Abstract
Antibodies to glutamic acid decarboxilase (GAD-Abs) are present in the serum of 60–80% of newly diagnosed type 1 diabetes (DM1) patients and patients with autoimmune polyendocrine syndrome (APS) associated with DM1. Higher titre of GAD-Abs are also present in the serum of 60% of patients with stiff-man syndrome (SMS) and all reported patients with cerebellar ataxia associated with polyendocrine autoimmunity (CAPA). Several studies suggest that GAD-Abs may play a critical role in the pathogenesis of SMS and CAPA but little is known about T-cell responsiveness to GAD-65 in these neurological diseases. To analyse cell-mediated responses to GAD, we studied the peripheral blood lymphocyte proliferation and cytokine responses to recombinant human GAD-65 in 5 patients with SMS, 6 with CAPA, 9 with DM1, 8 with APS and 15 control subjects. GAD-65-specific cellular proliferation was significantly higher in SMS than in CAPA, DM1, APS or controls. In contrast, only T cells from CAPA patients showed a significantly high production of interferon-γ after GAD stimulation, compared to all other patients and controls. No differences were found for IL-4 production. These results suggest that, despite similar humoral autoreactivity, cellular responses to GAD are different between SMS and CAPA, with a greater inflammatory response in CAPA, and this difference may be relevant to the pathogenesis of these diseases.
Keywords: GAD65, cellular responses, stiff-man syndrome, autoimmune cerebelar ataxia
INTRODUCTION
Glutamic acid decarboxylase (GAD) is the enzyme that catalyses the conversion of glutamic acid to γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the CNS. The enzyme is selectively expressed in GABA-ergic neurones and in extraneuronal tissues such as the pancreatic β-cells. GAD has been identified as a major autoantigen in type 1 diabetes mellitus (DM1) [1,2], an autoimmune disease resulting from the T cell-mediated destruction of pancreatic β-cells [3]. Autoantibodies to GAD65 (GAD-Abs) are detected in about 80% of newly diagnosed DM1 patients. These autoantibodies are present in the serum long before the appearance of clinical symptoms, but have low titre and no direct links to the pathogenesis of DM1 have been established. High GAD-Ab titre is detected in patients with type 1 and type 2 autoimmune polyendocrine syndrome (APS) with or without associated DM1 [4,5]. Higher levels of GAD-Abs are found in the serum and CSF of around 60% of patients with stiff-man syndrome (SMS), a rare disorder of the CNS characterized by progressive muscle rigidity with superimposed painful spasms [6,7], and in patients with late onset cerebellar ataxia associated with polyendocrine autoimmunity (CAPA) [8–12]. This disorder, previously classified as idiopathic, may represent a subgroup of ataxia of possible autoimmune origin.
It has been suggested that the high-titre GAD-Abs in SMS and CAPA patients could cause functional impairment of the GABA-mediated physiological process, thereby explaining the neurological simptoms [13,14]. The intracellular location of the antigen have questioned this relevance, although it is difficult to hypothesize a total absence of function for such high titre antibodies. Besides, a cell-mediated process could be playing an additional critical role in the pathogenesis of these diseases.
Autoimmune β cell destruction in DM1 is T cell dependent, and GAD-reactive T cells are diabetogenic in nonobese-diabetic (NOD) mice, an experimental model of DM1 [15,16]. A similar role for GAD-specific T-cell autoimmunity in human DM1 has been suggested [17,18] although the data are controversial and several reports have shown the absence of any significant peripheral T cell proliferation to GAD in DM1 [19,20]. Few data are available on cellular immunity to GAD in SMS [21–23] and none on the cellular responses to GAD in CAPA patients. Even in the absence of proliferation, cytokine responses to GAD may reflect the T-cell involvement in the autoimmune processes and a putative differential dominance of Th2 or Th1 profiles may in part account for the different clinical syndromes of the patient groups [24]. In this study, we have analysed peripheral T cell proliferation and cytokine production in response to recombinant human GAD in patients with the two neurological disorders (SMS and CAPA) associated to GAD autoimmunity, and compared their responses with that of patients with DM1 and APS.
PATIENTS AND METHODS
Patient selection
Blood samples were collected from 9 newly diagnosed DM1 patients preselected for ICA positivity; 8 patients with APS, all of them with DM1 and a high GAD-Ab titre; 5 patients with SMS; 6 patients with CAPA; 9 healthy control subjects of average age similar to the DM1 and APS patients (Control A) and 6 control subjects of average age matching the SMS and CAPA patients (Control B) (Table 1). Three of the 5 SMS patients and 5 of the 6 CAPA patients had late-onset DM1. At the time of sample collection, none of the patients were under immunosuppresive treatment. All samples were obtained after the donors had given their informed consent.
Table 1.
