Abstract
The aim of the study was to determine whether collagen-polyvinylpyrrolidone (collagen-PVP) modifies some proinflammatory responses in synovium cultures from rheumatoid arthritis (RA) patients. Synovium from 10 RA patients were cultured with or without 1% collagen-PVP. Tissues on the 3rd, 5th and 7th culture day were sectioned and stained by the Herovici technique. Total collagen and type I/III collagen ratios were evaluated by the Woessner micromethod and by interrupted gel electrophoresis, respectively. Collagenolytic activity was assessed by degradation of [3H]-collagen in supernatants. TIMP-1, IL-1β and TNF-α were determined in supernatants by ELISA, and the results were normalized by DNA concentration. IL-1β, TNF-α, IL-6, IL-8, MMP-1, TIMP-1, Cox-1, VCAM-1, ICAM-1 and Fas/APO95 expression was evaluated by immunohistochemistry. Apoptosis was detected by TUNEL technique. The histological analysis and electrophoresis revealed a 1·7-fold increase of type III collagen in a time-dependent fashion in collagen-PVP-treated cultures. Proinflammatory cytokines (IL-1β: 58 ± 9 versus 22 ± 10; TNF-α: 41 ± 6 versus 11 ± 3; IL-8: 59 ± 12 versus 29 ± 9; treated versus untreated), adhesion molecule (ICAM-1: 57 ± 11 versus 29 ± 15; VCAM-1: 49 ± 7 versus 21 ± 13; treated versus untreated) as well as Cox-1 (59 ± 10 versus 20 ± 3) expression was down-regulated in RA synovium treated. Meanwhile, TIMP-1 (36 ± 7 versus 57 ± 11) and Fas expression (20 ± 10 versus 55 ± 13) and apoptosis (14 ± 3 versus 55 ± 5) were up-regulated in treated cultures compared with controls. In supernatants, the collagenolytic activity, as well as IL-1β and TNF-α, levels were all down-regulated in treated cultures (two, three, fourfold, respectively). The addition of collagen-PVP to synovium-induced down-modulation of some inflammatory parameters and an increase in apoptosis of synovial cells. Perhaps this mechanism could contribute to inhibit outgrowth of pannus formation and to down-regulate inflammation of joints in patients with RA.
Keywords: adhesion molecules, apoptosis, collagen-PVP, proinflammatory cytokines, rheumatoid arthritis
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune disease of unknown aetiology, in which affected joints exhibit inflammation, synovial hyperplasia, fibrosis and eventually the degradation of articular cartilage and erosion of subchondral bone [1]. Initial histological features of RA are characterized by synovial lining hyperplasia, excessive angiogenesis and accumulation of mononuclear cells in the synovium. This histological picture may be associated with an imbalance between cell proliferation and cell death [2,3]. Indeed, it has been reported that Fas/Fas ligand (Fas L)-induced apoptotic synovial cells seems to be incapable of eliminating cells in the proliferative RA synovium [2,4,5]. Permanence of inflammatory cells in tissue and altered activation of circulating and resident connective tissue cells becomes self-perpetuating. This can also lead to chronic tissue destruction by the persistent release of matrix-degrading enzymes. The latter promotes and exacerbates the inflammatory response, as well as contributing to the invasion of the cartilage and bone by the pannocytes. The most important ones in RA are collagenase-1 or matrix metalloproteinase-1 (MMP-1), stromelysin-1 (MMP-3), gelatinase B (MMP-9) and collagenase-3 (MMP-13) [6,7]. All of them are synthesized in response to proinflammatory cytokines such as IL-1β and TNF-α[8,9], or in response to activation of cell adhesion molecules (CAMs) [10–13]. The activity of MMPs is down-regulated to some extent by tissue inhibitors of metalloproteinase (TIMPs), which forms a stochiometric complex. Thus, connective tissue destruction in RA is due to an imbalance between the production of the MMPs and of specific TIMPs [14].
The hypercellularity that contributes to the progressive tissue destruction is originated by synoviocyte adhesion, migration, proliferation and mainly by the enhanced recruitment of blood-borne cells into inflamed joints. These processes depend upon the expression of a number of CAMs modulated by cytokines [15–18]. CAMs, with an altered expression in synovium from RA patients, include members of the integrin family (α1–6, αv, αd, β1–5, etc.), and particularly the α2 integrin or leucocyte adhesion molecules (CD11a, CD11b and CD11c) [15,16,19], as well as the immunoglobulin gene superfamily (ICAM-1, ICAM-2, ICAM-3, LFA-3 and VCAM-1) [17,18].
Due to abnormal immune responses observed in RA, and that the type I collagen-polyvinylpyrrolidone or collagen-PVP has demonstrated anti-inflammatory properties [20–22], we evaluated the effects of the addition of collagen-PVP to synovial tissue cultures from RA patients. This biodrug is made of a γ-irradiated mixture of pepsinized porcine type I collagen and PVP. This biopolymer has been demonstrated to modulate the chronic inflammatory process and to improve skin wound repair and bone fractures in rats [23]. Intralesional injection of biodrug once per week during 1–3 months in human hypertrophic scars or scleroderma lesions diminishes pruritus, pain, erythema, volume and inflammatory infiltrates. It causes the tissue architecture to resemble normal skin. Moreover, the biodrug modulates ECM turnover, mainly types I and III collagen, and down-regulates the expression level of IL-1β, TNF-α, PDGF and VCAM-1 [20,21]. Also, our group has demonstrated that 1% collagen-PVP modifies collagen turnover in synovium cultures from RA patients [22].
Based on the results that collagen-PVP down-regulates some proinflammatory cytokines, adhesion molecules and collagen turnover in skin fibrotic disorders associated with chronic inflammation, we suspected that the biodrug could have the same effect on inflamed synovial tissue cultures from RA patients.
PATIENTS AND METHODS
Patients
The synovium of 10 patients with RA was included in this study. All of them fulfilled the American College of Rheumatology criteria for the diagnosis of RA [24]. Nine of them were female with a mean age of 50 ± 17 years (mean ± SD; range 33–67 years). The mean duration of their disease was 15 ± 6 years. Disease-modifying antirheumatic drugs and non-steroidal anti-inflammatory drugs were prescribed to all patients before total knee or hip replacement surgery. As controls, five synovial tissues from non-RA patients were obtained during fracture surgery. Two of them were female, with a mean age of 40 ± 21 years (range 19–61 years) and analgesics and non-steroidal anti-inflammatory drugs were prescribed to all patients before surgery. All samples were obtained with informed consent from patients and institutional approval.
