Abstract
The aim of the present study was to evaluate levels of soluble CD 163 in sera and fluids from rheumatoid arthritis (RA) patients and elucidate the mechanism that regulates the shedding of CD163. Levels of soluble CD163 in sera and fluids from RA patients were examined by a sandwich enzyme immunoassay and Western blotting. To determine the effects of tissue inhibitors of metalloproteinase (TIMPs) on the shedding of CD163 from monocytes/macrophages, levels of soluble CD163 in cultures of monocytes/macrophages and the expression of CD163 on monocytes/macrophages in the presence or absence of TIMPs were examined by a sandwich enzyme immunoassay and flow cytometry, respectively. The clinical marker that was most associated with serum levels of soluble CD163 was levels of CRP. TIMP-3, but not TIMP-1 or TIMP-2, inhibited the shedding of CD163 from monocytes/macrophages. It was shown that serum levels of soluble CD163 are a sensitive and reliable marker to monitor activated macrophages in synovitis from RA patients and the results imply that the responsible proteinase for the shedding of CD163 is not a member of the matrix metalloproteinases, but is likely to be a member of ADAMs.
Keywords: rheumatoid arthritis, soluble CD163, TIMP-3
INTRODUCTION
The CD163 molecule is expressed selectively on most macrophages in human tissue and at least 10–30% of monocytes [1–3]. This restricted expression suggests that the CD163 molecule is a useful marker to identify macrophages in tissue. The structure of CD163 gene belongs to the scavenger receptor family [4] and was shown recently to be a receptor for the haptoglobin–haemoglobin complex [5]. It has been reported that anti-CD163 antibody induces the secretion of proinflammatory cytokines in macrophages, and casein kinase II and protein kinase C are involved in this signalling mechanism [6,7], suggesting that the binding of haptoglobin–haemoglobin to the CD163 molecule could transduce intracellular signals in macrophages. Additionally, several lines of evidence suggest that the population of CD 163-positive macrophages has some function in down-regulating the inflammatory process [3,8,9].
Interestingly, the treatment of CD163-positive monocytes with phorbol 12-myristate 13-actate (PMA) has induced the shedding of membraneous CD163 [3,10]. This process was inhibited strongly by protease inhibitors. Thus, it is considered that soluble CD163 of sera and fluids could be a specific marker for the activation of tissue macrophages.
Recently, Sulahian et al. established the ELISA system for soluble CD163 [11] and it was reported that soluble CD163 is an abundant plasma protein valuable in monitoring patients with infection and myelomonocytic leukaemia [12]. However, there are no reports of levels of soluble CD163 in sera or fluids from inflammatory diseases.
A characteristic event in synovial tissues from rheumatoid arthritis (RA) patients is the infiltration of the synovial membrane by large numbers of highly activated macrophages. The degree of macrophage infiltration of rheumatoid joints has been reported to correlate with both the activity of the disease and the progression of joint destruction [13]. There is a need for a clinically useful macrophage activation marker. However, synovial biopsy is not available universally and macrophage-specific products are not plentiful.
The aim of the present study was to evaluate levels of soluble CD 163 in sera and fluids from RA patients and elucidate the mechanism that regulates shedding of CD163 by examining the effects of tissue inhibitors of metalloproteinases (TIMPs).
MATERIALS AND METHODS
Reagents
Anti-CD163 monoclonal antibodies (R20 and D7, IgG1) were produced by immunizing synovial cells from RA patients into Balb/c mice and fusing spleen cells from immunized mice with NS-1 cells, as described previously [14]. These antibodies were defined to be anti-CD163 by their reactivity to HEK293 cells transfected with pBKCMV inserted with full-length CD163 cDNA [2] by flow cytometric and Western blot analysis.
Study population
The mean age of healthy controls (16 women and two men) was 54 years (range 34–75). All 66 RA patients fulfilled the 1987ACR criteria for RA [15]. The mean age of the 49 patients (45 women and four men) in Fig. 3a (later) was 55 years (range 35–71). There were no significant differences regarding age and sex between healthy controls and 49 RA patients. In the 49 RA patients, three patients did not take any disease-modifying drugs, five patients took non-steroidal anti-inflammatory drugs, and others took either methotrexate, d-penicillamine, gold salt, salazopyrazone, bucillamine, less than 7·5 mg of predonisone or combinations of these. Synovial fluids and sera from 18 RA patients were used for the analysis of paired samples.