Description and HLA typing of patients
| ¶Autoantibody titre | HLA typing | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient code | Diagnosis† | Other AI pathologies‡ | Sex | §Age at sampling | §Age at diagnosis | GAD Ab | IA-2 Ab | DRB1* | DQB1* |
| DM1 | DM 1 | – | F | 19 | 19 | 515 | 37 | 04, – | 0302, – |
| DM2 | DM 1 | – | M | 15 | 15 | 9 | <0·20 | 03, 03 | 0201, – |
| DM3 | DM 1 | – | F | 23 | 23 | 58 | 0·20 | 03, 04 | 0201, 0302 |
| DM4 | DM 1 | – | F | 33 | 33 | 160 | 14 | 04, 13 | 0603, 0302 |
| DM5 | DM 1 | – | F | 25 | 25 | 600 | 5·20 | 03, 13 | 0201, 0609 |
| DM6 | DM 1 | – | F | 24 | 24 | 200 | 33·80 | 03, 07 | 0201, 0202 |
| DM7 | DM 1 | – | M | 30 | 30 | 27 | 0·86 | 03, 04 | 0201, 0302 |
| DM8 | DM 1 | – | F | 18 | 18 | 18 | 0·30 | 03, 03 | 0201, 0201 |
| DM9 | DM 1 | – | M | 16 | 16 | 54 | 4·80 | 04, 03 | 0302, 0201 |
| DM12 | APS | GD, VI | F | 61 | 60 | 1100 | 3·90 | 16, 03 | 0502, 0201 |
| DM13 | APS | VI, HT | F | 61 | 59 | 30000 | 0·20 | 04, 07 | 0202, 0302 |
| DM14 | APS | TPO, GPC | F | 42 | 38 | 1850 | <0·20 | 15, 04 | 0302, 0602 |
| DM16 | APS | TPO, GPC | F | 37 | 34 | 4000 | 1·20 | 03, 07 | 0201, 0202 |
| DM17 | APS | GD | F | 27 | 26 | 1100 | 0·15 | 01, 03 | 0501, 0201 |
| DM18 | APS | Vl, HT | F | 34 | 18 | 5200 | 0·3 | 01, 04 | 0501, 0301 |
| DM20 | APS | TPO | F | 24 | 18 | 1300 | 0·25 | 03, 04 | 0302, 0201 |
| DM21 | APS | VI, TPO, GPC | F | 22 | 8 | 5200 | 0·35 | 03, 04 | 0201, 0302 |
| SMS1¶¶ | SMS | DM 1 | F | 56 | 46 | 42000 | 0, 01 | 03, 08 | 0402, 0201 |
| SMS2 | SMS | VI | F | 77 | 72 | 67000 | 0, 15 | 13, 16 | 0603, 0502 |
| SMS3¶¶ | SMS | GD | F | 66 | 60 | 31333 | 0, 02 | 04, 03 | 0302, 0201 |
| SMS4 | SMS | DM 1 | F | 62 | 61 | 8700 | 0, 25 | 03, 07 | 0201, 0202 |
| SMS5 | SMS | DMI, HT | F | 38 | 37 | 4900 | 0, 03 | 03, 07 | nd |
| ACA1 | CAPA | DM 1 | F | 53 | 53 | 2300 | 2·90 | 03, 04 | 0302, 0201 |
| ACA2§§ | CAPA | DM 1 | F | 59 | 51 | 14000 | 0·10 | 01, 12 | 0301, 0501 |
| ACA3§§ | CAPA | DM 1, PA | F | 79 | 74 | 70000 | 0·15 | 01, 13 | 0501, 0604 |
| ACA4 | CAPA | HT | F | 40 | 39 | 22400 | 9·90 | 04, 04 | 0301, 0302 |
| ACA5§§ | CAPA | DM 1 | F | 63 | 61 | 39500 | 0·20 | 03, 07 | 0202, 0201 |
| ACA6 | CAPA | DM 1, GD, MG | F | 48 | 46 | 100000 | 0·25 | nd | 0603, 0503 |
| C1 | Control A | – | M | 17 | – | <0·20 | 0·10 | 15, 07 | nd |
| C2 | Control A | – | M | 14 | – | 0·20 | 0·15 | 03, – | nd |
| C3 | Control A | – | M | 19 | – | 0·30 | 0·20 | 07, 11 | 0202, 0301 |
| C4 | Control A | – | F | 21 | – | 0·40 | 0·25 | 08, 13 | 0202, – |
| C5 | Control A | – | F | 28 | – | <0·20 | 0·30 | 14, 17 | nd |
| C6 | Control A | – | F | 23 | – | 0·25 | 0·35 | 04, 15 | 0302, 0602 |
| C7 | Control A | – | M | 16 | – | 0·35 | 0·32 | 01, 13 | 0301, 0501 |
| C8 | Control A | – | F | 26 | – | 0·45 | 0·12 | 04, 07 | nd |
| C9 | Control A | – | F | 16 | – | <0·20 | 0·28 | 16, 12 | nd |
| C10 | Control B | – | F | 57 | – | <0·20 | nd | nd | nd |
| C11 | Control B | – | F | 59 | – | <0·20 | nd | nd | nd |
| C12 | Control B | – | F | 64 | – | <0·20 | nd | 07, 13 | 0202, 0603 |
| C13 | Control B | – | F | 53 | – | <0·20 | nd | nd | nd |
| C14 | Control B | – | F | 53 | – | <0·20 | nd | 03, 11 | 0201, 0301 |
| C15 | Control B | – | F | 57 | – | <0·20 | nd | 11, 13 | 0301, 0602 |
DM 1, type 1 diabetes mellitus; APS, type 2 autoimmune polyendocrine syndrome; SMS, stiff-man syndrome; CAPA, cerebellar ataxia associated with polyendocrine autoimmunity; Control A, control donors with 20 years average age; Control B, control donors with 57 years average age.
AI, autoimmune; HT, Hashimoto's thyroiditis; GD, Graves’ disease; VI, vitiligo; MG, myastenia gravis; PA, pernicious anaemia; TPO, antibodies to thyroid peroxidase; GPC, antibodies to gastric parietal cells.
Age in years.
U/ml. nd, not done.
described in reference [37].
described in reference [11].
