Abstract
The type 2 helper T cell (TH2) cytokine interleukin (IL)-4 is thought to play a central role in the early stages of asthma. In an effort to develop an antibody treatment for asthma that neutralizes the effects of IL-4, a murine monoclonal antibody, 3B9, was generated with specificity for human IL-4. In vitro studies demonstrated that 3B9 inhibited IL-4-dependent events including IL-5 synthesis, TH2 cell activation and up-regulation of immunoglobulin E expression. 3B9 was then humanized (pascolizumab, SB 240683) to reduce immunogenicity in humans. SB 240683 demonstrated species specificity for both monkey and human IL-4 with no reactivity to mouse, rat, cow, goat or horse IL-4. Pascolizumab inhibited the response of human and monkey T cells to monkey IL-4 and effectively neutralized IL-4 bioactivity when tested against several IL-4-responsive human cell lines. Affinity studies demonstrated rapid IL-4 binding by pascolizumab with a slow dissociation rate. In vivo pharmacokinetic and chronic safety testing in cynomolgus monkeys demonstrated that pascolizumab was well tolerated, and no adverse clinical responses occurred after up to 9 months of treatment. Three monkeys developed an anti-idiotypic response that resulted in rapid pascolizumab clearance. However, in the chronic dosing study the antibody response was transient and not associated with clinical events. In conclusion, pascolizumab is a humanized anti-IL-4 monoclonal antibody that can inhibit upstream and downstream events associated with asthma, including TH2 cell activation and immunoglobulin E production. Clinical trials are under way to test the clinical efficacy of pascolizumab for asthma.
Keywords: asthma, interleukin-4, monoclonal antibody, preclinical safety, toxicology
INTRODUCTION
Although the cause of asthma has not been defined completely, it is clear that asthma is the result of a series of cellular and cytokine-mediated events that induce chronic airway inflammation. Interleukin (IL)-4 is thought to be a key cytokine in the early stages of asthma because of its role in regulating B-cell isotype switching to immunoglobulin (Ig)E production, eosinophil chemotaxis and the development of effector T-cell responses [1,2], Interleukin-4 is produced by T lymphocytes, activated mast cells and basophils. Along with other cytokines (including IL-5 and IL-13), IL-4 can induce the development of allergic inflammatory diseases and can promote the differentiation of undifferentiated helper T cells (TH0) into type 2 helper T cells (TH2) [3,4]. These TH2 cells, in turn, secrete a pattern of cytokines including IL-3, IL-4, IL-5, IL-13 and granulocyte-macrophage colony-stimulating factor [5] that initiate and perpetuate the asthmatic inflammatory response leading to airway inflammation, obstruction and hyperresponsiveness characteristic of chronic asthma [6]. Both IL-4 and IL-5 mRNA and protein are elevated in asthmatic airway tissues [7,8].
Inhibition of IL-4 activity could potentially reduce the pulmonary inflammation and remodelling that define chronic persistent asthma. Because TH2 differentiation is IL-4-dependent, IL-4 neutralization may inhibit the development of TH2 cells and the subsequent events that lead to allergic inflammation [9]. Also, as IL-4 induces IL-5 synthesis, IL-4 neutralization may also block IL-5-dependent pulmonary eosinophilia. Inhibition of IL-4 may also reduce aberrant IgE production and subsequent IgE-mediated, mast cell-dependent inflammation. Furthermore, because IL-4 up-regulates collagen and fibronectin synthesis in subepithelial fibroblasts (leading to airway remodelling), inhibiting IL-4 may prevent long-term reduction in pulmonary function [10]. Finally, animal studies have revealed that IL-4 knockout mice sensitized to antigen were unable to develop allergic eosinophilic airway infiltration and did not produce antigen-specific IgE following exposure to aerosolized antigen [11]. Additionally, these mice failed to develop airway hyperresponsiveness following chronic aerosol exposure to antigen.
Interleukin-13 is related closely to IL-4 and has similar downstream functions. Produced by activated TH2 cells, TH0 cells, mast cells and dendritic cells, IL-13 can also stimulate IgE production by B cells [12]. However, because T cells do not express IL-13 receptors, IL-13 does not promote TH2 responses or suppress TH1 cell differentiation as does IL-4 [13]. Because of this difference in function, it is possible that suppressing IL-4 will prevent IL-13 up-regulation. Thus, although other cytokines are involved in the development of asthma, the neutralization of IL-4 alone may be sufficient to decrease eosinophil accumulation in the airways and to reduce lung airway remodeling in asthmatic patients.
Studies in murine models of asthma have demonstrated that both anti-IL-4 antibodies and soluble IL-4 receptors can block the downstream events associated with asthma. The administration of an aerosolized, soluble, murine recombinant IL-4 receptor inhibited IL-4 activity in mice and prevented the development of allergen-induced and allergen-dependent immediate hypersensitivity responses [14]. Similarly, in a murine model of atopic asthma, mice treated with anti-IL-4 monoclonal antibody (MoAb) prior to active sensitization with ovalbumin showed lower serum IgE levels compared with saline-treated controls [15].
Pascolizumab (SB 240683) is a humanized anti-IL-4 MoAb developed originally by GlaxoSmithKline (Philadelphia, PA, USA) and currently in development at Protein Design Laboratories, Inc. (Fremont, CA, USA). Pascolizumab blocks the interaction of IL-4 with its receptor (Fig. 1), thereby inhibiting the early events of asthma including TH2 cell differentiation, eosinophilia and IgE up-regulation. Preclinical data indicate that blocking these events in vivo may prevent airway inflammatory cell infiltration and remodelling in asthmatic patients. In the current study, the biological activity and preclinical pharmacology of pascolizumab and its parent antibody, 3B9, were evaluated. As a prelude to clinical trials, the safety and toxicity profile of pascolizumab was evaluated in cynomolgus monkeys, the only species in which antibody cross-reactivity was demonstrated.
