD rat 0, 7, 14, 21 and 28 days after OVA sensitization (n = 6) or PBS (n = 6). ▪ BN/OVA; • BN/PBS; □ SD/OVA; ○ SD/PBS.
Presence of neutrophils, macrophages and mast cells in the lung after OVA sensitization and challenge
The presence of neutrophils, macrophages and mast cells were analysed in frozen lung sections or cytospins of lung cell isolates, 18 hours after OVA challenge. Table 1 shows that in both rat strains the number of neutrophils was increased in response to the OVA protocol. OVA treatment had no effect on frequencies of macrophages or mast cells when compared to treatment with PBS in the BN rat. However, it did up regulate numbers of macrophages in the SD rat.
Table 1.
Numbers of neutrophils, macrophages and mast cells in the lung of both rat strains
| Rat strain | Treatment | Neutrophils (per microscopic field) | Macrophages (per microscopic field) | Mast cells (per tissue section) |
|---|---|---|---|---|
| BN | OVA | 48 ± 38* (P = 0·0091) | 57 ± 28 | 12 ± 10 |
| BN | PBS | 3 ± 2 | 52 ± 9 | 21 ± 14 |
| SD | OVA | 24 ± 13 (P = 0·036) | 79 ± 12 (P = 0·0032) | 32 ± 16 |
| SD | PBS | 2 ± 1 | 21 ± 12 | 64 ± 24 |
14 microscopic fields per tissue section counted
Significant values when compared to PBS treated animals.
Frequencies of T-cell subsets and B cells in isolated lung cells after OVA sensitization and challenge
To compare frequencies of different T-cell subsets and B cells after OVA treatment in both rat strains, leucocytes isolated from the lung were analysed for expression of αβT-cell receptor, γδT-cell receptor, CD4, CD8 or IgM (B cells) by three colour flow cytometry. As shown in Table 2, frequencies of the various T-cell subsets or B cells of rats treated with OVA were not significantly different when compared to rats treated with PBS. In the BN rat, higher CD4/CD8 ratio's were found than in the SD rat.
Table 2.
Percentages of T-cell subsets en B cells, isolated from the lung
| Rat strain | Treatment | αβ TCR (%)† | γδ TCR (%) | CD4+ T cells (%) | CD8+ T cells (%) | B cells (%) |
|---|---|---|---|---|---|---|
| BN | OVA | 29·5 ± 4 | 0·4 ± 0·1 | 23·7 ± 1·1 | 2·1 ± 0·1 | 57·5 ± 1·9 |
| BN | PBS | 32·0 ± 3 | 0·5 ± 0·2 | 29·9 ± 1·3 | 3·1 ± 1·2 | 53·1 ± 2·5 |
| SD | OVA | 26·5 ± 5 | 0·7 ± 0·3 | 18·7 ± 4·8 | 7·1 ± 0·7 | 58·7 ± 10·3 |
| SD | PBS | 27·5 ± 4 | 0·6 ± 0·2 | 20·0 ± 1·4 | 7·8 ± 1·5 | 55·5 ± 7·7 |
Cells expressed as percentage of lymphocytes isolated from lung
Expression of P-selectin, E-selectin, ICAM-1 and VCAM-1 on lung vessels after OVA sensitization and challenge
As shown in Table 3, P-selectin expression on endothelium was upregulated in the SD rat after the OVA protocol when compared to the PBS control. P-selectin expression also increased in the BN rat, but this increase was not significant. E-selectin was up regulated on endothelium in both strains after OVA treatment. VCAM-1 was up regulated in the BN rat but not in the SD rat. ICAM-1 expression was high in both control animals and animals treated with the OVA protocol.
Table 3.