Fig. 3.

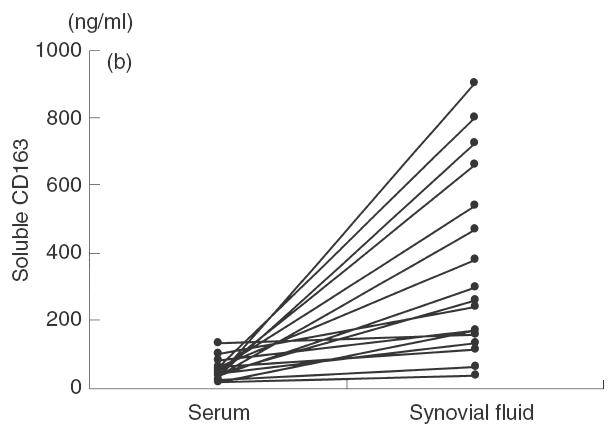
Levels of soluble CD163 in sera and synovial fluids. (a) Levels of soluble CD163 were measured in sera from healthy donors and RA patients as described in Materials and methods. Symbols represent individual samples. Lines represent the mean value in each group. The asterisk in (a) indicates statistically significant difference at P < 0·0001. (b) Levels of soluble CD163 were measured in paired sera and synovial fluids. Symbols represent individual samples and the lines connect paired samples in RA patients.
Samples
Whole peripheral blood was drawn from healthy donors and RA patients for serum, and synovial fluid was drawn from swelling knee joints of RA patients. Blood and synovial fluids were allowed to clot for 2 h at room temperature, then centrifuged at 500 g for 10 min. Samples were frozen at −80°C until use.
Monocytes from healthy donors were prepared as described previously [16]. Peripheral blood counts, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and immunoglobulin G (IgG) were measured by routine methods in the hospital clinical laboratory. Informed consent was obtained from all donors in accordance with the human investigation committee requirements of Kagoshima Red Cross Hospital.
Preparation of recombinant soluble CD163 and native soluble CD163 from RA synovial fluids
For the purification of recombinant soluble CD163, polymerase chain reaction (PCR) products of CD163 encoding Kozak and coding sequences without membrane and cytoplasmic domains were amplified (positions −25–2811) from pBK-CMV inserted with the full-length CD163 cDNA [2] by using the following primers: 5′-GAAGTTATAAATCTTTGGAATGAGC-3′ (forward), 5′-CACACGTCCAGAACAGGAAGT-3′ (reverse). The reaction was carried out for 25 cycles at 94°C for 1 min, 55°C for 1 min and 72°C. This PCR product was ligated to the pIB/V5-His vector (Invitrogen, Carlsbad, CA, USA). For the sequence of inserted PCR products, HincII digested four fragments were ligated to pBluescript II SK(–) (Stratagene, LaJolla, CA, USA). Sequencing of the cDNA indicated that G at position 874 was replaced by T, compared to the previous report [2]. Sf21 cells were transfected with the vector inserted with CD163 using Insectin-Plus (Invitrogen) and CD163-transfected cells were screened at 100 µg/ml of blasticidine S (Invitrogen). The supernatants of blasticidine-resistant Sf21 cells were collected and purified by talon metal affinity resins according to the manufacture's protocol (Clontech, Palo Alto, CA, USA) or D7 antibody-coated beads.
For the purification of native soluble CD163, the synovial fluid from RA patients was precipitated with 60% (NH4)2SO4. The precipitate was dissolved and dialysed in 0·01 m phosphate buffer, pH 7·4, 140 mm NaCl (PBS), and the dialysed sample was bound to D7 antibody-coated beads and eluted from D7 antibody-coated beads by 10 mm sodium acetate buffer, pH 2·5. The purity of recombinant and native soluble CD163 was identified by silver-stained gel after SDS-PAGE and the concentration of purified recombinant and native soluble CD163 was determined by a protein assay kit (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as standard.
Establishment of ELISA system of soluble CD163
Concentrations of soluble CD163 were determined by a sandwich ELISA system as established by Sulahian et al. [11], using the combination of Mac 2–158 and biotinylated R20 instead of biotinylated RM3/1. Diluted samples ranging from 1 : 20 to 1 : 50 were measured in duplicate.