Fig. 1.
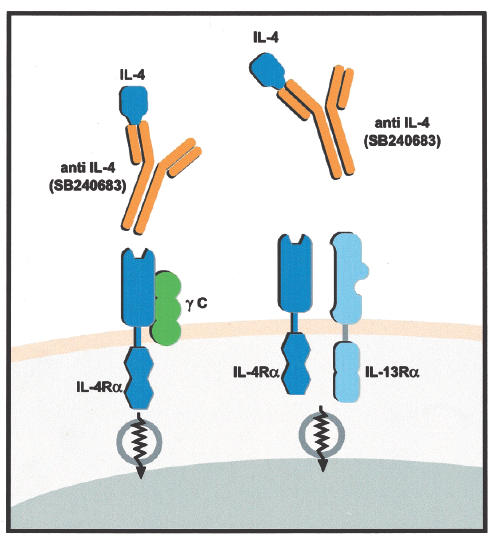
Effect of pascolizumab binding of IL-4 inhibits IL-4 interaction with the alpha chain of the IL-4 receptor. IL = interleukin; anti-IL-4 = antibody against IL-4; γ C = gamma signalling chain of the IL-4 receptor; IL-4Rα = IL-4 receptor alpha; IL-13Rα = IL-13 receptor alpha.
MATERIALS AND METHODS
Humanization of 3B9
Pascolizumab (SB 240683) is a fully humanized MoAb constructed using conventional molecular techniques to graft complementarity-determining regions from the parent murine antihuman IL-4 antibody (3B9) into human IgG1 kappa heavy- and light-chain frameworks. Substitutions were made to retain antibody activity and to reflect human Ig group/subgroup preferences (SMART™ technology; Protein Design Laboratories, Inc., Fremont, CA, USA).
Activity of pascolizumab in vitro
AffinityStudies
The kinetics of antibody binding to IL-4 were analysed at 25°C in a BIAcore biosensor. Pascolizumab was attached to the solid phase with protein A. Binding of IL-4 in solution was determined by change in refractive index. On and off rates of the antibody binding as well as the dissociation constant (Kd) were calculated. The interaction between IL-4 and pascolizumab was characterized by isothermal titration microcalorimetry and biosensor analysis.
IL-4-dependent T-cell proliferation
Human peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-paque centrifugation and incubated in 4-(2-hydroxyethyl)-1-piperazine-ethanesulphonic acid-buffered RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 1%l-glutamine and 1% penicillin-streptomycin plus 10 µg/ml phytohaemagglutinin (PHA) for 72 h at 37°C. The PHA-activated blasts were cultured (105 cells/ml) in round-bottom microtitre plate wells with human IL-4 (2 ng/ml) and pascolizumab for 3 days. Samples were pulsed with 0·5 µCi [3H]-thymidine and processed for scintillation counting in a 1205 Betaplate™ (EG & G Wallac, Inc., Gaithersburg, MD, USA).
IL-4-dependent TF-1 cell proliferation
TF-1 cells (American Type Culture Collection, a human erythroleukaemic cell line responsive to IL-4) were cultured in 4-(2-hydroxyethyl)-1-piperazine-ethanesulphonic acid-buffered RPMI-1640 medium supplemented with FBS, l-glutamine, and antibiotics. Round-bottom 96-well microtitre plates were seeded with 2·5 × 103 cells/well and incubated for 48 h in the presence of serially diluted antibody plus IL-4 (1 ng/ml). Cultures were pulsed with [3H]-thymidine and processed for scintillation counting.
IL-4-dependent IgE synthesis
Human PBMCs were separated by centrifugation over Ficoll-paque, washed, and resuspended to 1·25 × 106 cells/ml in supplemented RPMI 1640 medium. Cell suspensions (800 µl) were aliquoted into a 48-well plate along with IL-4 (3 ng/ml), hydrocortisone succinate (1 µm) and pascolizumab. Samples were incubated for 14 days at 37°C in an atmosphere of 5% CO2. At the end of the culture period, supernatants were removed and IgE concentrations measured by enzyme-linked immunosorbent assay (ELISA).
IL-4-dependent human spleen B-cell proliferation
B cells were purified from human tonsils or spleens using standard techniques and frozen in liquid nitrogen until required. Cells were resuspended to 2 × 106 viable cells per ml and incubated with phorbol 12,13-dibutyrate (10−8m) and anti-IgM antibody (1 µg/ml) for 45 min at 37°C. Cells were washed, aliquoted into microtitre plate wells and incubated with IL-4 (2 ng/ml) plus pascolizumab for 72 h. B-cell proliferation was measured by [3H]-thymidine incorporation.
IL-4-dependent CD23 expression on B cells
Purified tonsil B cells were incubated with IL-4 (1 ng/ml) plus pascolizumab for 48 h in RPMI-1640 medium with 15% FBS. Membrane-expressed CD23 Fc receptor type epsilon (FceRII; The Binding Site, Birmingham, UK) was determined by flow cytometric analysis.
Inhibition of IL-4–dependent TH2 cell differentiation by 3B9
Peripheral blood mononuclear cells from tetanus toxoid (TT)-sensitive donors were purified on Ficoll-paque and resuspended to 3 × 106 cells/ml in AIM-V medium with 15% FBS, 1 mm sodium pyruvate, and 40 nm 2-mercaptoethanol. Cells were cultured for 5 days at 37°C with adsorbed tetanus vaccine (1 : 375 dilution), IL-4 (1–2 ng/ml) and MoAb 3B9. Supernatants were removed and frozen prior to testing for IL-5. Cell proliferation was determined by [3H]-thymidine incorporation. Interleukin-5 and interferon gamma (IFN-γ) were measured with commercial ELISA kits (R&D Systems, Minneapolis, MN, USA).