Tissue cultures
All RA specimens showed a variable content of inflammatory cells, as determined histologically. Fibrotic stage specimens were included in the study. RA and non-RA synovial tissue was separated from fat, bone and cartilage tissues, and was cut into small pieces of approximately 7 mm3. After washing with RPMI-1640 medium (HyQ cell culture Reagents, Logan, UT, USA), 50–60 randomized explants were placed on polycarbonate membranes (Nucleopore Costar, Cambridge, MA, USA) in sterile 24-well plates (Costar). Five hundred µl of RPMI-1640 medium containing 10% FCS, and 100 U penicillin/0·1 mg streptomycin/0·25 µg amphotericin B/ml (Sigma Chemical Co., St Louis, MO, USA), and with or without 1% dialysed collagen-PVP (0·6 µg/ml of collagen) were added to each culture well in duplicate. The rationale for this concentration of collagen-PVP was based on previous assays [20,22]. We analysed different concentrations of collagen-PVP (1, 3, 5 and 10%) on the effect of CAM expression. We have found that 1% collagen-PVP was the minimal concentration that induced the best down-regulation effect on the CAM expression, where 3, 5 and 10% of the biodrug had almost the same effect. The plates were incubated at 37°C in a 5% CO2/95% air incubator with humidified atmosphere. Culture media and tissues were recovered on the 3rd, 5th and 7th days. Media were replaced daily with fresh medium, with or without collagen-PVP. Each supernatant or tissue sample was stored at −70°C until assayed.
Collagen content
Tissues were prepared by grinding for 1 min in a polytron homogenizer. Two hundred and fifty µl of the samples were hydrolysed by adding 250 µl of 12 N HCl. The samples were sealed in small ampoules and hydrolysed for 48 h at 104°C. The content was then evaporated and dissolved in 100 µl of water. Five µl were taken and diluted with 195 µl of water. Hydroxyproline oxidation was initiated by incubation during 20 min at room temperature with 100 µl of 0·05 m chloramine T (Merck, Darmstadt, Germany). It was then destroyed by incubation during 5 min with 100 µl of 3·15 m perchloric acid. Finally, 100 µl of 20% p-dimethylaminobenzaldehyde solution (Sigma) were added. Samples were placed in a 60°C water bath for 30 min and then cooled. The developed colour was stable for at least 1 h. The absorbancy to the solutions was determined spectrophotometrically at 557 nm. The hydroxyproline values were determined directly from the standard curve [25].
Histology
In this technique a mixture of picric acid, methyl blue and acid fuchsin is used to distinguish type I from type III collagen. This method stains type I collagen red and type III collagen blue [26].
Interrupted gel electrophoresis
Ninety µl of synovial tissue homogenates and 1 mg of pepsin in 0·5 m acetic acid were incubated for 96 h at 4°C. Samples were dialysed against 5 mm acetic acid for 24 h and the pepsin-insoluble material was spun down at 10 000 g for 5 min. Twenty µl of samples were diluted 1 : 1 (v/v) with sample buffer and boiled for 5 min. Thirty-five µl of this solution were run on 7·5% polyacrylamide gels containing 0·1% SDS. Electrophoresis was performed at 70 mA, until the front dye had entered 7 mm into the resolving gel. The current was then switched off and wells were filled with 5 µl of 20%β-mercaptoethanol (v/v). Electrophoresis was resumed after 15 min. Gels were run for another hour [27] and silver-stained and the types I and III collagen bands were analysed by densitometry.
Measurement of DNA
Tissue homogenates were prepared by grinding for 1 min in a polytron homogenizer in phosphate-saline (0·05 m NaPO4, 2·0 m NaCl) buffer, pH 7·4, and sonicated briefly. One hundred µl of each homogenate were mixed with 2·8 ml of phosphate–saline buffer and 0·1 µg/ml of H33258 Hoechst reagent. A standard curve was prepared with calf thymus DNA (Merck) within a range of 100 ng/ml to 1 µg/ml of DNA. Spectrofluorophotometric measurements were made at 360 nm for excitation and 460 nm for emission [28].
Collagenolytic activity
Rat N-(propionate-2,3–[3H])-labelled type I collagen (400 cpm) (Amersham Life Science, Buckinghamshire, UK) were mixed with 140 µl of supernatants, in 0·050 m Tris-HCl, pH 7·8, containing 5 mm CaCl2 or 10 mm EDTA. Assay mixtures were incubated for 24 h at 35°C and reactions were stopped with o-phenantroline and centrifuged. Supernatants were placed in Bray's solution to assess the amount of radioactivity in a liquid scintillation counter (Beckman model LS 1801, Fullerton, CA, USA). A blank containing water instead of culture supernatant was run with each determination. Calcium-dependent collagenolytic activity was calculated as the difference between total collagenolytic activity (CaCl2 buffer) and calcium-independent collagenolytic activity (EDTA buffer). Collagenase activity was expressed as cpm/24 h/µg of DNA at 35°C [29].
Measurement of TIMP-1
The concentration of TIMP-1 was assessed by a one-step sandwich enzyme immunoassay (ELISA) system (Amersham) in diluted 1 : 5–1 : 20 supernatants. The ELISA system was capable of measuring not only free TIMP-1, but also TIMP-1 complexed with either proMMP-9 or the active forms of MMP-1, MMP-2, MMP-3 and MMP-9. In this ELISA system, free TIMP-1 has the same reactivity as complexed TIMP-1.
Measurement of cytokines
IL-1β and TNF-α were determined in supernatants of cultures with an ELISA kit according to the manufacturer's instructions (Amersham).
Immunoperoxidase staining
The antibodies used were either mouse antihuman ICAM-1 or VCAM-1 monoclonal IgG at 10 µg/ml (Genzyme Corp., Cambridge, MA, USA), goat antihuman IL-1β, TNF-α, MMP-1, TIMP-1 or Fas monoclonal IgG at 10 µg/ml (Santa Cruz, CA, USA), rabbit antihuman IL-8, IL-6 or Cox-1 polyclonal IgG at 10 µg/ml (Santa Cruz). The negative control reaction was performed with a 1 : 100 dilution of normal human serum, instead of the primary antibody. The reactive blank was incubated with phosphate buffer saline–egg albumin (Sigma) instead of the primary antibody. Both controls exclude non-specific staining or endogenous enzymatic activities. At least two different sections were examined for each tissue in a double blind analysis. CAM, MMP-1, TIMP-1, Cox-1, Fas/Apo95 and cytokine expression was assessed by estimating positively stained cells in blood vessels and in stromal cells along one field selected randomly. Results are expressed as the mean ± standard deviation (s.d.) of the percentage of immunoreactive cells [20,30].
Detection of DNA strand breaks in apoptotic synovial cells by in situ nick translation
Apoptotic cells were detected with TUNEL kit according to the manufacturer's instructions (Roche Diagnostics GmbH, Mannheim, Germany). The negative control was incubated with nucleotide mixture instead of the TUNEL reaction mixture. At least two different sections were examined for each synovial tissue in all cultures in a double blind analysis. Apoptotic cells were assessed by estimating positively stained cells in blood vessels and in stromal cells along one field selected randomly. Results are expressed as the mean ± s.d. of the percentage of reactive cells.
Statistical analysis
All experiments were made at least in duplicate. Statistical analysis was performed by paired Student's t-test. Data were expressed as the mean ± s.d. P-values smaller than or equal to 0·05 were considered significant.