Treatment of macrophages with TIMPs
Monocytes from healthy donors were treated with dexamethasone (Sigma, Tokyo, Japan) as described by Hogger et al. [10]. After 2 days, cells were treated with recombinant TIMP-1, purified natural TIMP-2 and recombinant TIMP-3 as described previously [17] for 20 min and then stimulated with 100 nm of PMA (Sigma) for 2 h at 37°C. Cells were stained with R20 and followed with by FITC-conjugated antimurine IgG. Stained cells were analysed by flow cytometry. The culture supernatants were collected and of amounts of soluble CD163 were measured.
Immunoprecipitation
An aliquot of 500 µl of serum and synovial fluid from the same patient and the culture supernatant were immunoprecipitated with D7-coated beads as described previously [18]. The immunoprecipitates were analysed on SDS polyacrylamide 10% gels under non-reducing conditions and proteins were transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia, Tokyo, Japan) by Western blotting and followed by biotinylated R20 antibody and streptavidin-peroxidase (Zymed, South San Francisco, CA, USA). The blots were incubated in ECL reagents (Amersham) and the chemiluminescence of the blot was detected on Hyperfilm ECL (Amersham).
Statistical analysis
The Mann–Whitney U-test was used to examine the differences of serum soluble CD163 values among the groups shown in Fig. 3 (later).
The univariate regression analysis was used to examine the correlation between serum levels of soluble CD163 and clinical markers in Fig. 4 (later). Furthermore, stepwise regression analysis was used to identify clinical marker (s) best associated with serum levels of soluble CD163.
Fig. 4.
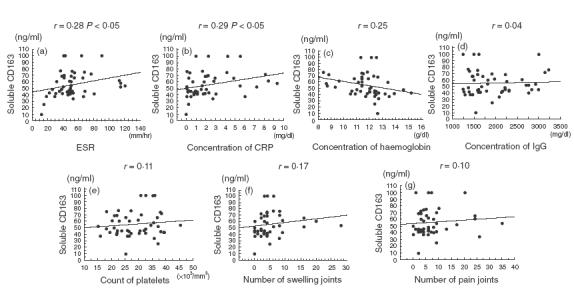
Correlation between levels of serum soluble CD163 and clinical parameters. Symbols represent individual patients and the lines represent regression lines. Statistical correlation is significant at (a) and (b).
Differences with a confidence level of>95% were considered statistically significant.
RESULTS
Elevated levels of soluble CD163 in RA sera and synovial fluids
In accordance with the presence of soluble CD163 in sera from healthy donors [11], we confirmed the presence of soluble CD163 in RA sera and synovial fluids by Western blot (Fig. 1). The concentration of soluble CD163 in the synovial fluid was higher than that in simultaneously obtained serum samples, and the molecular weight of these molecules was approximately 130 kDa, similar to that in the culture supernatant from monocytes/macrophages pretreated with PMA.
Fig. 1.

Soluble CD163 in RA serum and synovial fluid. The equal volume of serum and synovial fluid was immunoprecipitated with anti-CD163 antibody (D7)-conjugated sepharose. The immunoprecipitates were analysed as described in Material and methods. Lanes were as follows: lanes 1, 4: culture supernatant of monocytes/macrophages treated with PMA; lanes 2, 5: RA serum; lanes 3, 6: RA synovial fluid. Lanes were treated as follows: lanes 1, 2 and 3 were reacted with biotinylated anti-CD163 antibody (R20); lanes 4, 5 and 6 were reacted with biotinylated irrelevant antibody (IgG1).
To measure levels of soluble CD163 in sera and fluids from RA patients, we established an ELISA system. In this ELISA system, the recombinant soluble CD163 was used as the standard protein. Figure 2 shows the purified recombinant soluble CD163 with a molecular weight of approximately 100 kDa. The assay had a sensitivity of 0·4 ng/ml and a linear range between 0·4 ng/ml and 20 ng/ml. The variation coefficients of intra- and interassay in this ELISA were within 10%. To exclude non-specific binding of rheumatoid factors in this ELISA system, CD14 antibodies (murine IgG1) [18] were also used as the capture antibody instead of anti-CD163 antibody. In the ELISA system using anti-CD14 antibody, we did not find any false positive RA sera and fluids. As shown in Fig. 3a, the level of soluble CD163 in sera from RA patients was significantly higher than that from healthy donors. When levels of soluble CD163 were compared between paired sera and synovial samples, levels of soluble CD163 in synovial fluids were consistently higher than those in paired sera (Fig. 3b). In one patient, the level of soluble CD163 in synovial fluid was 22 times higher than that in the respective serum sample.