Inhibition of in vivo human IgE production by MoAb 3B9
Six−8-week-old severe combined immunodeficient (SCID) mice received intraperitoneal (i.p.) injection of human PBMCs (3·5 × 107 cells/animal) from house dust mite-sensitive donors. Each mouse received five daily i.p. injections of recombinant human interleukin (rhIL)-4 (10 µg/mouse/day) starting on the day after reconstitution. Blood samples were obtained from the orbital plexus weekly (weeks 2–10). Sera were assayed for human IgE and IgG. Monoclonal antibody 3B9 (0·5–10 mg per mouse) was given as a single i.p. injection at the time of reconstitution.
Species specificity of 3B9 and pascolizumab
Species specificity was confirmed by 3B9 and pascolizumab inhibition of native and recombinant human IL-4 activity and recombinant murine, bovine or rat IL-4 in the T-cell proliferation assay. Monoclonal antibody 3B9 and pascolizumab were assessed for their recognition of supernatant components from PHA-activated guinea pig and dog spleen cells. Monoclonal antibody 3B9 binding to human and monkey IL-4 was analysed by standard ELISA. Pascolizumab inhibition of human and monkey T-cell responses to recombinant monkey IL-4 was measured.
Immunochemistry
Immunohistochemical analysis was performed to detect pascolizumab binding to normal adult male and female human tissue (31 tissue types; 1–3 donors/tissue type). Cryostat sections of surgical and autopsy specimens (fixed at 4°C in methanol/acetone) were incubated with biotinylated-pascolizumab followed by peroxidase-conjugated streptavidin. Following peroxidase development, sections were counterstained with haematoxylin. Controls included IL-4–coated sepharose beads and an isotype-matched, biotinylated-humanized IgG1 MoAb.
Single- and repeat-dose toxicity studies in cynomolgus monkeys
Cynomolgus monkeys received one intravenous (i.v.) dose of 0, 5, 50 or 500 mg/kg (three male and three female monkeys/group) of pascolizumab, followed by a second equal dose 1 month later (two male and two female monkeys/group). Changes in laboratory parameters (chemistry, urinalysis, haematological parameters including lymphocyte subsets and lymphocyte mitogen responses), electrocardiography, ophthalmological examination, histological parameters and body weight were noted in addition to adverse clinical responses for 1 month following each dose.
Chronic toxicity in cynomolgus monkeys
Three female and three male monkeys (including two female and two male monkeys per group from the previous study approximately 6 months after the last pascolizumab treatment) were administered monthly doses of pascolizumab (10 mg/kg subcutaneous [s.c.], 10 mg/kg i.v. or 100 mg/kg i.v.) or placebo for 9 months. Changes in laboratory and physical parameters (listed above) and adverse events were noted.
Assessment of anti-pascolizumab antibodies in monkey sera
Serum samples (diluted 1 : 5 in phosphate-buffered saline/0·1% bovine serum albumin) were incubated with TAG-pascolizumab (ruthenium-labelled), affinity-purified rabbit antipascolizumab antibody standard or unknown sera, and biotin–pascolizumab or biotin−3B9 for 1 h, followed (without washing) by streptavidin-coated paramagnetic microbeads for 30 min. Biotin-labelled pascolizumab would allow detection of anti-isotypic and anti-idiotypic antibody responses and biotin-labelled 3B9 would allow detection of anti-idiotypic responses. ORIGEN® Assay Buffer was added and electrochemiluminescent responses recorded on an ORIGEN® Analyser (IGEN International, Gaithersburg, MD, USA). The dynamic range for both assays was 47–6000 ng/ml.
Determination of pascolizumab in monkey plasma
The presence of pascolizumab in monkey plasma was determined using a selective electrogenerated chemiluminescence immunoassay similar to that described previously [16]. The lower limit of detection for pascolizumab was 50 ng/ml. Pharmacokinetic parameters, including the antibody half-life, peak and trough concentrations, area under the curve and maximal drug concentration, were calculated.
Animal care and use and human tissue use
All study designs were reviewed and approved by the Institutional Animal Care and Use Committees. Definitive toxicity studies were conducted in accordance with Good Laboratory Practices for Nonclinical Laboratory Studies (21 CFR, part 58). Human blood was collected from volunteer donors in the laboratory following approved consenting procedures, and unused samples were discarded.
RESULTS
In vitro characterization of the IL-4 epitope bound by 3B9 and pascolizumab
Cynomolgus monkey IL-4 has an amino acid sequence identity with human IL-4 of 93%. The IL-4 amino acid sequence in other species, including mouse, rat, cow, goat and horse, shows less homology to the human sequence. Additionally, the amino acid sequence of the IL-4 epitope bound by 3B9 and SB 240683, encompassing residues Ala70-Trp81 present in the C helix of IL-4, is 100% identical between human and monkey. Neither 3B9 nor pascolizumab recognizes IL-4 from mouse, rat, cow, goat or horse with low homology in this region.