RESULTS
Collagen-PVP effect on DNA and total collagen content in synovial tissue cultures from non-RA and RA patients
To evaluate whether collagen-PVP produces an effect either on cell metabolism or cell concentration, we measured the DNA content. There were no statistically significant differences in DNA content when treated and control cultures were compared (Table 1). It is important to mention that DNA content in non-RA synovium was eight to 10-fold lower than that determined in RA synovial tissue.
Table 1.
Collagen-PVP effect on DNA in synovial tissue cultures from non-RA and RA patients
| Sample (mean ± SD)* | Treatment Culture day | – 3rd | – 5th | – 7th | + 3rd | + 5th | + 7th |
|---|---|---|---|---|---|---|---|
| Non- RA | 0·50 ± 0·08 | 0·47 ± 0·08 | 0·46 ± 0·08 | 0·40 ± 0·07 | 0·51 ± 0·08 | 0·45 ± 0·08 | |
| RA | 4·27 ± 1·20 | 3·92 ± 1·11 | 5·45 ± 1·68 | 4·69 ± 1·48 | 4·71 ± 1·64 | 3·84 ± 1·21 |
The results depict mean ± s.d. of DNA content (µg/ml) in synovial tissue cultures from five non-RA and 10 RA patients each sample performed in quadruplicate synovial tissue homogenates. (−) without collagen-PVP; (+) with 1% collagen-PVP.
In order to determine whether collagen-PVP modifies the total collagen content, the latter was quantified in control and treated cultures. We did not find any statistical difference in collagen content between treated and untreated synovial tissue cultures (data not shown).
Histological findings in synovial tissue cultures from non-RA and RA patients
Morphological evaluation of non-RA synovium showed unaltered tissue architecture on the 3rd and 7th culture days (Fig. 1a). The addition of collagen-PVP to non-RA synovium cultures did not modify tissue architecture, fibre collagen thickness or type I/III collagen proportion (Fig. 1b). However, histological evaluation of synovial tissue from RA control cultures showed a variable content of inflammatory cells and fibrosis with abundant type I collagen (red fibres in Fig. 1c) at the 3rd and 7th culture days. In contrast, paired 1% collagen-PVP-treated cultures showed recovery of type III collagen (blue fibers in Fig. 1d at the 7th culture day).
Fig. 1.
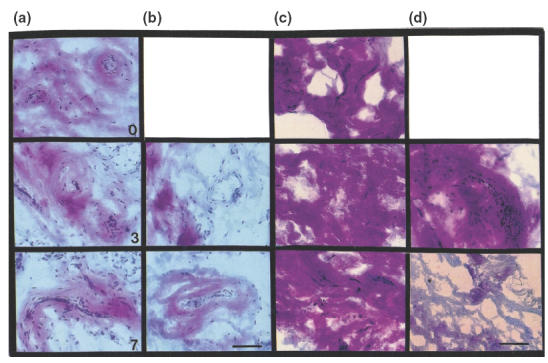
Collagen-PVP effect on tissue architecture of synovium in culture. Photomicrographs of synovial tissue cultures with or without 1% collagen-PVP, stained with the Herovici technique. (a) Non-RA synovial control cultures on the 0, 3rd and 7th culture days. (b) Non-RA synovium collagen-PVP-treated cultures during days 3 and 7. (c) RA synovial tissue incubated without treatment during days 0, 3 and 7, respectively. The predominant extracellular matrix component is type I collagen (fibres in red). (d) RA synovium treated with collagen-PVP during days 3 and 7, respectively. There is an increase of type III collagen (fibres in blue). Scale bar: 100 µm.
Relative percentage of types I and III collagen in synovial tissue cultures from non-RA and RA patients
In order to confirm the change in the relative proportions of types I and III collagen, duplicates of synovium homogenates at each point of culture were evaluated by interrupted gel electrophoresis and densitometric analysis. Under reducing conditions, the interchain disulphide bonds are cleaved, releasing α1(III) monomers of collagen, which migrate more slowly than α1(I) chains of collagen. Figure 2a shows the fine band of α1(III) in the synovial tissue control cultures. Densitometric analysis showed that the addition of collagen-PVP to non-RA synovium cultures did not produce any modification in the relative percentage of type I or type III collagen (Fig. 2b). However, RA synovium cultures treated on the 7th day displayed a 1·7-fold increase in the α1(III) band in a time-dependent fashion (P < 0·009, treated versus untreated cultures; Fig. 2c).
Fig. 2.
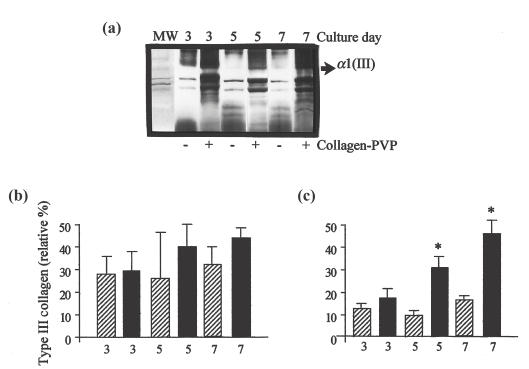
Collagen-PVP effect on types I and III collagen content in synovial tissue cultures. (a) SDS-PAGE of types I and III collagen of representative RA synovial tissue cultures with (+) or without (−) collagen-PVP on the 3rd, 5th and 7th culture days, respectively. MW: Type I collagen standard. (b) Type III collagen relative percentage in non-RA synovial tissue cultures. (c) Type III collagen relative percentage in RA synovial tissue cultures. Statistical differences between control and collagen-PVP-treated groups were obtained on the 5th (*P = 0·009) and the 7th culture days (*P = 0·00007). Data are the mean ± s.e.m. of synovial tissue cultures from five non-RA and 10 RA patients, each performed in duplicate.  , Type III collagen in control group; ▪, Type III collagen in collagen-PVP group.
, Type III collagen in control group; ▪, Type III collagen in collagen-PVP group.
Collagenolytic activity of synovial tissue from RA patients
During the progression of RA the proteolytic activity at the site of inflammation is increased. Thus, synovia from 10 patients with RA and five non-RA patients were examined immunohistochemically in detail using specific antisera to MMP-1. The RA synovia showed a considerably high range in MMP-1 distribution compared with non-RA synovia. There was no difference between treated versus control group (Fig. 3d,e). On the other hand, in RA supernatants from collagen-PVP-treated cultures, levels of total collagenolytic activity were 1·6-fold lower than those in control cultures (P < 0·05; Fig. 3a). Calcium-dependent collagenases (MMPs) in supernatants from synovial tissue-treated cultures were measured by the difference between the degradation of [3H]-collagen in a CaCl2 buffer and in an EDTA buffer (total collagenolytic activity – calcium-independent collagenases). This proteolytic activity exhibited slightly lower levels in supernatants from biocompound-treated cultures than untreated-ones (Fig. 3b). Collagenase activity of proteinases that did not require calcium for their stability (putatively elastase and/or G cathepsin) was 2·2-fold lower compared to untreated tissue cultures (P < 0·008; Fig. 3c).