Recombinant glutamic acid decarboxylase production
The 65kD isoform of human GAD was expressed in Sf9 cells using a baculovirus-based vector. Infected cells from 750 ml culture were homogenized in Triton-X114, and the detergent phase was separated and submitted to anion-exchange chromatography on DEAE-sepharose as described elsewhere [25]. This GAD65 preparation was used in immunoblotting, proliferation and cytokine assays. Negative control preparations were derived from SF9 cells infected with wild-type baculovirus and prepared exactly as for GAD65 (SF9). Cytosol extracts of human pancreas exocrine tissue homogenized in sucrose buffer (0·32 m, pH 7·4) and ultracentrifuged at 24 000g (IP-3) were also used as negative controls (not shown).
Autoantibodies
The presence of GAD-Abs was assessed by radioimmunoassay (RIA), as previously described [11] and islet cell antibodies (ICA), by standard immunofluosrescence methods [26]. Antibodies to IA-2 (IA-2 Abs) were measured by radioimmunoassay using 125I-labelled human recombinant tyrosine phosphatase [27] and a commercial kit (CIS Biointernational, Gif-sur-Yvette, France).
Peripheral blood mononuclear cells (PBMC) proliferation assays
Mononuclear cells were isolated from heparinized peripheral blood by density gradient centrifugation (Lymphoprep, Nycomed, Oslo, Norway) and cryopreserved until needed. Proliferation assays were performed in triplicate in 96-well round-bottom plates (Costar, Cambridge, MA, USA) previously seeded with 50 μl/well antigen preparation in IMDM medium (Gibco-Life Technologies, UK) at 3× concentration and stored at −20°C. The plates were thawed at 37°C for 1 min just before the assay. For each sample, 1 × 105 cells in 100 μl culture medium supplemented with 15% autologous serum were added per well. Antigens used were: purified GAD 65 protein (10 μg/ml); Sf9 preparation (10 μg/ml) and medium as negative controls, as well as IP-3 preparations (not shown); and IL-2 (20 U/ml) and PHA (3 μg/ml, not shown) as positive controls. The proliferative response was measured by 3H] thymidine (1 μCi/well) uptake, added 16 h before harvesting on day 5 of culture. Responses are reported as mean cpm ± SD values of triplicate cultures. Stimulation index (mean cpm with antigen/mean cpm in the absence of antigen) was also calculated.
HLA typing
HLA typing was performed using standard PCR-SSO [28] techniques with DNA isolated from blood samples.
Monoclonal antibodies
The monoclonal antibodies (moAbs) used for membrane staining were PerCP-conjugated Leu-4 (anti-CD3), FITC-anti-HLA-DR, PE-anti-HLA-DR and IgG1/IgG2 isotype controls, all from Becton Dickinson (San Jose, CA, USA). For cytoplasmic detection of cytokines, PE-anti-IL-4 (clone 8D4-8), FITC-anti-IFN-γ (clone B27) and FITC- anti-IL-2 (clone MQ1–17H12) auto- antibodies (PharMingen, San Diego, CA, USA), were used. All moAbs were used at the manufacturers recommended concentrations. Anti-GAD 65 moAb GAD6 (Developmental Studies Hybridoma Bank, University of Iowa, USA) was used as positive control for GAD detection assays.
Detection of cytoplasmic cytokines by flow cytometry
PBMC (1 × 106) were cultured for 3 days in 24-well polystyrene tissue culture plates (Costar) with or without 10 μg/ml of purified GAD protein in 2 ml IMDM medium supplemented with 10% pooled A+ serum. Intracellular cytokines were detected after blocking secretion using Brefeldin A (Epicentre Technologies, Madison, WI, USA) a relatively non toxic but potent inhibitor of intracellular transport. BFA was added to the PBMC for the final 4 h of culture at a concentration of 10 μg/ml. The cells were then incubated with a fluorocrome conjugated moAb specific for CD3 and HLA-DR in staining buffer (1% FCS, 0·1% w/v NaN3 in PBS) for 30 min at 4°C, fixed with 4% paraformaldehyde in PBS for 20 min at 4°C, washed and incubated with PE-IL-4, FITC-IL-2 or FITC-IFN-γ moAb in permeabilization solution (1%FCS, 0·1% w/v NaN3 0·5% saponin in PBS) for 1 h at 4°C and analysed by flow cytometry in an FACSCalibur flow cytometer (Becton Dickinson). The results were analysed with the CellQuest® program and the negative control standard for each cytokine was based on the CD3 negative cell population from each cell preparation.
Detection of cytokines by ELISA
PBLs were cultured as describe above; supernatants were harvested after 72h of culture without the addition of BFA, and tested with a commercial enzyme-linked immunosorbent assay for IFN-γ (Biotrak, Amersham International, Little Chalfont, UK) and IL-4 (Bender Medsystems,Vienna, Austria).
Statistical analysis
Comparison of the different parameters between the groups was done by one-way anova test. When significant differences were detected by anova, the Newman-Kewls test was applied. The parameters analysed were GAD-Ab titre, stimulation index (SI) and cpm for proliferation, and total number of CD3+ cells positive for each cytokine (cytokine positive cells).
RESULTS
Humoral autoimmunity
The titre of GAD-Abs in the sera from SMS patients (mean, 30 786 U/ml ± 25 472) and from CAPA patients (mean, 41 700 U/ml ± 36 851) was significantly higher than in DM1 (mean, 186 U/ml ± 221) or APS patients (mean, 6234 U/ml ± 9766) (P < 0·005) (Table 1). All the sera from the SMS, CAPA, DM1 and APS patients were ICA positive and control sera were all ICA negative (data not shown). IA-2 Abs were present in 5 of the 9 DM1 patients (mean, 19 U/ml ± 15·49; range, 4·8–37 U/ml); 2/8 APS patients (mean, 2·55 U/ml ± 1·90; range, 1·2–3·9 U/ml); 2/6 patients with CAPA (mean, 6·4 ± 4·94 U/ml; range, 2·9– 9·9 U/ml) and in none of the 5 SMS patients. Correlation analysis revealed inverse tendencies of GAD and IA-2 antibody titres in these groups of patients.