Affinity studies
The affinity of the interactions between pascolizumab and IL-4, measured by titration microcalorimetry, indicated that binding of pascolizumab to IL-4 occurred at a molar ratio of 2 IL-4 molecules per 1 antibody molecule, with a Kd of 45 pm at 25°C. Pascolizumab was stable against thermal unfolding to at least 65°C. Biosensor studies showed rapid binding to IL-4 by pascolizumab and a slow dissociation phase. The kon and koff values were 3·4 × 106 M−1s−1 and 2 × 10−4s−1, respectively, and the Kd was 59 pm. BIAcore biosensor studies were also performed using immobilized IL-4 and soluble IL-4 receptor alpha chain-Fc. Pascolizumab inhibited the binding of IL-4 to its receptor with a 50% inhibitory concentration (IC50) of approximately 10 nm. Greater than 90% inhibition was achieved with 250 nm pascolizumab.
In vitro pharmacology of 3B9 and pascolizumab
A series of in vitro studies were conducted with the parent MoAb (3B9) and pascolizumab to determine their effects on IL-4–dependent downstream events and to confirm that the humanization process did not alter significantly the binding affinity of the antibody.
IL-5 synthesis
In vitro studies demonstrated that IL-5 syn-thesis by human PBMCs in response to the recall antigen TT was significantly increased in the presence of exogenous rhIL-4 (1–2 ng/ml; Table 1). In contrast, rhIL-4 did not influence cell proliferation or the production of the TH1 cytokine, IFN-γ. These results confirm that IL-4 is capable of preferentially stimulating the production of TH2 cytokines (e.g. IL-5) over TH1 cytokines (e.g. IFN-γ) in response to antigen. Monoclonal antibody 3B9 was tested in this model for its ability to inhibit IL-4-dependent IL-5 synthesis by PBMCs in the presence of TT (Fig. 2). Antigen-driven, IL-4-dependent IL-5 synthesis was inhibited by MoAb 3B9 in a dose-dependent manner with an IC50 = 730 pm, providing evidence that antibody neutralization of IL-4 can down-regulate TH2 cell cytokine production.
Table 1.
Effect of IL-4 on tetanus toxoid PBMC proliferation and cytokine production
| Treatment | Proliferation, cpm × 103 (s.e.m.) | IL-5, pg/ml (s.e.m.) | IFN-γ, pg/ml (s.e.m.) |
|---|---|---|---|
| Medium alone | 46·5 (3·3) | 122 (15) | 1148 (264) |
| IL-4 | 48·8 (3·0) | 298 (20)* | 1983 (258) |
IL = interleukin; PBMC = peripheral blood mononuclear cell; cpm = counts per minute; SEM = standard error of the mean; IFN-γ = interferon gamma.
P < 0·001 versus medium control.
Fig. 2.
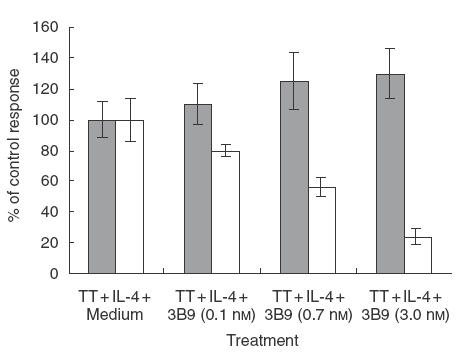
Effect of MoAb 3B9 on antigen-driven IL-4-dependent T-cell proliferation and IL-5 synthesis. Results are expressed as percentage of the control response obtained with TT-stimulated cells in the presence of IL-4. IL = interleukin; TT = tetanus toxoid.  , Cell proliferation; □, IL-5 production.
, Cell proliferation; □, IL-5 production.
T-cell proliferation
To confirm that the humanized antibody retained the ability to inhibit IL-4-dependent functions and to confirm the cross-species specificity of the antibody, pascolizumab was tested for its ability to inhibit IL-4-dependent T-cell proliferation using human or monkey T cells (Fig. 3a). Pascolizumab inhibited the response of human and monkey T cells to recombinant monkey IL-4 with mean IC50 values of 268 pm and 587 pm, respectively. Although the mean IC50 values were lower for inhibition of human and monkey T cells to recombinant human IL-4 (157 pm and 234 pm, respectively), pascolizumab was able to inhibit IL-4-induced activation in both species.
Fig. 3.
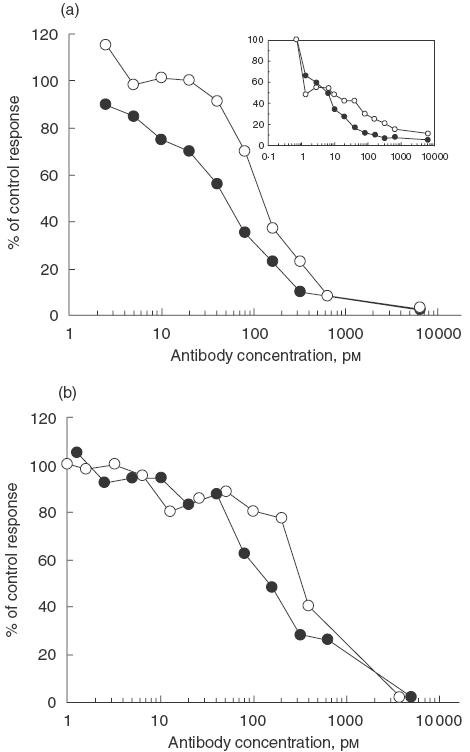
Effect of pascolizumab on human (a), cynomolgus monkey (inset) and TF-1 cell (b) IL-4-dependent T-cell proliferation. Results are expressed as percentage of the control response obtained with IL-4-stimulated cells. •, MoAb 3B9; ○, pascolizumab.