Fig. 3.
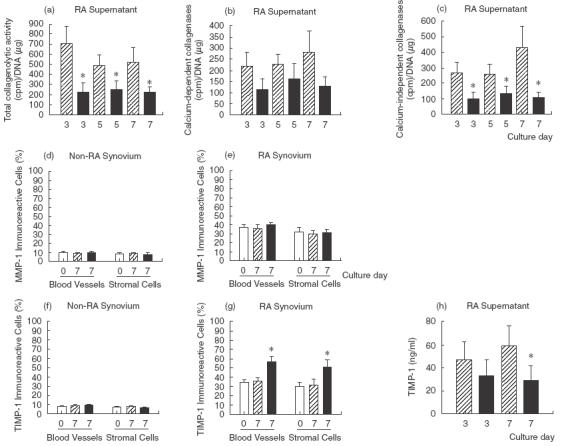
Collagen-PVP effect on collagenase activity and TIMP-1 expression in synovial tissue cultures. (a) Total collagenolytic activity. Data were statistically significant with *P = 0·008, *P = 0·055 and *P = 0·05 for the 3rd, 5th and 7th culture days, respectively. (b) Calcium-dependent collagenolytic activity. (c) Calcium-independent collagenolytic activity. Collagen-PVP-treated groups were compared with untreated cultures and the differences were statistically significant with *P = 0·008, *P = 0·006 and *P = 0·002 for the 3rd, 5th and 7th culture days, respectively. Collagenase activity was expressed as cpm/24 h/µg of DNA at 35°C. Data are mean ± s.e.m. of synovial tissue cultures from 10 RA patients, each performed in duplicate. (d) MMP-1 immunoreactive cells on non-RA synovial tissue. (e) MMP-1 immunoreactive cells on RA synovial tissue. (f) TIMP-1 immunoreactive cells on non-RA synovial tissue. (g) TIMP-1 immunoreactive cells on RA synovial tissue. Data are mean ± s.e.m. of synovial tissue cultures from five non-RA and 10 RA patients, each performed in duplicate, where of each tissue at least two sections were evaluated. (h) TIMP-1 concentration in synovial tissue supernatant with or without collagen-PVP treatment (*P = 0·04 on the 7th culture day). Data are the mean ± s.e.m. of synovial tissue cultures from 10 RA patients, each performed in duplicate. □, Initial tissue;  , control; ▪, collagen-PVP.
, control; ▪, collagen-PVP.
TIMP-1 concentration in synovial tissue cultures from RA patients
Sections of RA synovium stained with anti-TIMP-1 showed strong immunoreactivity in blood vessel (36·0 ± 7·4 versus 57·1 ± 11·2, untreated versus treated; P < 0·05) and stromal cells (31·7 ± 13·9 versus 51·8 ± 15·2, untreated versus treated; P < 0·05) in collagen-PVP-treated synovial tissue from RA patients (Fig. 3g). Collagen-PVP has no effect on synovium from non-RA patients (7·8 ± 1·0 versus 9·1 ± 1·2 for blood vessels and 7·1 ± 0·6 versus 6·4 ± 1·1 for stromal cells, untreated versus treated; Fig. 3f). However, TIMP-1 levels in supernatants from control cultures contained 1·7-fold higher levels of the glycoprotein than treated cultures on the 7th day (P = 0·04; Fig. 3h).
Adhesion molecule expression in synovial tissue cultures from non-RA and RA patients
In order to establish whether ICAM-1 and VCAM-1 molecules, inflammatory markers, were modified by collagen-PVP treatment, they were detected in synovium. The ICAM-1 expression in cultures from non-RA patients was similar between control and collagen-PVP-treated group (11–17%; Table 2). However, in treated cultures from RA patients, both ICAM-1 and VCAM-1 molecules showed lower levels of intensity and immunoreactivity than control cultures (Table 2). The levels were statistically significant for ICAM-1 on the 7th culture day in blood vessels (57·3 ± 10·6 versus 29·2 ± 15·2, control versus treated; P = 0·03) and stromal cells (31·0 ± 11·4 versus 18·3 ± 11·0; P = 0·04) and the percentage of positive ICAM-1 cells from treated cultures was similar to that determined for normal synovial tissue cultures (Table 2) [30]. In addition, VCAM-1 expression in blood vessels and stromal cells from treated cultures also showed a substantial down-regulation (48·6 ± 7·0 versus 21·4 ± 13·1 for blood vessels and 38·9 ± 11·6 versus 17·1 ± 7·7 for stromal cells, treated versus untreated cultures at 7th culture day; P < 0·05).
Table 2.
Collagen-PVP effect on molecule expression on blood vessel cells and stromal cells from synovial tissue cultures from non-RA and RA patients†
| Non-RA | RA | ||||||
|---|---|---|---|---|---|---|---|
| Culture day Treatment | 0 − | 7th − | 7th + | 0 − | 7th − | 7th + | |
| Antibody to | Cell type | ||||||
| ICAM-1 | Blood vessel | 12·0 ± 0·8 | 11·6 ± 0·9 | 13·0 ± 0·7 | 64·0 ± 4·8 | 57·3 ± 10·6 | 29·2 ± 15·2* |
| Stroma | 11·0 ± 1·8 | 10·7 ± 1·1 | 10·0 ± 1·2 | 28·6 ± 14·0 | 31·0 ± 11·4 | 18·3 ± 11·0* | |
| VCAM-1 | Blood vessel | 16·0 ± 3·5 | 17·0 ± 1·6 | 14·8 ± 0·9 | 53·5 ± 6·7 | 48·6 ± 7·0 | 21·4 ± 13·1* |
| Stroma | 15·6 ± 0·2 | 16·0 ± 0·8 | 15·3 ± 1·4 | 36·1 ± 11·2 | 38·9 ± 11·6 | 17·1 ± 7·7* | |
| IL-6 | Blood vessel | 7·9 ± 0·6 | 8·7 ± 1·0 | 8·0 ± 1·8 | 26·4 ± 20·2 | 27·4 ± 19·0 | 10·4 ± 6·3 |
| Stroma | 7·3 ± 1·5 | 8·5 ± 2·0 | 9·0 ± 2·7 | 12·2 ± 10·6 | 14·5 ± 9·0 | 6·1 ± 3·8 | |
| Cox-1 | Inflammatory | 8·0 ± 1·5 | 8·3 ± 2·6 | 7·6 ± 2·0 | 57·7 ± 17·1 | 58·5 ± 10·0 | 20·0 ± 2·5* |
| FAS/ | Blood vessel | 22·0 ± 1·5 | 23·4 ± 6·1 | 21·8 ± 3·8 | 20·2 ± 5·4 | 20·2 ± 9·9 | 55·2 ± 12·5* |
| Apo95 | Stroma | 15·5 ± 4·6 | 19·8 ± 5·2 | 20·0 ± 4·2 | 22·0 ± 5·2 | 26·1 ± 8·9 | 17·5 ± 3·7 |
| TUNEL | Blood vessel | 14·9 ± 5·2 | 14·4 ± 3·8 | 16·0 ± 5·3 | 18·1 ± 7·74 | 13·9 ± 2·9 | 54·9 ± 4·7* |
| Stroma | 13·5 ± 2·8 | 14·5 ± 3·7 | 14·5 ± 3·6 | 13·8 ± 4·8 | 14·7 ± 4·3 | 35·6 ± 9·7* | |
The results depict mean ± s.d. of molecule expression (percentage of immunoreactive cells determined by immunohistochemistry) in synovial tissue cultures from five non-RA and 10 RA patients each performed in duplicate where of each tissue at least two sections were evaluated. (−) without collagen-PVP; (+) with 1% collagen-PVP; (ND) Not Done
P < 0·05; 0 versus 7th culture day.