Expression of P-selectin, E-selectin, ICAM-1 and VCAM-1 on lung vessels in both rat strains
| Rat strain | Treatment | P-selectin (%)† | E-selectin (%) | ICAM-1 (%) | VCAM-1 (%) |
|---|---|---|---|---|---|
| BN | OVA | 71 ± 9 | 62 ± 9 (n = 0·029)* | 98 ± 1 | 63 ± 9 (n = 0·01) |
| BN | PBS | 58 ± 7 | 35 ± 9 | 97 ± 1 | 26 ± 16 |
| SD | OVA | 58 ± 12 (n = 0·032) | 60 ± 8 (n = 0·044) | 97 ± 1 | 52 ± 13 |
| SD | PBS | 35 ± 9 | 49 ± 3 | 98 ± 1 | 41 ± 5 |
All data are expressed as percentage of positive vessels
Significant values when compared to PBS treated animals.
DISCUSSION
In this study we found that the Th2-predisposed BN rat had a more pronounced allergic airway response after OVA sensitization and challenge than the SD rat.
The first parameter of allergic airway inflammation that we studied was increase of the number of eosinophils in lung tissue. We found that the increase of lung eosinophils after allergen challenge was higher in the BN rat than in the SD rat. One explanation for that could be higher production of IL-5 in the BN rat. The Th2 phenotype of the BN rat would suggest more IL-5 production in response to OVA than in the SD rat. This would be in line with experiments done by Renzi et al.[14] which showed higher expression of IL-5 mRNA in the lung of BN rats after OVA challenge in comparison with SD rats. At present, as IL-5 in the rat cannot be measured at the protein level, we did not measure this cytokine. Another explanation for the high increase of eosinophils in the lung of the BN rat could be the higher expression of VCAM-1 on airway endothelium in the BN rat when compared to the SD rat. Peripheral blood eosinophils can constitutively bind VCAM-1 by very late antigen-4 (VLA-4) and enter the lung [15].
In clinical asthma, variable and often weak expression of VCAM-1 on airway endothelium has been found, with no increase over that of control subjects [16]. However, a consistent increase of VCAM-1 has been observed after antigen challenge with a correlation with eosinophil influx [17]. IL-4 is known to up regulate expression of VCAM-1 [18]. The observed increase of VCAM-1 expression after OVA immunization and challenge in BN rats could therefore be explained by the supposed Th2-like phenotype of this rat strain.
The second parameter of allergic airway inflammation that we studied was the IL-4/IFNγ profile as determined as numbers of cytokine producing cells using the ELISPOT assay. We found an increased Th2 response after OVA sensitization and challenge in the lungs of the BN rat. This Th2 response was less pronounced in the SD rat as the OVA protocol did not down regulate numbers of IFNγ producing cells in this rat strain. In contrast, Renzi et al.[14] found in the SD rat increased numbers of cells expressing IL-2- and IFNγ-mRNA, 8 h after OVA challenge. We did not find such a Th1-like response after OVA in this rat strain at the protein level.
In serum, both the total- and OVA-specific IgE levels were studied at several time points after sensitization. As expected, we found higher amounts of (OVA-specific) IgE produced in the BN rat than in the SD rat after OVA sensitization. The obvious explanation for this would be that BN rats produce higher levels of IL-4 by peripheral circulating cells when compared to the SD rat. However, when testing spleen cells of both rat strains on IL-4 producing cells by Elispot assay, we did not find higher numbers of IL-4 producing cells in the BN rat (data not shown). Another of many possible explanations could be the high CD4/CD8 T-cell ratio in the BN rat (Table 2). CD8+ T cells have been shown to down regulate the IgE response to OVA [19]. Their down regulatory effect is mediated, at least in part, by the production of IFNγ[20].