Neutralization of IL-4 bioactivity
Pascolizumab and 3B9 inhibition of IL-4 bioactivity is summarized in Table 2. Both antibody treatments inhibited the proliferation of human T cells (Fig. 3a), human B cells and TF-1 cells (Fig. 3b). Interleukin-4-dependent IgE production by human PBMCs (Fig. 4a) and up-regulation of Fc∈RII (CD23; Fig. 4b) on human B cells were also blocked by both antibodies. These in vitro analyses indicate that the antigen-binding affinity and pharmacodynamic properties were retained during the humanization of 3B9 to pascolizumab.
Table 2.
Pharmacological activity of pascolizumab in vitro*
| Antibody | T-cell proliferation IC50, pm | TF-1 cell proliferation IC25, pm | IgE production IC50, pm | CD23 expression IC50, pm | Tonsil B-cell proliferation IC50, pm |
|---|---|---|---|---|---|
| Pascolizumab | 157 | 694 | 278 | 950 | 377 |
| (113–262)5 | (334–1000)3 | (240–311)2 | (600–1300)2 | 3/4 | |
| MoAb 3B9 | 31 | 87 | 612 | 147 | 134 |
| (11–60)10 | (27–139)3 | (370–1070)6 | (53–272)3 | (80–231)4 |
IC50 = 50% inhibitory concentration; IC25 = 25% inhibitory concentration.
Mean (range)n.
Fig. 4.
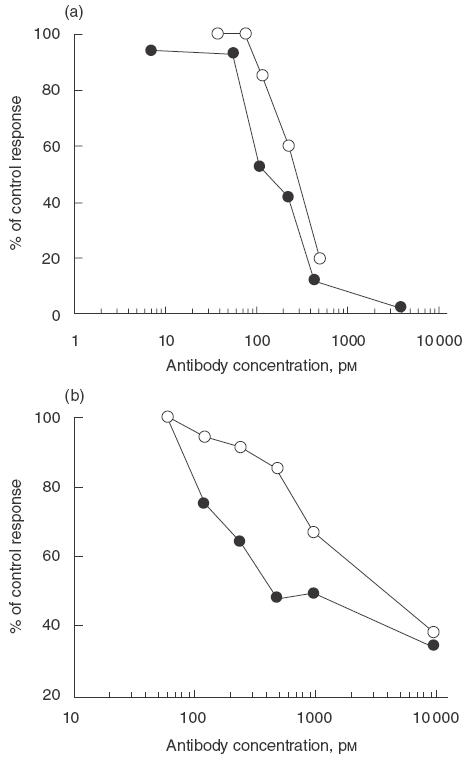
Effect of MoAb 3B9 and pascolizumab on IL-4-dependent IgE synthesis from human PBMCs (a) and surface expression of CD23 on purified human tonsillar B cells (b). Results are expressed as percentage of the control response obtained with IL-4-stimulated cells. •, MoAb 3B9; ○, pascolizumab.
Inhibition of IgE production by 3B9
Because IgE production is up-regulated following B-cell stimulation by IL-4, the inhibition of IL-4-dependent IgE production by MoAb 3B9 was examined in a SCID mouse model. In this model, SCID mice that had been administered immune cells from house dust mite-sensitive human donors were administered IL-4 (10 µg/mouse/day), causing a > twofold increase in IgE production within 4 weeks. Treatment of mice with MoAb 3B9 at the time of reconstitution inhibited IgE production in a dose-dependent manner (Fig. 5). The highest dose of MoAb 3B9 (10 mg) rendered IgE levels undetectable at all time-points. Serum IgG levels were unaffected by 3B9 treatment (data not shown).
Fig. 5.
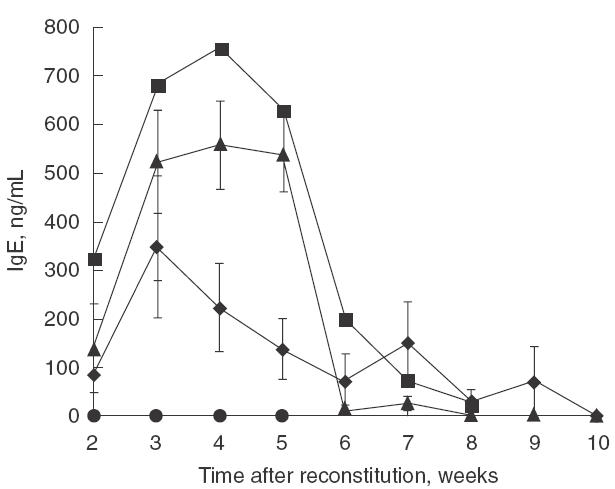
Effect of MoAb 3B9 on the production of IgE from SCID mice reconstituted with immune cells from atopic volunteers. Six−8-week-old SCID mice received purified PBMCs from house dust mite-sensitive donors, dosed with rhIL-4 for 4 days and a single IP MoAb 3B9 injection at the time of PBMC administration. ▴, IL-4; ▪, IL-4 + MoAb 3B9 (0·5 mg);  , IL-4 + MoAb 3B9 (1.0 mg); •, IL-4 + 3B9 (10 mg).
, IL-4 + MoAb 3B9 (1.0 mg); •, IL-4 + 3B9 (10 mg).
Tissue cross-reactivity
Immunohistochemistry studies demonstrated no pascolizumab staining in frozen sections from 37 normal human tissue types. These results suggest that pascolizumab may not be appropriate for immunohistochemistry on frozen tissue, or that the IL-4 concentrations in the tissues were below the level of sensitivity necessary for detection of pascolizumab binding.