Proinflammatory cytokine production by synovial tissue cultures from RA patients
In RA many factors are involved in synovial inflammation, where the cytokines such as IL-1, TNF-α, IL-6 and IL-8, have emerged as regulatory factors of particular importance. In order to establish the effect of collagen-PVP on the expression of these cytokines, we determined these proteins in the synovium by immunohistochemistry. Results showed that IL-8 was expressed at significantly higher levels in non-treated synovial tissue cultures from RA patients (58·6 ± 11·7 for blood vessels and 43·1 ± 10·2 for stromal cells) than in non-RA (9·8 ± 3·0 for blood vessels and 9·3 ± 2·4 for stromal cells) and RA-treated cultures (29·4 ± 8·5 for blood vessels and 17·7 ± 3·8 for stromal cells; Fig. 4a,b). Meanwhile, IL-6 did not show any difference between RA-treated cultures and control cultures (Table 2).
Fig. 4.
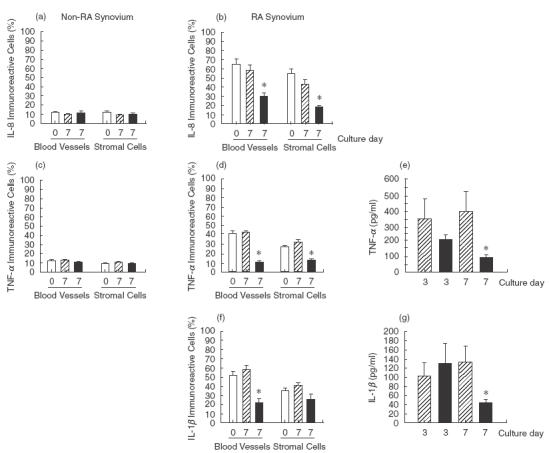
Effect of collagen-PVP on proinflammatory cytokine expression in synovial tissue cultures. (a) IL-8 immunoreactive cells on blood vessels and stromal cells from non-RA patients. (b) IL-8 immunoreactive cells in synovial tissue from RA patients (*P < 0·05). (c) TNF-α immunoreactive cells on blood vessels and stromal cells from non-RA patients. (d) TNF-α immunoreactive cells on blood vessels and stromal cells from RA patients (*P < 0·05). Data are mean ± s.e.m. of synovial tissue cultures from five non-RA and 10 RA patients, each performed in duplicate, where of each tissue at least two sections were evaluated. (e) TNF-α production (*P = 0·03) on the 7th day. Data are the mean ± s.e.m. of synovial tissue cultures from 10 RA patients, each performed in duplicate. (f) IL-1β immunoreactive cells on blood vessels and stromal cells from RA patients (*P < 0·05). Data are mean ± s.e.m. of synovial tissue cultures from five non-RA and 10 RA patients, each performed in duplicate, where of each tissue at least two sections were evaluated. (g) IL-1β concentration in supernatants from RA synovium cultures (*P = 0·05). Data are the mean ± s.e.m. of synovial tissue cultures from 10 RA patients, each performed in duplicate. □, Initial tissue;  , control; ▪, collagen-PVP.
, control; ▪, collagen-PVP.
Because collagenolytic activity, CAMs and TIMP-1 expression levels were down-modulated with collagen-PVP treatment, we suspected that the production of IL-1β and TNF-α was modified. The exogenous addition of the biodrug to non-RA tissue cultures did not produce any effect on TNF-α expression in blood vessels (12·8 ± 1·8 versus 10·7 ± 2·3, untreated versus treated cultures) nor stromal cells (10·7 ± 1·4 versus 9·6 ± 1·6, untreated versus treated; Fig. 4c). TNF-α expression was down-modulated by collagen-PVP in tissue from RA patients at statistical significant levels compared with non-treated cultures (42·1 ± 4·5 versus 11·2 ± 3·3 for blood vessels and 31·3 ± 4·1 versus 12·4 ± 3·7 for stromal cells, P < 0·05; Fig. 4d). Moreover, collagen-PVP down-regulated TNF-α protein concentration in supernatants (406·8 ± 137·0 versus 101·6 ± 14·6 pg/ml; untreated versus treated, P < 0·02; Fig. 4e). The same down-regulated pattern of IL-1β expression was observed in RA tissue treated with collagen-PVP (58·3 ± 8·5 versus 22·0 ± 9·8 for blood vessel cells and 40·8 ± 7·3 versus 25·5 ± 5·7 for stromal cells; untreated versus treated, P < 0·05; Fig. 4f) and protein concentration in supernatants (133·5 ± 35·3 versus 44·8 ± 6·3 pg/ml; untreated versus treated, P < 0·03; Fig. 4g).
Effect of collagen-PVP on Cox-1 expression
Cox-1 is a constitutively enzyme that synthesized prostaglandins pathway arachidonic acid metabolism. Prostaglandins probably contribute to synovial inflammation by increasing local blood flow and potentiating the effects of mediators such as bradykinin and IL-1 that induce vasopermeability. In this vein, the immunohistochemistry showed that collagen-PVP induced a negative modulation on the expression of Cox-1 in RA tissue compared with untreated cultures (58·5 ± 10·0 versus 20·0 ± 2·5, untreated versus treated, P < 0·05; Table 2). However, biodrug did not show effect on non-RA tissue (8·3 ± 2·6 versus 7·6 ± 2·0, untreated versus treated; Table 2).