Fig. 2.
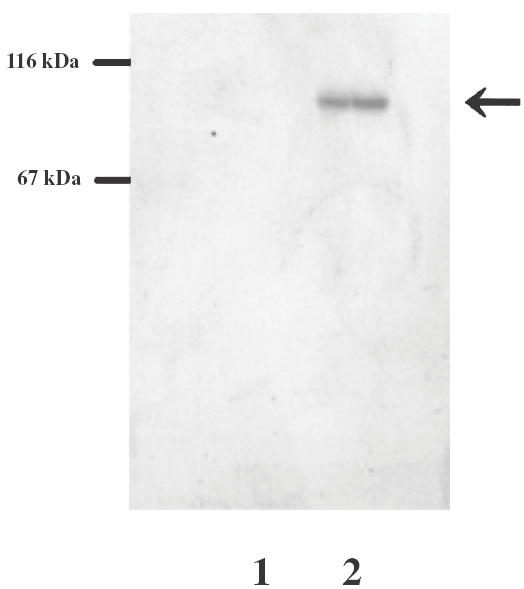
SDS-PAGE analysis of recombinant soluble CD163. The culture supernatant of Sf21 cells transfected with PIB-His-CD163 were immunoprecipitated with anti-CD163 antibody-conjugated sepharose and electrophoresed on a 10% polyacrylamide gel. The gels was silver-stained. Eluates from anti-CD163-conjugated sepharose incubated with the culture supernatant with PIB-His vector alone (lane 1) or PIB-His-CD163cDNA (lane 2). The arrow indicates recombinant soluble CD163.
Correlation between serum levels of soluble CD163 and clinical markers in RA patients
We examined the correlations between serum levels of soluble CD163 and clinical markers in RA patients. As shown in Fig. 4, weak correlations were found between levels of soluble CD163 and levels of ESR or levels of CRP. On the other hand, there was no correlation with haemoglobin levels, IgG levels, platelet numbers, numbers of swelling joints or numbers of painful joints. When stepwise regression analysis was used to identify clinical marker(s) best associated with serum levels of soluble CD163, levels of CRP were most associated with serum levels of soluble CD163 (P = 0·042).
Inhibition of the shedding of CD163 from monocytes/macrophages by TIMP-3
It was demonstrated that PMA induced the shedding of CD163 from monocytes and this cleavage was prevented by protease inhibitor cocktail against serine-, cysteine- and metalloproteases [10]. To characterize further the effects of proteinase inhibitors on the shedding of CD163, the effects of TIMPs were examined with monocytes/macrophages on the production of soluble CD163 in the culture mediums following PMA treatment. As shown in Fig. 5, TIMP-3 but not TIMP-1 or TIMP-2 prevented a decrease in CD163 expression on monocytes/macrophages treated with PMA. Consistent with this finding, the release of soluble CD163 into the culture medium was suppressed by TIMP-3 with an IC50 value at approximately 70 nm, but not TIMP-1 and TIMP-2 (Fig. 6).
Fig. 5.
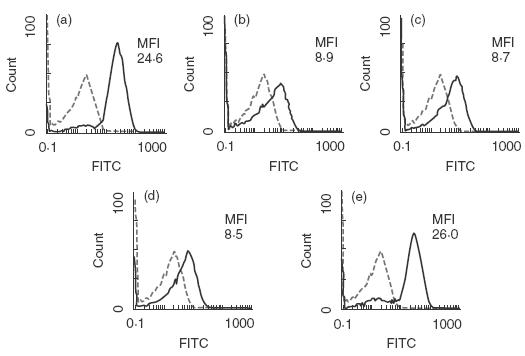
Inhibition of shedding of CD163 by TIMP-3. Monocytes/macrophages pretreated with glucocorticoid were treated with (a, b) buffer, (c) TIMP-1, (d) TIMP-2 and (e) TIMP-3 at a concentration of 0·25 µm. Subsequently (b, c, d and e) were activated with PMA to induce shedding of CD163. The expression of CD163 was analysed by the flow cytometry as described in Materials and methods. Broken lines show control staining and MFI is the abbreviation of mean fluorescence intensity of stained cells. The data are representative of three experiments.