Pharmacokinetics and safety of pascolizumab in cynomolgus monkeys
The pharmacokinetics and safety of pascolizumab were evaluated in cynomolgus monkeys as a prelude to testing in humans. Following the first dose of pascolizumab, a transient hyperemia, facial flushing lasting less than 1 h, was observed one of six cynomolgus monkeys that received 50 mg/kg and in two of six monkeys that received 500 mg/kg. No other adverse events were observed during this period. One monkey of each sex from each treatment group was necropsied at the end of the observation period. There were no drug-related histopathological findings in these monkeys. The remaining monkeys were administered a second dose of antibody and monitored for an additional month. Both antibody doses were well tolerated. Following the first dose of pascolizumab, one monkey developed anti-idiotypic antipascolizumab antibodies that resulted in enhanced plasma clearance of pascolizumab. No additional monkeys developed antipascolizumab antibodies.
In a chronic dosing study to assess toxicity and pharmacokinetics of i.v. and s.c. dosing, cynomolgus monkeys were administered pascolizumab monthly for 9 months. There were no significant changes in laboratory analyses, electrocardiogram measurements, ophthalmic observations, histology or body weight parameters (data not shown). There were no effects on lymphocyte numbers or subsets or responsiveness to mitogenic stimulation. There was no effect on circulating IgE levels in these non-allergic monkeys. A transient antibody response (including anti-idiotypic antibodies) was detected in two female monkeys after receiving the first 10-mg/kg i.v. dose, which was not evident following the second, third or fourth monthly doses. There were no adverse clinical responses in these antibody-positive monkeys; however, rapid serum elimination of pascolizumab was observed in the two monkeys expressing anti-idiotypic antibody (Fig. 6). A similar effect of rapid pascolizumab clearance was observed in one monkey, dosed with 10 mg/kg s.c., that did not test positive for anti-idiotypic antibodies, due presumably to residual levels of circulating pascolizumab that would compete in the assay. By the fifth dose, plasma pascolizumab concentration profiles in these monkeys were indistinguishable from the other monkeys in the same dose groups.
Fig. 6.
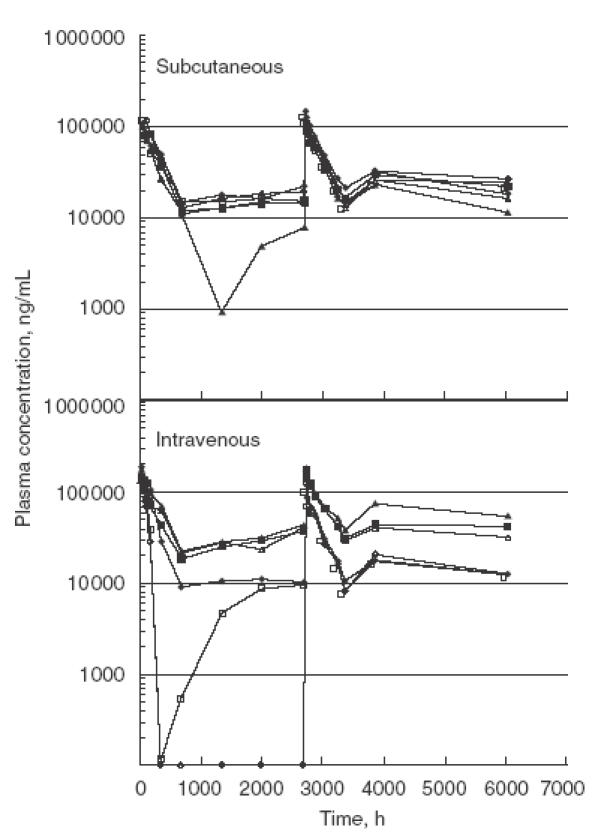
Plasma concentration versus time profiles for pascolizumab on the 9-month toxicity study. The peaks present between 0 and 600 h and again between 2700 and 3500 h represent full pharmacokinetic profiles after the first and fifth doses. The remaining time-points represent the trough plasma concentrations of pascolizumab present in plasma samples collected immediately prior to each monthly dose. There was no change in the clearance profile after repeat dosing. A slight accumulation was observed in the trough plasma concentrations between the first and fifth doses. Symbols represents individual monkeys: filled symbols represent males; open symbols represent females.
After chronic dosing, the terminal half-life of the antibody was 8·9 ± 1·4 days. The highest trough concentrations of antibody were reached after approximately 4 monthly doses. There was a slight accumulation of the drug at the 100-mg/kg dose level (Table 3; Fig. 6). The area under the curve following both the 10-mg/kg s.c. and i.v. dosing was similar, indicating 100% bioavailability of antibody after s.c. delivery. Pascolizumab was detected successfully in bronchoalveolar lavage (methods described in Hart et al. [17]) from four of 12 monkeys that received 10 mg/kg s.c. or i.v. (range 40–80 ng/ml) and in all the monkeys that received 100 mg/kg i.v. (range 350–900 ng/ml).
Table 3.
Preclinical pharmacokinetics of pascolizumab following chronic monthly dosing for 9 months in cynomolgus monkeys
| Males (n = 3) | Females (n = 3) | |||||||
|---|---|---|---|---|---|---|---|---|
| Observed mean Cmax,* µg/ml | Mean AUC(0–4 week), µg h/ml | Observed mean Cmax,* µg/ml | Mean AUC(0–4 week), µg h/ml | |||||
| Dose group, mg/kg (route) | Dose 1 | Dose 5 | Dose 1 | Dose 5 | Dose 1 | Dose 5 | Dose 1 | Dose 5 |
| 10 (s.c.) | 105 | 117 | 30 669 | 32 043 | 113 | 119 | 30 136 | 33 382 |
| 10 (i.v.) | 164 | 174 | 38 921 | 43 178 | 152 | 155 | 40 588 | 33 850 |
| 100 (i.v.) | 1351 | 1771 | 286 688 | 384 808 | 1175 | 1879 | 257 995 | 364 559 |
Cmax = maximal drug concentration; AUC = area under the curve; s.c. = subcutaneous; i.v. = intravenous.