Expression of Fas/Apo95 and detection of DNA strand breaks in apoptotic synovial cells by in situ nick translation
It has been shown that Fas antigen is expressed on the surface of synovial cells and mediates cell death of the Fas-expressing synovial cells when stimulated with agonistic anti-Fas. However, defective apoptosis is associated intimately with RA, thus the function of the Fas/FasL system seems to be incapable of eliminating cells in the proliferative RA synovium. Due to this, we examined whether Fas antigen was expressed on synovium as well as the presence of apoptotic cells. We found that Fas/Apo95 was predominantly up-regulated on blood vessel cells from RA synovium collagen-PVP-treated (20·2 ± 9·9 versus 55·2 ± 12·5, untreated versus treated, P < 0·05; Table 2). When we applied the in situ cell death detection assay for RA synovial tissues to detect apoptotic cells, TUNEL technique showed an up-regulation mainly in blood vessel (13·9 ± 2·9 versus 54·9 ± 4·7, untreated versus treated) and stromal cells (14·7 ± 4·3 versus 35·6 ± 9·7, untreated versus treated; Table 2) treated with collagen-PVP.
DISCUSSION
We evaluated the anti-inflammatory effect of polymerized type I collagen (collagen-PVP), as well as collagen turnover in synovial tissue from RA patients, based on previous studies that have analysed the effects of exogenous ECM proteins on in vitro T cell responses [31,33] and in vivo on phase I and II clinical trials where has been recognized that types I, II or III collagen are capable to induce peripheral immune tolerance or suppression and thus down-regulate inflammation of RA joint [34,35].
Collagen association with PVP and the cross-linking favoured by γ-irradiation confers on it various physicochemical properties, such as the impossibility of forming gel when it is diluted in culture medium at 37°C and neutral pH. Moreover, electrophoretical analysis demonstrated a change in the relative mobility of collagen-PVP when compared with the mixture without γ-irradiation on the components alone. Also, bioassays have demonstrated that the components alone do not have the same properties than collagen-PVP, where PVP does not have any effect, as described previously [36]. Collagen-PVP has been shown to have immunomodulatory effects on some pathologies associated with chronic inflammatory processes [20,22]. For this reason, and based on a previous study of tissue from RA patients [22], we evaluated the exogenous addition of 1% collagen-PVP during 1 week to non-RA and rheumatoid synovium cultures. The biodrug addition to non-RA and RA cultures did not induce any change in DNA concentration or metabolism. However, the addition of the biodrug to RA synovial tissue cultures modified the histological and biochemical pattern of fibrosis, without changing the total collagen content. The biodrug induced the recovery of type III collagen at similar levels to those detected in normal synovial tissue. Collagen-PVP diminished the accumulation of dense and tightly packed type I collagen fibres and contributed to establish a similar tissue architecture to that observed in normal synovium. These data are relevant as matrix macromolecules are essential for the structure and integrity of the tissues. Furthermore, ECM components regulate several important cellular functions, including cell phenotype, differentiation, migration, mitogenic activity, cell activation, apoptosis, the synthesis of macromolecules and interstitial hydraulic resistance [36].
We also evaluated the proteolytic activity, particularly that attributable to the collagenases. Even though calcium-dependent collagenases decreased with biodrug treatment, the difference in proteolytic activity levels was not statistically significant. However, the calcium-independent collagenases, probably neutrophil elastase and/or cathepsin G, which shows enzymatic activity at neutral pH, was diminished at statistically significant levels. It is important to emphasize that the participation of other enzymes, not only MMPs but also calcium-independent collagenolytic proteases, may be important during disease progression. Thus, collagenase reduction probably contributes to avoid the most serious sequels of rheumatoid synovitis, the invasion of cartilage and bone at the chondrosynovial junction.
Moreover, TIMP-1 production by tissue cultures treated with the biodrug increased in synovium while it diminished in supernatants in a time-dependent manner. The latter may be due to a down-regulation of proinflammatory cytokines [38]. In this vein, TIMP-1 associated with tissue perhaps inhibits MMP action.
Cellular invasion and fibrosis are favoured by a direct relationship between the cell–cell and cell–matrix interactions through CAMs. It is well known that rheumatoid synovium overexpresses ICAM-1 and VCAM-1 [39,40]. These are dramatically up-regulated by proinflammatory cytokines in a number of cell types, including endothelium [41]. After treatment with collagen-PVP both molecules were down-regulated in blood vessel and stromal cells at similar levels to those determined in normal synovium [30]. Thus, collagen-PVP could decrease microvascular endothelial activation, with the consequent reduction of leucocyte trafficking to the synovial joint, suggesting that the anti-inflammatory effect of the biodrug may be due partially to the effect on endothelial and stromal CAMs.
Finally, we focused on three proinflammatory cytokines, IL-8, TNF-α and IL-1β, as they induce the activation of Cox-1 [43], and in consequence the increase of prostaglandin E2 production, as well as cartilage destruction [8,44] and bone resorption. These proinflammatory cytokines have also been related to the onset and exacerbations of disease activity of in vitro cultures [45,46] and of an in vivo rodent model [47,48]. Similarly, TNF-α and IL-1β induce the production of other proinflammatory cytokines such as GM-CSF, IL-6 and IL-8 [42,43]. Also, they increase the MMP activity [8,44] and ELAM-1, VCAM-1 and ICAM-1 expression in capillaries and venules in synovial biopsies [15–18]. Here, we determined that the biodrug diminished the IL-1β and TNF-α levels detected in tissue and supernatants from RA synovium cultures, thus participating in the interruption of the inflammatory process through direct effect on cellular metabolism.
Based on these results, we suggest that collagen-PVP added to RA synovium cultures, modulates collagen turnover, because the biodrug decreases collagenolytic activity, as well as TIMP-1 production, and increases the amount of type III collagen to similar levels observed in normal synovium. The chronic inflammatory process is altered by collagen-PVP action, as described previously [20,21], due presumably to the down-regulation of IL-1β and TNF-α, as both cytokines are capable of inducing the expression and activation of collagenolytic enzymes, as well as inducing proliferation and migration of synovial cells via CAMs and inducing Cox-1 activation. Also, down-production of TNF-α and IL-1β seems to stimulate synovial cell death via apoptosis in synovium cultures; the latter may contribute to inhibition of the outgrowth of synovial cells that leads eventually to hyperplasia or pannus formation and the destruction of RA joints [49].
We infer that collagen-PVP mechanism of action might be mediated through regulation of certain transcription factors such as NF-κB and AP-1. In particular, NF-κB regulates the expression of proinflammatory enzymes, cytokines, chemokines, immunoreceptors and CAMs as well as apoptosis; it has been often termed a ‘central mediator of the immune response’[50–52]. Because of this key role, we suggest that collagen-PVP could be contribute considerably to the anti-inflammatory effects observed through the down-regulation of NF-κB. However, it is necessary to perform the appropriate experiments to explore this possibility.
In conclusion, we showed that polymerized collagen induced a down-modulation but not an inhibition of inflammatory parameters in rheumatoid synovium. This effect probably allowed a gradual and better recovery of synovial tissue homeostasis. Future studies should focus in early RA and on other pro- and anti-inflammatory cytokines, their receptors and the concomitant estimation of cytokine inhibitors, and also analysis of specific proteases that may be regulated by the biocompound, as well as on the study of the molecular pathways of collagen-PVP effects on the cell. Whether the anti-inflammatory features of collagen-PVP will permit the utilization of this biodrug in the treatment of RA is still unknown.