Fig. 6.
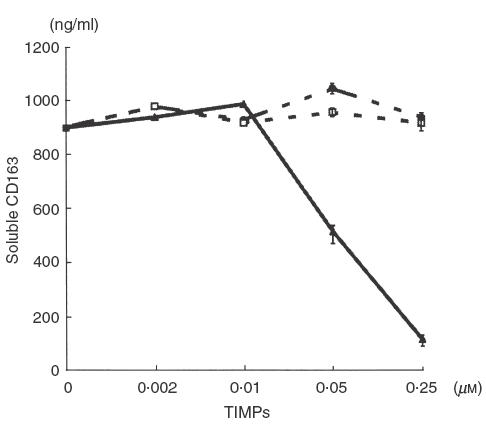
Decreased soluble CD163 production from monocyte/macrophage treated with TIMP-3. Monocytes/macrophages pretreated with glucocorticoid were treated with buffer, TIMP-1, TIMP-2 and TIMP-3 at the indicated concentration and were then activated with PMA to induce shedding of CD163. Levels of soluble CD163 in these cultures supernatants were measured by an ELISA as described in Materials and methods. The values were expressed as the mean ± standard error (at concentrations of 0·05 and 0·25 µm). The data are representative of three experiments. •, TIMP-1; □, TIMP-2; ▴, TIMP-3.
DISCUSSION
This study has demonstrated that levels of soluble CD163 in the sera of RA were higher than those of healthy donors. Furthermore, levels of soluble CD163 in RA synovial fluids were consistently higher than those in paired sera, supporting the hypothesis that a large portion of soluble CD163 originates from synovial tissue macrophages.
The clinical marker that was most associated with serum levels of soluble CD163 was levels of CRP. It has been known that the variation of CRP is a reliable marker of RA activity [19]. Additionally, previous reports have demonstrated elevated serum levels of cytokines such as TNF-α, IL-1, IL-6, IL-8 and soluble TNF receptor [19–22], soluble CD14 [18,20], neopterine [20], lysozyme [23], MRP8 and MRP14 [24] in active RA patients. CRP and cytokines reflect systemic rather than local activity in inflammation process. Weak correlations between serum levels of soluble CD163 and levels of CRP might be explained by this point. Soluble CD14, lysozyme, neopterin, MRP8 and MRP14 come from activated monocytes, neutrophils, and/or activated T cells, in addition to the production from activated macrophages. Therefore, strictly speaking these serum markers do not necessarily reflect the activity of macrophages. In fact, in our previous studies we have shown that levels of soluble CD14 in RA synovial fluids were not necessarily higher from those in paired sera [18], suggesting that levels of soluble CD14 in sera may reflect the activation state of peripheral blood monocytes in addition to synovial macrophages. In this regard, elevated serum levels of soluble CD163 in RA patients are a useful marker that reflects activated synovial macrophages. Further examination will be necessary to define the clinical benefits of soluble CD163.
In vitro, cleavage of the cell surface molecule is often enhanced by activation of protein kinase C by a phorbol ester, is generally close to the extracellular face of plasma membrane and is sensitive to peptide hydroxamate metalloproteinase inhibitors [25], but how shedding is regulated remains largely unknown. TIMP-3 was shown to have inhibitory activity against MMPs and aggrecanase 1 and 2 [17]. In addition, TIMP-3 inhibits the shedding of cell surface-anchored molecules such as TNF-α, l-selectin, IL-6 receptor and syndecans -1 and -4, which is thought to be catalysed by membrane-bound metalloproteinases, ADAMs [26–29]. In the present experiments, TIMP-3 prevented the shedding of CD163 within concentrations shown to be effective for the inhibition of these metalloproteinases and aggrecanases [17]. TIMP-3 was demonstrated to be present in the RA synovial lining layer [30] and up-regulated by TGF-β, platelet-derived growth factor, epidermal growth factor and IL-1α[31] that are also present in rheumatoid synovium. Thus, the present finding suggests that the interaction of ADAMs and TIMP-3 may be involved in the shedding of CD163. We are currently investigating which metalloproteinases are responsible for CD163 shedding.