Cmax values were generally observed at the first sampling time, approximately 24 h post-dosing.
DISCUSSION
In the present study, we have characterized the in vitro activity, pharmacokinetics and safety of a humanized anti-IL-4 MoAb, pascolizumab (SB 240683) and its parent murine MoAb, 3B9. Monoclonal antibody 3B9 inhibited IL-4-dependent events, including IL-5 synthesis, TH2 cell activation and IgE up-regulation. The humanized pascolizumab MoAb retained the species specificity, avidity and IL-4 neutralizing activity of 3B9. Titration microcalorimetry and BIAcore biosensor studies demonstrated rapid IL-4 binding by pascolizumab and slow dissociation. Taken together, the in vitro data suggest that pascolizumab specifically inhibits IL-4; therefore, in vivo testing was performed to define the preliminary safety of pascolizumab.
Because the in vitro studies demonstrated specificity of pascolizumab for cynomolgus monkey IL-4, the pharmacokinetics and safety of pascolizumab were assessed in cynomolgus monkeys. These in vivo studies demonstrated that pascolizumab treatment was well tolerated with slight antibody accumulation achieved after chronic dosing due to the long plasma half-life. There were no drug-related immunotoxicological or histopathological findings. There was no effect on circulating IgE levels in monkeys treated with pascolizumab; however, the monkeys had not been sensitized prior to initiation of the safety studies and thus did not have elevated levels of IgE. These in vivo and in vitro studies provide the rationale for testing the safety and efficacy of pascolizumab in humans.
Existing treatments for asthma provide indirect evidence that IL-4 inhibition may decrease the asthmatic response. Theophylline and other phosphodiesterase inhibitors may decrease IL-4 production in inflammatory cells, due possibly to increasing intracellular 3′,5′-adenosine monophosphate [18]. Additionally, allergen-specific immunotherapy reduces IL-4 expression in circulating lymphocytes [19], and prednisolone reduces the number of IL-4 and IL-5 mRNA-expressing cells and increases IFN-γ mRNA [20], suggesting an immunological shift to a TH1 response. Therefore, it is hypothesized that direct inhibition of IL-4 with a specific antibody may prevent T-cell maturation to the TH2 phenotype, which would in turn prevent secretion of TH2 cytokines including IL-4 and IL-5 in asthmatic patients. Furthermore, anti-IL-4 therapy might also reduce IgE production, inhibit mast cell growth, reduce eosinophil chemotaxis to the airway epithelium and decrease inflammatory destruction of the airways. Inhibition of IL-4 with a soluble form of the IL-4 receptor is currently being evaluated in the clinic with promising early results, including effects on forced expiratory volume in 1 s and on asthma symptom scores [21].
A major obstacle to the successful use of antibody therapy is the host immune response to treatment. Humanization of murine MoAbs for clinical use dramatically reduces their immunogenicity, thereby reducing the immune response in humans [22]. However, as occurred in this study, administration of humanized antibodies to monkeys can induce antibody responses [23]. The antibody response can vary from neutralizing antibodies that enhance clearance of the MoAb from circulation, to more severe anaphylactic responses [24]. In the current study, an antibody response occurred in only 12·5% of the monkeys (one in the two-dose regimen and two in the 9-month regimen) with no adverse events apparently related to the antipascolizumab response. In the 9-month dosing regimen, the antipascolizumab response was transient and was not detectable following the second, third or fourth doses. It is possible that the anti-idiotypic immune response was modulated via the pharmacological activity of the anti-IL-4 antibody on humoral immune responses [1]. Although the antipascolizumab antibodies did cause rapid serum elimination of pascolizumab, the pharmacokinetic profile of pascolizumab following the disappearance of antipascolizumab was comparable to that in the monkeys that did not develop an anti-antibody response. It is predicted that pascolizumab will be less immunogenic in humans than it was in monkeys; however, anti-idiotypic responses may still occur. As in the primate study, it is believed that such transient antibody responses will not interfere with long-term treatment of humans with therapeutic levels of pascolizumab.
In summary, in vitro studies confirm the specificity of pascolizumab for human and primate IL-4. In vitro, the antibody inhibits the downstream events associated with IL-4 stimulation, including IgE and CD23 up-regulation and T-cell proliferation, and may therefore be of potential benefit in the treatment of chronic asthma. Toxicology studies in cynomolgus monkeys confirm that the antibody is well tolerated during acute and chronic dosing. Furthermore, the high binding affinity, long half-life and low immunogenicity of the humanized anti-IL-4 antibody suggest that further evaluation in human clinical trials is warranted for pascolizumab.
Acknowledgments
The authors gratefully acknowledge the assistance and support of Rodd Polsky, Charles Hottenstein, Carol Silverman, Elisabeth Gore, Donna Williams and Mike Semanik in the conduct of this work. The authors also thank Drs Charles Davis and Peter Bugelski for their helpful discussions over the course of the studies reported here.