Acknowledgments
We thank Dr Virgilio Hernández Cuevas for providing some of the clinical specimens used in this study; Alejandro Quintana Díaz BSc and Miguel Morales PhD for technical assistance; Edgar Krötzsch PhD, Carlos Rosales Ledezma PhD and Marco José-Valenzuela PhD for their critical and careful review of the manuscript and Ana Luisa Weckmann MSc for correcting the English version of the manuscript. This work was partially supported by grants from the Universidad Nacional Autónoma de México (PADEP 030308 and 030352; PUIS) and from ÁSPID S.A. de C.V. (LDL-94).
REFERENCES
- 1.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–439. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS, Yeo M, Zvifler NJ. Apoptosis in rheumatoid arthritis synovium. J Clin Invest. 1995;96:1631–8. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sugiyama M, Tsukazaki T, Yonekura A, et al. Localisation of apoptosis and expression of apoptosis related proteins in the synovium of patients with rheumatoid arthritis. Ann Rheum Dis. 1996;55:442–9. doi: 10.1136/ard.55.7.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakajima T, Aono H, Hasunuma T, et al. Apoptosis and functional Fas antigen in rheumatoid arthritis synoviocytes. Arthritis Rheum. 1995;38:485–91. doi: 10.1002/art.1780380405. [DOI] [PubMed] [Google Scholar]
- 5.Tsuboi M, Eguchi K, Kawakami K, et al. Fas antigen expression on synovial cells was down-regulated by interleukin 1β. Biochem Biophys Res Commun. 1996;218:280–6. doi: 10.1006/bbrc.1996.0049. [DOI] [PubMed] [Google Scholar]
- 6.Hembry RM, Bagga MR, Reynolds JJ, Hamblen DL. Immunolocalisation studies on six matrix metalloproteinases and their inhibitors, TIMP-1 and TIMP-2, in synovia from patients with osteo- and rheumatoid arthritis. Ann Rheum Dis. 1995;54:25–32. doi: 10.1136/ard.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamanaka H, Matsuda Y, Tanaka M, et al. Serum matrix metalloproteinase 3 as a predictor of the degree of joint destruction during the six months after measurement, in patients with early rheumatoid arthritis. Arthritis Rheum. 2000;43:852–8. doi: 10.1002/1529-0131(200004)43:4<852::AID-ANR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8.West-Mays JA, Strissel KJ, Sadow PM, Fini ME. Competence for collagenase gene expression by tissue fibroblasts requires activation of an interleukin 1α autocrine loop. Proc Natl Acad Sci. 1995;92:6768–72. doi: 10.1073/pnas.92.15.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Migita K, Eguchi K, Kawabe Y, et al. TNF-α-mediated expression of membrane-type matrix metalloproteinase in rheumatoid synovial fibroblasts. Immunol. 1996;89:553–7. doi: 10.1046/j.1365-2567.1996.d01-789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larjava H, Lyons J, Salo R, et al. Anti-integrin antibodies induce type IV collagenase gene expression in keratinocytes. J Cell Physiol. 1993;157:190–200. doi: 10.1002/jcp.1041570125. [DOI] [PubMed] [Google Scholar]
- 11.Romanic AM, Madri JA. The induction of 72 kD gelatinase in T cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol. 1994;125:1165–78. doi: 10.1083/jcb.125.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rükonen T, Westermarck J, Koivisto L, et al. Integrin α2β1 is a positive regulator of collagenase (MMP-1) and collagen α1(I) gene expression. J Biol Chem. 1995;270:13548–52. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- 13.Migita K, Eguchi K, Tominaga M, et al. β2-Microglobulin induces stromelysin production by human synovial fibroblasts. Biochem Biophys Res Comm. 1997;239:621–5. doi: 10.1006/bbrc.1997.7366. [DOI] [PubMed] [Google Scholar]
- 14.Murphy G, Willenbrock F, Crabbe T, et al. Greenwald RA, Golub LM, editors. Regulation of matrix metalloproteinase activity. In: Inhibition of matrix metalloproteinases. Therapeutic potential. 732:31–41. doi: 10.1111/j.1749-6632.1994.tb24722.x. [DOI] [PubMed] [Google Scholar]
- 15.Pirila L, Heino J. Altered integrin expression in rheumatoid synovial lining type B cells. In vitro cytokine regulation of α1β1, α6β1, and αvβ5 integrins. J Rheumatol. 1996;23:1691–8. [PubMed] [Google Scholar]
- 16.Rinaldi N, Barth TFE, Weis D, et al. Loss of laminin and of the laminin receptor integrin subunit α6 in situ correlates with cytokine induced down regulation of α6 on fibroblast-like synoviocytes from rheumatoid arthritis. Ann Rheum Dis. 1998;57:559–65. doi: 10.1136/ard.57.9.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindsley HB, Smith DD, Cohick CB, et al. Proinflammatory cytokines enhance human synoviocyte expression of functional intercellular adhesion molecule-1 (ICAM-1) Clin Immunol Immunopathol. 1993;68:311–20. doi: 10.1006/clin.1993.1132. [DOI] [PubMed] [Google Scholar]
- 18.Kienzle G, Von Kempis J. Vascular cell adhesion molecule 1 (CD106) on primary human articular chondrocytes. Functional regulation of expression by cytokines and comparison with intercellular adhesion molecule 1 (CD54) and very late activation antigen 2. Arthritis Rheum. 1998;41:1296–305. doi: 10.1002/1529-0131(199807)41:7<1296::AID-ART21>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Rinaldi N, Schwarz-Eywill M, Weis D, et al. Increased expression of integrins on fibroblast-like synoviocytes from rheumatoid arthritis in vitro correlates with enhanced binding to extracellular matrix proteins. Ann Rheum Dis. 1997;56:45–51. doi: 10.1136/ard.56.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krötzsch-Gómez FE, Furuzawa-Carballeda J, Reyes-Márquez R, et al. Cytokine expression is down-regulated by collagen-polyvinylpyrrolidone in hypertrophic scars. J Invest Dermatol. 1998;111:828–34. doi: 10.1046/j.1523-1747.1998.00329.x. [DOI] [PubMed] [Google Scholar]
- 21.Barile L, Furuzawa-Carballeda J, Krötzsch-Gómez FE, et al. Comparative study of collagen-polyvinylpyrrolidone versus triamcinolone acetate in systemic sclerosis. Clin Exp Rheumatol. 1998;16:370. [Google Scholar]
- 22.Furuzawa-Carballeda J, Alcocer-Varela J, Díaz de León L. Collagen-PVP decreases collagen turnover in synovial tissue cultures from rheumatoid arthritis patients. Ann NY Acad Sci. 1999;878:598–603. doi: 10.1111/j.1749-6632.1999.tb07738.x. [DOI] [PubMed] [Google Scholar]