HLA-typing
All DM1 and APS patients carried the HLA-DRB1*0301, DQB1*0201 (DR3, DQw2) and/or HLA-DRB1*04, DQB1*0302 (DR4, DQ8) susceptibility haplotypes (Table 1) [29]. Four of the 5 SMS patients were DR3, DQw2 and these included all patients with late-onset DM1 in addition to SMS. In contrast, only 2/6 CAPA patients were DR3, DQw2 and only one was DR4, DQ8. No association was found between the presence of late-onset DM1 in these patients and any HLA allele.
Anti-GAD cellular immunity
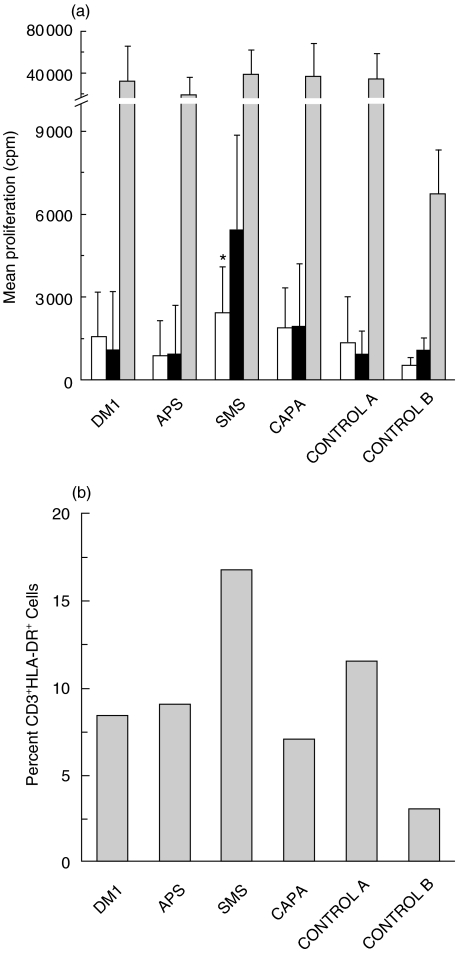
The proliferative response to GAD from 9 newly diagnosed DM1 patients, 8 APS patients, 4 SMS patients, 5 patients with CAPA and 13 healthy controls was tested after incubating cultures of patients’ PBMCs with purified GAD protein for 5 days. The results are shown in Fig. 1a. None of the cell samples proliferated in the absence of antigen or with any of the negative controls. Only 1 of the 9 DM1 patients showed proliferative response to GAD protein (6708cpm, SI = 9·4), with a very low average response for the whole group (1095cpm, SI = 2·2), similar to that observed in control A (914cpm, SI = 2·1) and control B groups (1063cpm, SI = 2·6). The response of APS patients to GAD (943cpm, SI = 2·04) and of the 5 CAPA patients (1912cpm, SI = 1·75) were also low. In contrast, 3 of the 4 tested SMS patients proliferated in the presence of GAD protein, showing a significantly higher response (mean 5437cpm, SI = 10·3) than the control subjects (P < 0·005). The results are representative of at least two experiments. The presence of antibodies did not affect the proliferative responses, since the same level of proliferation was observed with autologous and with human pooled A+ sera (data not shown). The percentage of activated T cells after 3-day culture with GAD, analysed by the coexpression of CD3 and HLA-DR was higher in SMS patients than in the other groups (Fig. 1b) but these differences were only significant if compared to the age matched control B group (P < 0·01).
Fig. 1.
Patients’ PBMC proliferative response to GAD protein. (a) ▪ proliferation to GAD, shown for each patient and control groups;  ;positive control proliferation to IL-2; □ responses to negative control antigen preparation (Sf9). Data are expressed as mean cpm from triplicate cultures. *P < 0.005. (b) The values represent the average number of activated cells in each patient group, as measured by flow cytometric analysis of CD3 and DR coexpression after 3 day-culture of patients’ PBL with GAD (see Fig. 3). Number of patients tested in each group: DM1 (n = 9), APS (n = 8), CAPA (n = 5), SMS (n = 4), Control A (n = 7) and Control B (n = 6).
;positive control proliferation to IL-2; □ responses to negative control antigen preparation (Sf9). Data are expressed as mean cpm from triplicate cultures. *P < 0.005. (b) The values represent the average number of activated cells in each patient group, as measured by flow cytometric analysis of CD3 and DR coexpression after 3 day-culture of patients’ PBL with GAD (see Fig. 3). Number of patients tested in each group: DM1 (n = 9), APS (n = 8), CAPA (n = 5), SMS (n = 4), Control A (n = 7) and Control B (n = 6).