REFERENCES
- 1.Ricci M. IL-4: a key cytokine in atopy. Clin Exp Allergy. 1994;24:801–12. doi: 10.1111/j.1365-2222.1994.tb01803.x. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Brusselle GG, Tournoy KG, Lambrecht BN, Kips JC. Cytokines and their receptors as therapeutic targets in asthma. Clin Exp Allergy. 1998;28(Suppl. 3):1–5. [PubMed] [Google Scholar]
- 3.Coyle AJ, Bertrand C, Tsuyuki S, et al. IL-4 differentiates naive CD8+ T cells to a ‘Th2-like’ phenotype. a link between viral infections and bronchial asthma. Ann NY Acad Sci. 1996;796:97–103. doi: 10.1111/j.1749-6632.1996.tb32571.x. [DOI] [PubMed] [Google Scholar]
- 4.Coyle AJ, Le Gros G, Bertrand C, et al. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–9. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 5.Parronchi P, Macchia D, Piccinni MP, et al. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci USA. 1991;88:4538–42. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ricci M, Matucci A, Rossi O. IL-4 as a key factor influencing the development of allergen-specific Th2-like cells in atopic individuals. J Invest Allergol Clin Immunol. 1997;7:144–50. [PubMed] [Google Scholar]
- 7.Ying S, Humbert M, Barkans J, et al. Expression of IL-4 and IL-5 mRNA and protein product by CD4+ and CD8+ T cells, eosinophils, and mast cells in bronchial biopsies obtained from atopic and nonatopic (intrinsic) asthmatics. J Immunol. 1997;158:3539–44. [PubMed] [Google Scholar]
- 8.Humbert M, Durham SR, Ying S, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against ‘intrinsic’ asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med. 1996;154:1497–504. doi: 10.1164/ajrccm.154.5.8912771. [DOI] [PubMed] [Google Scholar]
- 9.Garlisi CG, Kung TT, Wang P, et al. Effects of chronic anti-interleukin-5 monoclonal antibody treatment in a murine model of pulmonary inflammation. Am J Respir Cell Mol Biol. 1999;20:248–55. doi: 10.1165/ajrcmb.20.2.3327. [DOI] [PubMed] [Google Scholar]
- 10.Buttner C, Skupin A, Reimann T, et al. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997;17:315–25. doi: 10.1165/ajrcmb.17.3.2279. [DOI] [PubMed] [Google Scholar]
- 11.Pauwels RA, Brusselle GJ, Kips JC. Cytokine manipulation in animal models of asthma. Am J Respir Crit Care Med. 1997;156:S78–S81. doi: 10.1164/ajrccm.156.4.12-tac-1. [DOI] [PubMed] [Google Scholar]
- 12.Wills-Karp M, Luyimbazi J, Xu X, et al. Interleukin-13. central mediator of allergic asthma. Science. 1998;282:2258–61. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 13.Oettgen HC, Geha RS. IgE in asthma and atopy: cellular and molecular connections. J Clin Invest. 1999;104:829–35. doi: 10.1172/JCI8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nasert S, Millner M, Herz U, et al. Therapeutic interference with interferon-γ (IFN-γ) and soluble IL-4 receptor (sIL-4R) in allergic diseases. Behring Inst Mitt. 1995;96:118–30. [PubMed] [Google Scholar]
- 15.Zhou CY, Crocker IC, Koenig G, Romero FA, Townley RG. Anti-interleukin-4 inhibits immunoglobulin E production in a murine model of atopic asthma. J Asthma. 1997;34:195–201. doi: 10.3109/02770909709068189. [DOI] [PubMed] [Google Scholar]
- 16.Zia-Amirhosseini P, Minthorn E, Benincosa LJ, et al. Pharmacokinetics and pharmacodynamics of SB-240563, a humanized monoclonal antibody directed to human interleukin-5, in monkeys. J Pharmacol Exp Ther. 1999;291:1060–7. [PubMed] [Google Scholar]
- 17.Hart TK, Cook RM, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of mepolizuMoAb (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys. J Allergy Clin Immunol. 2001;108:250–7. doi: 10.1067/mai.2001.116576. [DOI] [PubMed] [Google Scholar]
- 18.Tohda Y, Nakahara H, Kubo H, Muraki M, Fukuoka M, Nakajima S. Theophylline suppresses the release of interleukin-4 by peripheral blood mononuclear cells. Int Arch Allergy Immunol. 1998;115:42–6. doi: 10.1159/000023828. [DOI] [PubMed] [Google Scholar]
- 19.O'Brien RM, Byron KA, Varigos GA, Thomas WR. House dust mite immunotherapy results in a decrease in Der p 2-specific IFN-gamma and IL-4 expression by circulating T lymphocytes. Clin Exp Allergy. 1997;27:46–51. [PubMed] [Google Scholar]
- 20.Bentley AM, Hamid Q, Robinson DS, et al. Prednisolone treatment in asthma. Reduction in the numbers of eosinophils, T cells, tryptase-only positive mast cells, and modulation of IL-4, IL-5, and interferon-gamma cytokine gene expression within the bronchial mucosa. Am J Respir Crit Care Med. 1996;153:551–6. doi: 10.1164/ajrccm.153.2.8564096. [DOI] [PubMed] [Google Scholar]
- 21.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001;2:66–70. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaswani SK, Hamilton RG. Humanized antibodies as potential therapeutic drugs. Ann Allergy Asthma Immunol. 1998;81:105–15. doi: 10.1016/S1081-1206(10)62794-9. [DOI] [PubMed] [Google Scholar]
- 23.Newman R, Alberts J, Anderson D, et al. ‘Primatization’ of recombinant antibodies for immunotherapy of human diseases: a macaque/human chimeric antibody against human CD4. Biotechnology (NY) 1992;10:1455–60. doi: 10.1038/nbt1192-1455. [DOI] [PubMed] [Google Scholar]
- 24.Hakimi J, Chizzonite R, Luke DR, et al. Reduced immunogenicity and improved pharmacokinetics of humanized anti-Tac in cynomolgus monkeys. J Immunol. 1991;147:1352–9. [PubMed] [Google Scholar]