We found accumulation of neutrophils in the lung in both rat strains after allergen challenge. This increase of neutrophils was related to increased expression of E-selectin on airway endothelium in both rat strains. It has been described that neutrophils bind with greater avidity to E-selectin than eosinophils [17,21,22]. In situations where large amounts of inflammatory cytokines are generated, such as may occur after allergen challenge, during exacerbations or generally in more severe disease, neutrophils would be expected to be recruited by E-selectin and VCAM-1 would be the dominant receptor mediating eosinophil capture [16]. Neutrophils are not consistently found in lung biopsies of patients with asthma, except in cases of sudden-onset fatal asthma [23]. However, it has been described that in marked contrast to eosinophils, neutrophils do not persist in the lung, likely because of their relatively short survival and rapid clearance [13]. This might explain why neutrophils are rarely abundant in biopsies.
In our study, the number of lymphocytes did not increase after allergen challenge in both rat strains. This is in line with the study from Schneider et al.[13] in which numbers lymphocytes did not increase within 24 h in bronchoalveolar lavage fluid (BALF) of BN rats after treatment with OVA. In human studies [24,25], and studies in mouse models [26] allergic airway disease has been associated with increased infiltration of lymphocytes (i.e. CD4+ T cells). The discrepancy between these studies could be due to the different compartments of the lung in which lymphocytes are measured (i.e. BALF and biopsies in mouse and human studies, and lung cell isolates in our study) or the presence of bronchus associated lymphoid tissue (BALT) in the lungs of our rats. Lymphocytes from BALT contributed to a large extent to the number of lymphocytes isolated from the lung. In mouse and man, BALT is rarely found [27].
In conclusion, we have found that after sensitization and antigen challenge, the allergic airway inflammation was more pronounced in the BN rat than in the SD rat. However, although the SD rat is not Th2 predisposed, it still was able to respond in a Th2-like (allergic) way to the OVA antigen. This suggests that factors other than genetics contribute to allergic disease.
Acknowledgments
The authors thank Pieter Klok and Anthony Groothuismink for excellent technical assistance and Dr Ewoud Dubois for critically reading and helpful suggestions in reviewing the English version of the manuscript. This work was supported by a grant of the Netherlands Asthma Foundation (97·42).
REFERENCES
- 1.Jeffery PK. Pathology of asthma. Br Med Bull. 1992;48:23–39. doi: 10.1093/oxfordjournals.bmb.a072537. [DOI] [PubMed] [Google Scholar]
- 2.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 3.Lukacs NW, Strieter RM, Kunkel SL. Leukocyte infiltration in allergic airway inflammation. Am J Respir Cell Mol Biol. 1995;13:1–6. doi: 10.1165/ajrcmb.13.1.7598934. [DOI] [PubMed] [Google Scholar]
- 4.Duff AL, Platts-Mills TA. Allergens and asthma. Pediatr Clin North Am. 1992;39:1277–91. doi: 10.1016/s0031-3955(16)38445-0. [DOI] [PubMed] [Google Scholar]
- 5.Kay AB. Biological properties of eosinophils. Clin Exp Allergy. 1991;21(Suppl. 3):23–9. doi: 10.1111/j.1365-2222.1991.tb01760.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time-trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. [DOI] [PubMed] [Google Scholar]
- 7.Sandford A, Weir T, Pare P. The genetics of asthma. Am J Respir Crit Care Med. 1996;153:1749–65. doi: 10.1164/ajrccm.153.6.8665031. [DOI] [PubMed] [Google Scholar]
- 8.Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am Rev Respir Dis. 1990;142:1351–8. doi: 10.1164/ajrccm/142.6_Pt_1.1351. [DOI] [PubMed] [Google Scholar]