Furthermore, it was postulated that the haptoglobin–haemoglobin complex activates macrophages, judging from the finding that anti-CD163 antibody induced the secretion of proinflammatory cytokines in macrophages [6]. Elevated levels of soluble CD163 in vivo may inhibit these functions of the haptoglobin–haemoglobin complex and consequently suppress RA activity. Interestingly, it has been shown that glucocorticoid and IL-10 with anti-inflammatory properties induced the expression of CD163 and the population of CD163-positive macrophages secrete proteins with anti-inflammatory properties [3,8]. Taken together with previous findings, it is supposed that the CD163 molecule may be anti-inflammatory in vivo.
In summary, it has been shown that CD163 level is a sensitive and reliable marker to monitor activated macrophages in synovitis from RA patients.
Acknowledgments
This work was supported by the grant from the Ministry of Japan Culture, Education and Science. The authors would like to thank Mai Yamamoto for her excellent assistance in the preparation of manuscript.
REFERENCES
- 1.Law SKA, Micklem KJ, Shaw JM, et al. A new macrophage differentiation antigen which is a member of the scavenger receptor superfamily. Eur J Immunol. 1993;23:2320–51. doi: 10.1002/eji.1830230940. [DOI] [PubMed] [Google Scholar]
- 2.Hogger P, Dreier J, Droste A, Buck F, Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163) J Immunol. 1998;161:1891–900. [PubMed] [Google Scholar]
- 3.Sulahian TH, Hogger P, Wahner AE, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–21. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- 4.Ritter M, Buechler C, Langmann T, Schmitz G. Genomic organization and chromosomal localization of the human CD163 (M130) gene: a member of the scavenger receptor cystein-rich superfamily. Biochem Biophys Res Commun. 1999;260:466–74. doi: 10.1006/bbrc.1999.0866. [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 6.Van den Heuvel MM, Tensen CP, van As JH, et al. Regulation of CD163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–66. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- 7.Ritter M, Buechler C, Kapinsky M, Schmitz G. Interaction of CD163 with the regulatory subunit of casein kinaseII (CKII) and dependence of CD163 signalling on CKII and protein kinase C. Eur J Immunol. 2001;31:999–1009. doi: 10.1002/1521-4141(200104)31:4<999::aid-immu999>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Hamann W, Floter A, Schmultzler W, Zwadlo-Klarwasser G. Characterization of a novel anti-inflammatory factor produced by RM3/1 macrophages derived from glucocorticoid treated human monocytes. Inflam Res. 1995;44:535–40. doi: 10.1007/BF01757358. [DOI] [PubMed] [Google Scholar]
- 9.Hogger P, Sorg C. Soluble CD163 inhibits phorbol ester-induced lymphocyte proliferation. Biochem Biophys Res Commun. 2001;288:841–3. doi: 10.1006/bbrc.2001.5845. [DOI] [PubMed] [Google Scholar]
- 10.Droste A, Sorg C, Hogger P. Shedding of CD163, a novel regulatory mechanism for a member of the scavenger receptor cystein-rich family. Biochem Biophys Res Commun. 1999;256:110–3. doi: 10.1006/bbrc.1999.0294. [DOI] [PubMed] [Google Scholar]
- 11.Sulahian TH, Hintz KA, Wardwell K, Guyre PM. Development of an ELISA to measure soluble CD163 in biological fluids. J Immunol Meth. 2001;252:25–31. doi: 10.1016/s0022-1759(01)00328-3. [DOI] [PubMed] [Google Scholar]
- 12.Moller HJ, Peterslund NA, Graversen JH, Moestrup SK. Identification of the hemoglobin scavenger receptor/CD163 as a natural soluble protein in plasma. Blood. 2002;99:378–80. doi: 10.1182/blood.v99.1.378. [DOI] [PubMed] [Google Scholar]