Cytokine production by T cells after three-day culture with GAD protein
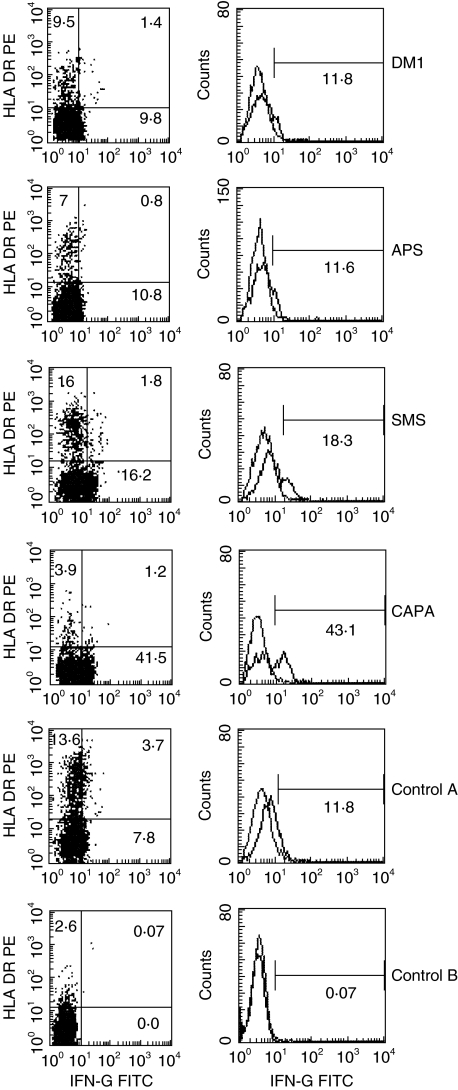
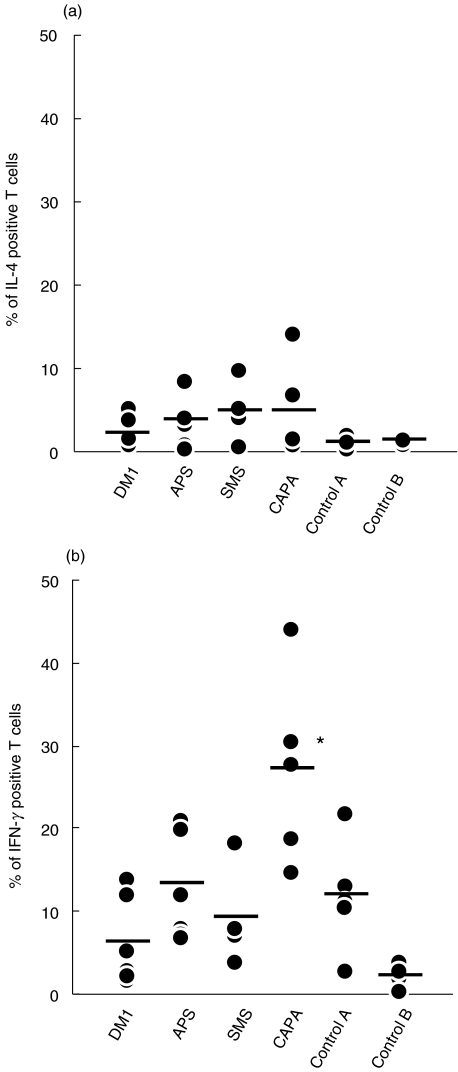
In order to establish a pattern of response to GAD in the different groups of patients, we analysed the synthesis of cytoplasmic IL-4 and IFN-γ by PBLs in response to GAD. Time course experiments were done to establish the optimal time (3 days) of IFN-γ and IL-4 production by activated T cells obtained from the patients (data not shown). After 3 days of in vitro culture in the presence or absence of GAD 65 protein, the number of CD3+ IL-4+ or IFN-γ+ cells, representative of dominant Th2 or Th1 patterns, were analysed. The results are shown in Table 2 and Figs 2 and 3. Only CD3 positive cells were analysed, using standard negative controls (isotype matched labelled immunoglobulins). An example of the analysis of IFN-γ expression in one patient of each group is shown in Fig. 2. No significant differences were found between the patients and controls in the number of IL-4+ CD3+ cells detected. However, a clear difference was observed for IFN-γ production. T cells from CAPA patients produced significantly more IFN-γ (mean percentage of IFN-γ+ cells in CAPA patients = 27·06) than control A (12·03%, P < 0·005) and age-matched control B subjects (2·05%, P < 0·001), DM1 (6·21%, P < 0·005), APS (13·42%, P < 0·005) or SMS patients (9·21%, P < 0·001). The number of IFN-γ positive cells was higher than the average control, in all CAPA patients (Fig. 3). Detectable IFN-γ expression was confirmed by ELISA (two experiments done, not shown). As expected, the number of IL-2+ activated T cells was higher in SMS patients than in the other groups but the differences were not significant (not shown).
Table 2.
Cytokine produccion by T cells of the patients
| Percentage of positive T cells | ||
|---|---|---|
| Patients† | IL-4 | IFN-γ |
| DM 1 | 2·12 | 6·21 |
| (0·71–5·19) | (1·75–13·8) | |
| APS | 3·77 | 13·42 |
| (0·8–8·5) | (7·1–20·81) | |
| SMS | 4·88 | 9·22 |
| (0·67–9·8) | (3·7–18·3) | |
| CAPA | 4·73 | 27·06* |
| (0·84–14) | (14·73–43·12) | |
| Control A | 1·03 | 12·04 |
| (0·22–1·8) | (2·83–21·65) | |
| Control B | 1·3 | 2·05 |
| (0·75–1·71) | (0·31–3·94) | |
The results are expressed as average and (range) number of CD3+ cytokine+ cells after 3 day stimulation with GAD.
P < 0·005.
DM 1, type 1 diabetes mellitus; APS, autoimmune type 2 polyendocrine syndrome; SMS, stiff-man syndrome; CAPA, cerebellar ataxia associated with polyendocrine autoimmunity; Control A, control donors with 20 years average age; Control B, control donors with 57 years average age.
Fig. 2.