- 9.Pauwels R, Bazin H, Platteau B, Van Der SM. The influence of antigen dose on IgE production in different rat strains. Immunology. 1979;36:151–7. [PMC free article] [PubMed] [Google Scholar]
- 10.Martin JG, Xu LJ, Toh MY, Olivenstein R, Powell WS. Leukotrienes in bile during the early and the late airway responses after allergen challenge of sensitized rats. Am Rev Respir Dis. 1993;147:104–10. doi: 10.1164/ajrccm/147.1.104. [DOI] [PubMed] [Google Scholar]
- 11.Baumgarth N, Kelso A. Functionally distinct T cells in three compartments of the respiratory tract after influenza virus infection. Eur J Immunol. 1996;26:2189–97. doi: 10.1002/eji.1830260934. [DOI] [PubMed] [Google Scholar]
- 12.Hylkema MN, Pater JM, Kampinga J, Nieuwenhuis P, Groen H. Single expression of CD45RC and RT6 in correlation with T-helper 1 and T-helper 2 cytokine patterns in the rat. Cell Immunol. 2000;199:89–96. doi: 10.1006/cimm.1999.1607. [DOI] [PubMed] [Google Scholar]
- 13.Schneider T, van Velzen D, Moqbel R, Issekutz AC. Kinetics and quantitation of eosinophil and neutrophil recruitment to allergic lung inflammation in a brown Norway rat model. Am J Respir Cell Mol Biol. 1997;17:702–12. doi: 10.1165/ajrcmb.17.6.2849. [DOI] [PubMed] [Google Scholar]
- 14.Renzi PM, al Assaad AS, Yang J, Yasruel Z, Hamid Q. Cytokine expression in the presence or absence of late airway responses after antigen challenge of sensitized rats. Am J Respir Cell Mol Biol. 1996;15:367–73. doi: 10.1165/ajrcmb.15.3.8810641. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Sterbinsky SA, Bickel CA, Zhou DF, Kovach NL, Bochner BS. Regulation of alpha 4 integrin-mediated adhesion of human eosinophils to fibronectin and vascular cell adhesion molecule-1. J Allergy Clin Immunol. 1997;99:648–56. doi: 10.1016/s0091-6749(97)70027-7. [DOI] [PubMed] [Google Scholar]
- 16.Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J Allergy Clin Immunol. 1999;104:917–26. doi: 10.1016/s0091-6749(99)70069-2. [DOI] [PubMed] [Google Scholar]
- 17.Bochner BS, Schleimer RP. The role of adhesion molecules in human eosinophil and basophil recruitment. J Allergy Clin Immunol. 1994;94:427–38. doi: 10.1016/0091-6749(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 18.Hickey MJ, Granger DN, Kubes P. Molecular mechanisms underlying IL-4-induced leukocyte recruitment in vivo. a critical role for the alpha 4 integrin. J Immunol. 1999;163:3441–8. [PubMed] [Google Scholar]
- 19.Kemeny DM, Diaz-Sanchez D. The role of CD8+ T cells in the regulation of IgE. Clin Exp Allergy. 1993;23:466–70. doi: 10.1111/j.1365-2222.1993.tb03232.x. [DOI] [PubMed] [Google Scholar]
- 20.Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 21.Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, l-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–39. [PubMed] [Google Scholar]
- 22.Sriramarao P, Norton CR, Borgstrom P, DiScipio RG, Wolitzky BA, Broide DH. E-selectin preferentially supports neutrophil but not eosinophil rolling under conditions of flow in vitro and in vivo. J Immunol. 1996;157:4672–80. [PubMed] [Google Scholar]
- 23.Sur S, Crotty TB, Kephart GM, et al. Sudden-onset fatal asthma. Am Rev Respir Dis. 1993;148:713–9. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 24.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Invest. 1999;104:985–93. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yurovsky VV, Weersink EJ, Meltzer SS, et al. T-Cell repertoire in the blood and lungs of atopic asthmatics before and after ragweed challenge. Am J Respir Cell Mol Biol. 1998;18:370–83. doi: 10.1165/ajrcmb.18.3.2935. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima H, Iwamoto I, Tomoe S, et al. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am Rev Respir Dis. 1992;146:374–7. doi: 10.1164/ajrccm/146.2.374. [DOI] [PubMed] [Google Scholar]
- 27.Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68:1–8. doi: 10.1159/000028109. [DOI] [PubMed] [Google Scholar]