- 13.Bulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage population and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–241. doi: 10.1002/art.1780390116. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama T, Yamada A, Kay J, et al. Activation of CD4 cells by fibronectin and anti-CD3 antibody: a synergic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989;170:1133–48. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Athritis Rheum. 1987;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Matsuyama T, Nakashima N, Matsuda T, Nakamura H, Uchida S, Abe T. Induction of multinucleated giant cells from rheumatoid arthritis (RA) synovial adherent cells by anti-DR antibody. Clin Exp Immunol. 1994;98:257–63. doi: 10.1111/j.1365-2249.1994.tb06135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–4. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 18.Yu S, Nakashima N, Xu BH, et al. Pathological significance of elevated soluble CD14 production in rheumatoid arthritis: in the presence of soluble CD14, lipopolysaccharides at low concentrations activate RA synovial fibroblasts. Rheumatol Int. 1998;17:237–43. doi: 10.1007/s002960050041. [DOI] [PubMed] [Google Scholar]
- 19.Bodolato R, Oppenheim JJ. Role of cytokines, acute phase proteins, and chemokines in the progression of rheumatoid arthritis. Semin Arthritis Rheum. 1996;26:526–38. doi: 10.1016/s0049-0172(96)80041-2. [DOI] [PubMed] [Google Scholar]
- 20.Horneff G, Sack U, Kalden JR, Emmrich F, Burmester GR. Reduction of monocyte–macrophage activation markers upon anti-CD4 treatment. Decreased levels of IL-1, IL-6, neopterin and soluble CD14 in patients with rheumatoid arthritis. Clin Exp Immunol. 1993;91:207–131. doi: 10.1111/j.1365-2249.1993.tb05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohshima S, Saeki Y, Mima T, et al. Long-term follow-up of the changes in circulating cytokines, soluble cytokine receptors, and white blood cell subset counts in patients with rheumatoid arthritis (RA) after monoclonal anti-TNF alpha antibody therapy. J Clin Immunol. 1999;19(5):305–13. doi: 10.1023/a:1020543625282. [DOI] [PubMed] [Google Scholar]
- 22.Charles P, Elliott MJ, Davis D, et al. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999;163:1521–8. [PubMed] [Google Scholar]
- 23.Torsteinsdottir I, Hakansson L, Hallgren R, et al. Serum lysozyme: a potential marker of monocyte/macrophage activity in rheumatoid arthritis. Rheumatology. 1999;38:1249–54. doi: 10.1093/rheumatology/38.12.1249. [DOI] [PubMed] [Google Scholar]
- 24.Frosch M, Strey A, Vogel T, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43:628–37. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 25.Hooper NM, Turner AJ. Membrane protein secretases. Biochem Soc Trans. 1999;27:211–57. doi: 10.1042/bst0270255. [DOI] [PubMed] [Google Scholar]
- 26.Smith MR, Kung H, Durum SK, Colburn NH, Sun Y. TIMP-3 induces cell death by stabilizing TNF-alpha receptors on the surface of human colon carcinoma cells. Cytokine. 1997;9:770–80. doi: 10.1006/cyto.1997.0233. [DOI] [PubMed] [Google Scholar]
- 27.Borland G, Murphy G, Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of l-selectin from leukocytes. J Biol Chem. 1999;274:2810–5. doi: 10.1074/jbc.274.5.2810. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves PG, Wang F, Antcliff J, et al. Human myeloma cells shed the interleukin-6 receptor: inhibition by tissue inhibitor of metalloproteinase-3 and a hydroxamate-based metalloproteinase inhibitor. Br J Haematol. 1998;101:694–702. doi: 10.1046/j.1365-2141.1998.00754.x. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and-4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3 sensitive metalloproteinase. J Cell Biol. 2000;148:811–24. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takizawa M, Ohuchi E, Yamanaka H, et al. Production of tissue inhibitor of metalloproteinases 3 is selectively enhanced by calcium pentosan polysulfate in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2000;43:812–20. doi: 10.1002/1529-0131(200004)43:4<812::AID-ANR11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 31.Fabunmi RP, Baker AH, Murray EJ, Booth RF, Newby AC. Divergent regulation by growth factors and cytokines of 95kDa and 72kDa gelatinase and tissue inhibitors of metalloproteinase-1, -2, and -3 in rabbit aortic smooth muscle cells. Biochem J. 1996;315:335–42. doi: 10.1042/bj3150335. [DOI] [PMC free article] [PubMed] [Google Scholar]