An example of the flow cytometry analysis of cytoplasmic IFN-γ expression. Data from one patient of each group are shown. Patients’ PBMC were cultured with purified GAD antigen for 3 days, stained with the corresponding fluorescence-labelled antibodies, and the data analysed by flow cytometry as follows: CD3 + cells were selected (gated) for analysis of the expression of HLA-DR, as a marker of T cell activation, and of cytoplasmic IFN-γ (dot blot figures on left). Quadrants were set based on the histogram analysis by comparing with the control (right).
Fig. 3.
Cytoplasmic expression of cytokines by patients’ T cells after 3-day stimulation with GAD. Total PBMC were gated by FSC and CD3 expression to identify T cells, and samples were analysed as in Fig. 2 for (a) IL-4 or (b) IFN-γ positivity. The dots represent percentages of cytokine positive T cells for each patient in the different groups. Number of patients tested in each group: DM1 (n = 7), APS (n = 6), CAPA (n = 5), SMS (n = 4), Control A (n = 5) and Control B (n = 5). *P < 0.005.
DISCUSSION
The main finding of the present study is that GAD-specific cell-mediated immunity displayed by patients with SMS is different from that of patients with CAPA despite a very high titre of GAD-Abs in both syndromes and other similarities such as high incidence of DM1 and clinical and serological evidences of other organ-specific autoimmune manifestations. Although derived from the study of a small number of patients, these observations give additional support to the concept of differential immunopathogenesis in these two neurological disorders.
IA-2-Abs, which are a useful serological marker for DM1 [30], were absent in the SMS patients, in agreement with previous reports [31], but present in 2/6 CAPA patients. The distributions of IA-2 and GAD-Ab titres showed inverse tendencies, suggesting that IA-2 reactivity might be more specifically associated with DM1 than GAD-specific responses.
The number of patients studied was too small to draw any conclusions about HLA allele associations. However, the data suggested further differences between SMS and CAPA. As expected, all DM1 and APS patients were positive for the haplotypes associated to susceptibility to DM1, DRB1*0301 DQB1*0201 (DR3 DQw2) or DRB1*04 DQB1*0302 (DR4 DQw3) [29]. Similarly, all but one of the SMS patients carried the DRB1*0301 DQB1*0201 haplotype. The only SMS patient without DR3 or DR4 was non diabetic and expressed DQB1*0603, an allele negatively associated with DM1 (relative risk 0·18) [29]. A strong association of DQB1*0201, but not DRB1*0301, with SMS had been previously reported in a relatively large series of patients (n = 18) [32]. These results contrasted with those obtained with CAPA patients, where only 2/6 were DRB1*0301 DQB1*0201 and only one was DR4, regardless of the presence of DM1. Although not statistical analysis would be reliable with this small sample size, these data could suggest differences in the genetic background for these two neurological diseases.
The present study confirms previous data by our own group and others showing that proliferation to GAD65 in PBMC from newly diagnosed DM1 and APS patients is not different from controls and does not induce a clear pattern of cytokine expression [19]. The number of T cells infiltrating human diabetic pancreas is not very large [33,34] and this would explain the absence of a detectable pool of GAD-responding cells in peripheral blood. However, PBL responses to some GAD peptides have been clearly demonstrated in DM1 patients [23] and, in accordance with other authors, we have been able to isolate GAD-specific cell lines from DM1 PBL after several rounds of in vitro stimulation see [35,36] and M. Costa et al. unpublished data]. In contrast, we found a high and specific peripheral proliferative response to GAD65 in SMS patients but the production of cytokines by the responding cells did not allow the definition of the response as Th1 or Th2. A group of SMS patients reported by Lohman et al.[23] also responded positively to GAD; in addition, the humoral response to GAD in SMS but not in DM1 included IgG4 and IgE isotypes, suggesting a strong Th2 response. Other data [22] showed positive T cell proliferation to GAD65 in two SMS patients with a mixed Th0-like response of GAD-specific T cell clones after PMA stimulation (low IFN-γ, low IL-4, IL-10). A positive response to GAD has also been reported in another SMS patient, with clear and specific IFN-γ production [21]. In our experiments, we detected both IFN-γ and IL-4, but the levels were not significantly higher than in controls or in any of the other groups of patients. Such mixed profiles would be expected in periphery if both pathologic and regulatory T cell clones are involved in the response.
A different pattern of cellular immune responses was obtained in CAPA patients. The proliferative response to GAD65 was low, but T cells from these patients produced significantly more IFN-γ after GAD65 stimulation than the T cells from any of the other groups. The increased IFN-γ/IL-4 ratio in CAPA patients suggest a predominant Th1 response to GAD. However, the high level of IFN-γ production in the absence of cellular proliferation in these patients is intriguing. Although not all samples were tested in time course experiments, no responses were observed in two patients tested on days 2 and 3 after stimulation (data not shown), so no hidden early response was apparent. We could be dealing with an already expanded nonproliferative effector population, indicating that the peripheral T cell response to GAD in CAPA patients may be mediated by cells producing an inflammatory cytokine capable of promoting the differentiation into active cytotoxic cells or activating inflammatory reactions in situ. The relative relevance of cellular versus humoral anti-GAD responses in the pathogenesis of SMS and CAPA is so far unclear. The precise effects of GAD-Abs in these diseases are largely unknown. There is in vitro evidence that GAD-Abs in SMS can reduce GAD enzyme activity and GABA synthesis [14]. The results of the present study showing a CD4+ T cell mediated response, although with no clear bias towards a Th2 subset, do not rule out this hypothesis.
Our results support that CAPA may involve an inflammatory Th1 cell response to GAD. Although GAD-Abs from one patient with CAPA induced a selective suppression of GABA-ergic transmission using isolated rat cerebellar slices [13], this effect of antibodies could be additional to in situ inflammation effects. Cerebellar atrophy has been detected by MR in some of these patients [8,11–13], suggesting a destructive process, but the lack of autopsies from any CAPA patient prevents any conclusive hypothesis as to what are the pathogenic mechanisms involved.