- 23.Chimal-Monroy J, Bravo-Ruiz T, Furuzawa-Carballeda GJ, et al. Collagen-PVP accelerates new bone formation of experimentally induced bone defects in rat skull and promotes the expression of osteopontin and SPARC during bone repair of rat femora fractures. Ann NY Acad Sci. 1998;857:232–6. doi: 10.1111/j.1749-6632.1998.tb10120.x. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The american rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1998;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this iminoacid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 26.Herovici C. Polychrome stain for differentiating precollagen from collagen. Stain Technol. 1963;38:204–5. [PubMed] [Google Scholar]
- 27.Sykes B, Puddle B, Francis M, Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Comm. 1976;72:1472–81. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- 28.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–52. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 29.Murawaki Y, Yamada S, Koda M, Hirayama C. Collagenase and collagenolytic cathepsin in normal and fibrotic rat liver. J Biochem. 1990;108:241–4. doi: 10.1093/oxfordjournals.jbchem.a123187. [DOI] [PubMed] [Google Scholar]
- 30.Furuzawa-Carballeda J, Alcocer-Varela J. Interleukin-8, interleukin-10, intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 expression levels are higher in synovial tissue from patients with rheumatoid arthritis than in osteoarthritis. Scand J Immunol. 1999;50:215–22. doi: 10.1046/j.1365-3083.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 31.Easter DW, Hoyt DB, Ozkan AN. Immunosuppression by a peptide from the gelatin binding domain of human fibronectin. J Surg Res. 1988;45:370–5. doi: 10.1016/0022-4804(88)90133-3. [DOI] [PubMed] [Google Scholar]
- 32.Rybski JA, Lause DB, Reese AC. Effect of fibronectin on antigen-induced lymphoproliferation and antibody synthesis in rats. J Leukoc Biol. 1989;45:35–45. doi: 10.1002/jlb.45.1.35. [DOI] [PubMed] [Google Scholar]
- 33.Rüegg CR, Chiquet-Ehrismann R, Alkan SS. Tenascin, an extracellular matrix protein, exerts immunomodulatory activities. Proc Natl Acad Sci USA. 1989;86:7437–41. doi: 10.1073/pnas.86.19.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trentham DE, Dynesius-Trentham RA, Orav EJ, et al. Effects of oral administration of type II collagen on rheumatoid arthritis. Science. 1993;261:1727–30. doi: 10.1126/science.8378772. [DOI] [PubMed] [Google Scholar]
- 35.Sieper J, Kary S, Sörensen H, et al. Oral type II collagen treatment in early rheumatoid arthritis. A double-blind, placebo-controlled, randomized trial. Arthritis Rheum. 1996;39:41–51. doi: 10.1002/art.1780390106. [DOI] [PubMed] [Google Scholar]
- 36.Chimal-Monroy J, Bravo-Ruiz T, Krötzsch-Gómez FE, Díaz de León L. Implantes de Fibroquel aceleran la formación de hueso nuevo en defectos óseos inducidos experimentalmente en cráneos de rata. Rev Biomed. 1997;8:81–8. [Google Scholar]
- 37.Hynes RO, Lander AD. Contact and adhesive specifities in the associations, migration, and targeting of cells and axons. Cell. 1992;68:303–22. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- 38.Shingu M, Nagai Y, Isayama T, et al. The effects of cytokines on metalloproteinase inhibitors (TIMP) and collagenase production by human chondrocytes and TIMP production by synovial cells and endothelial cells. Clin Exp Immunol. 1993;94:145–9. doi: 10.1111/j.1365-2249.1993.tb05992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolhain RJEM, Tak PP, Dijkmans BAC, et al. Methotrexate reduces inflammatory cell numbers, expression of monokines and of adhesion molecules in synovial tissue of patients with rheumatoid arthritis. Br J Rheumatol. 1998;37:502–8. doi: 10.1093/rheumatology/37.5.502. [DOI] [PubMed] [Google Scholar]
- 40.Youssef PP, Triantafillou S, Parker A, et al. Variability in cytokine and cell adhesion molecule staining in arthroscopic synovial biopsies: quantification using color video image analysis. J Rheumatol. 1995;24:2291–8. [PubMed] [Google Scholar]
- 41.Cicuttini FM, Martin M, Boyd AW. Cytokine induction of adhesion molecules on synovial type B cells. J Rheumatol. 1994;21:406–12. [PubMed] [Google Scholar]
- 42.Paleolog EM, Hunt M, Elliott MJ, et al. Deactivation of vascular endothelium by monoclonal anti-tumor necrosis factor α antibody in rheumatoid arthritis. Arthritis Rheum. 1996;39:1082–91. doi: 10.1002/art.1780390703. [DOI] [PubMed] [Google Scholar]
- 43.Crofford LJ, Wilder RL, Ristimäki AP, et al. Cyclooxigenase-1 and -2 expression in rheumatoid synovial tissues. J Clin Invest. 1994;93:1095–101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sklatavala J, Sarsfield SJ, Townsend Y. Purification of two immunologically different leukocyte proteins that cause cartilage resorption lymphocyte activation and fever. J Exp Med. 1985;162:1208–15. doi: 10.1084/jem.162.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakisaka S, Suzuki N, Saito N, et al. Possible correction of abnormal rheumatoid arthritis synovial cell function by jun D transfection in vitro. Arthritis Rheum. 1998;41:470–81. doi: 10.1002/1529-0131(199803)41:3<470::AID-ART14>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 46.Gowen M, Wood DD, Ihrie EJ, et al. An interleukin-1 like factor stimulates bone resorption in vitro. Nature. 1983;306:378–81. doi: 10.1038/306378a0. [DOI] [PubMed] [Google Scholar]
- 47.Van De Loo AAJ, Arntz OJ, Van Den Berg VW. Flare-up of experimental arthritis in mice with murine recombinant IL-1. Clin Exp Immunol. 1992;87:196–202. doi: 10.1111/j.1365-2249.1992.tb02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cooper WO, Fava RA, Gates CA, et al. Acceleration of onset of collagen-induced arthritis by intra-articular injection of tumour necrosis factor or transforming growth factor-beta. Clin Exp Immunol. 1992;89:244–50. doi: 10.1111/j.1365-2249.1992.tb06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wakisaka S, Suzuki N, Takeba Y, et al. Modulation by proinflammatory cytokines of Fas/Fas ligand-mediated apoptotic cell death of synovial cells in patients with RA. Clin Exp Immunol. 1998;114:119–28. doi: 10.1046/j.1365-2249.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul HL. Activators and target genes of Rel/NF-kappa B transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 51.Tegeder I, Pfeilschifter J, Geisslinger G. Cyclooxigenase-independent actions of cyclooxigenase inhibitors. FASEB J. 2001;15:2057–72. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H-G, Huang N, Liu D, et al. Gene therapy that inhibits nuclear translocation of nuclear factor κB results in tumor necrosis factor a-induced apoptosis of human synovial fibroblasts. Arthritis Rheum. 2000;43:1094–105. doi: 10.1002/1529-0131(200005)43:5<1094::AID-ANR20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]