In conclusion, this preliminary study suggests that cell- mediated immune mechanisms may be different between SMS and CAPA both sharing a similar humoral response against GAD. This could be related to the different clinical and pathological manifestations of these patients.
Acknowledgments
The authors wish to thank Dr T. Dyrberg from Novo Nordisk, Denmark, for the recombinant GAD and wild type baculovirus constructs; Dr L. Serradell for Sf9 cells and help in the production of recombinant GAD; Drs C. Roura-Mir, M. Martí and R. Pujol-Borrell for support. We also thank Dr G. Ercilla and E. Palou for HLA typing, M. Bonastre for technical assistance and Drs M. Arias, J. Arpa, L. Brieva, C. de Andrés, J. Maestre, and J. J. Zarranz for referring patients. This work was supported by a grant from the Fundació La Marató de TV3 (1007/97) and in part by grant SGR 9500027 of the Generalitat de Catalunya. M. C. is a fellow of the Fundació La Marató de TV3.
REFERENCES
- 1.Baekkeskov S, Aanstoot HJ, Christgau S, et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990;347:151–6. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Zimmet P, Mackay IR, et al. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet. 1994;343:1383–5. doi: 10.1016/s0140-6736(94)92521-6. [DOI] [PubMed] [Google Scholar]
- 3.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–7. doi: 10.1016/s0092-8674(00)81106-x. [DOI] [PubMed] [Google Scholar]
- 4.Seissler J, Bieg S, Yassin N, et al. Association between antibodies to the MR 67,000 isoform of glutamate decarboxylase (GAD) and type 1 (insulin-dependent) diabetes mellitus with coexisting autoimmune polyendocrine syndrome type 2. Autoimmunity. 1994;19:231–8. doi: 10.3109/08916939409071348. [DOI] [PubMed] [Google Scholar]
- 5.Velloso LA, Winqvist O, Gustafson J. Autoantibodies against a novel 51 kDa islet antigen and glutamate decarboxylase isoforms in autoimmune polyendocrine syndrome type 1. Diabetologia. 1994;37:61–9. doi: 10.1007/BF00428779. [DOI] [PubMed] [Google Scholar]
- 6.Solimena M, Folli F, Denis-Donini S, et al. Autoantibodies to glutamic acid decarboxylase in a patient with stiff-man syndrome, epilepsy, and type I diabetes mellitus. N Engl J Med. 1988;318:1012–20. doi: 10.1056/NEJM198804213181602. [DOI] [PubMed] [Google Scholar]
- 7.Solimena M, Folli F, Aparisi R, Pozza G, De Camilli P. Autoantibodies to GABA-ergic neurons and pancreatic beta cells in stiff-man syndrome. N Engl J Med. 1990;322:1555–60. doi: 10.1056/NEJM199005313222202. [DOI] [PubMed] [Google Scholar]
- 8.Honnorat J, Saiz A, Giometto B, et al. Cerebellar ataxia with anti- glutamic acid decarboxylase antibodies. Arch Neurol. 2001;58:225–30. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- 9.Honnorat J, Trouillas P, Thivolet C, Aguera M, Belin MF. Autoantibodies to glutamate decarboxylase in a patient with cerebellar cortical atrophy, peripheral neuropathy, and slow eye movements. Arch Neurol. 1995;52:462–8. doi: 10.1001/archneur.1995.00540290050017. [DOI] [PubMed] [Google Scholar]
- 10.Giometto B, Miotto D, Faresin F, Argentiero V, Scaravilli T, Tavolato B. Anti-gabaergic neuron autoantibodies in a patient with stiff-man syndrome and ataxia. J Neurol Sci. 1996;143:57–9. doi: 10.1016/s0022-510x(96)00065-2. [DOI] [PubMed] [Google Scholar]
- 11.Saiz A, Arpa J, Sagasta A, et al. Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late onset insulin-dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology. 1997;49:1026–30. doi: 10.1212/wnl.49.4.1026. [DOI] [PubMed] [Google Scholar]
- 12.Abele M, Weller M, Mescheriakov S, Bürk K, Dichgans J, Klockgether T. Cerebellar ataxia with glutamic acid decarboxylase autoantibodies. Neurology. 1999;52:857–9. doi: 10.1212/wnl.52.4.857. [DOI] [PubMed] [Google Scholar]
- 13.Ishida K, Mitoma H, Song SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol. 1999;46:263–7. [PubMed] [Google Scholar]
- 14.Dinkel K, Meinck HM, Jury KM, Karges W, Ritcher W. Inhibition of γ-aminobutiric acid synthesis by glutamic acid decarboxylase autoantibodies in stiff-man syndrome. Ann Neurol. 1998;44:194–201. doi: 10.1002/ana.410440209. [DOI] [PubMed] [Google Scholar]
- 15.Tisch R, Yang XD, Singer SM, Liblau RS, Fugger L, McDevitt HO. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993;366:72–5. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- 16.Yoon JW, Yoon CS, Lim HW, et al. Control of autoimmune diabetes in NOD mice by GAD expression or suppression in β cells. Science. 1999;284:1183–7. doi: 10.1126/science.284.5417.1183. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson MA, Kaufman DL, Campbell L, et al. Response of peripheral-blood mononuclear cells to glutamate decarboxylase in insulin-dependent diabetes. Lancet. 1992;339:458–9. doi: 10.1016/0140-6736(92)91061-c. [DOI] [PubMed] [Google Scholar]
- 18.Honeyman MC, Cram DS, Harrison LC. Glutamic acid decarboxylase 67-reactive T cells. a marker of insulin-dependent diabetes. J Exp Med. 1992;177:535–40. doi: 10.1084/jem.177.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roep BO, Atkinson MA, van Endert PM, et al. Autoreactive T cell responses in insulin-dependent (Type 1) diabetes mellitus. Report of the first international workshop for standardization of T cell assays. J Autoimmun. 1999;13:267–82. doi: 10.1006/jaut.1999.0312. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki I, Cheung RK, Gaedigk R, et al. T cell activation and anergy to islet cell antigen in type I diabetes. J Immunol. 1995;154:1461–9. [PubMed] [Google Scholar]
- 21.Hummel M, Durinovic-Bello I, Bonifacio E, et al. Humoral and cellular immune parameters before and during immunosuppressive therapy of a patient with stiff-man syndrome and insulin dependent diabetes mellitus. J Neurol Neurosurg Psychiatry. 1998;65:204–8. doi: 10.1136/jnnp.65.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schloot NC, Batstra MC, Duinkerken G, et al. GAD-65 reactive T cells in a non-diabetic stiff-man syndrome patient. J Autoimmun. 1999;12:289–96. doi: 10.1006/jaut.1999.0280. [DOI] [PubMed] [Google Scholar]
- 23.Lohman T, Hawa M, Leslie RDG, Lane R, Picard J, Londei M. Immune reactivity to glutamic acid decarboxilase 65 in stiff-man syndrome and type 1 diabetes mellitus. Lancet. 2000;356:31–5. doi: 10.1016/S0140-6736(00)02431-4. [DOI] [PubMed] [Google Scholar]
- 24.Ellis TM, Atkinson MA. The clinical significance of an autoimmune response against glutamic acid decarboxylase. Nat Med. 1996;2:148–53. doi: 10.1038/nm0296-148. [DOI] [PubMed] [Google Scholar]
- 25.Moody AJ, Hejnæs KR, Marshall MO, et al. Isolation by anion-exchange of immunologically and enzymatically active human islet glutamic acid decarboxylase 65 overexpressed in SF9 insect cells. Diabetologia. 1995;38:14–23. doi: 10.1007/BF02369348. [DOI] [PubMed] [Google Scholar]
- 26.Vives M, Somoza N, Soldevila G, et al. Reevaluation of autoantibodies to islet cell membrane in IDDM. Failure to detect islet cell surface antibodies using human islet cells as a substrate. Diabetes. 1992;41:1624–31. doi: 10.2337/diab.41.12.1624. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki E, Yu L, Gianani R, et al. Evaluation of islet cell antigen (ICA) 512/IA-2 autoantibody radioassays using overlapping ICA512/IA-2 constructs. J Clin Endocrinol Metab. 1997;82:375–80. doi: 10.1210/jcem.82.2.3723. [DOI] [PubMed] [Google Scholar]
- 28.Soriano JB, Ercilla MG, Sunyer J, et al. HLA class II genes in soybean epidemic asthma patients. Am J Respir Crit Care Med. 1997;156:1–5. doi: 10.1164/ajrccm.156.5.9701064. [DOI] [PubMed] [Google Scholar]
- 29.Caillat-Zucman S, Djilali-Saiah I, Timsit J, et al. Insulin dependent diabetes mellitus (IDDM): 12th International Workshop Study. In: Charron D, editor. Genetic Diversity of HLA. Functional and Medical Implications. Paris: EDK Medical and Scientific International Publisher; 1997. pp. 389–97. [Google Scholar]
- 30.Lan MS, Wasserfall C, Maclaren NK, Notkins AL. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc Natl Acad Sci. 1996;25:6367–70. doi: 10.1073/pnas.93.13.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgenthaler NG, Seissler J, Achenbach P, et al. Antibodies to the tyrosine phosphatase-like protein IA-2 are highly associated with IDDM, but not with autoimmune endocrine diseases or stiff man syndrome. Autoimmunity. 1997;25:203–11. doi: 10.3109/08916939708994729. [DOI] [PubMed] [Google Scholar]
- 32.Pugliese A, Solimena M, Awdeh ZL, et al. Association of HLA-DQB1*0201 with stiff-man syndrome. J Clin Endocrin Metab. 1993;77:1550–3. doi: 10.1210/jcem.77.6.8263140. [DOI] [PubMed] [Google Scholar]
- 33.Somoza N, Vargas F, Roura-Mir C, et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor Vβ usage, and cytokine profile. J Immunol. 1994;153:1360–77. [PubMed] [Google Scholar]
- 34.Itoh N, Hanafusa T, Miyazaki A, et al. Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest. 1993;92:2313–22. doi: 10.1172/JCI116835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabata H, Kanai T, Yoshizumi H, et al. Characterization of self-glutamic acid decarboxylase 65-reactive CD4+ T-cell clones established from Japanese patients with insulin-dependent diabetes mellitus. Hum Immunol. 1998;59:549–60. doi: 10.1016/s0198-8859(98)00050-0. [DOI] [PubMed] [Google Scholar]
- 36.Roep BO. T-cell responses to autoantigens in IDDM. The search for the Holy Grail. Diabetes. 1996;45:1147–56. doi: 10.2337/diab.45.9.1147. [DOI] [PubMed] [Google Scholar]
- 37.Saiz A, Graus F, Valderiola F, Valls-Sole J, Tolosa E. Stiff-leg syndrome: a focal form of stiff-man syndrome. Ann Neurol. 1998;43:400–3. doi: 10.1002/ana.410430322. [DOI] [PubMed] [Google Scholar]