Abstract
Soy-based formulas are the most employed cow's milk substitutes in the treatment of cow's milk allergy in our country. Since adverse reactions have been reported in allergic patients as a consequence of exposure to soy proteins, we have investigated the possible cross-reactivity between components from soybean and cow's milk. A cow's milk specific polyclonal antiserum and casein specific monoclonal antibodies were used in immunoblotting and competitive ELISA studies to identify a 30-kD component from soybean that cross-reacts with cow's milk caseins. Its IgE binding capacity was tested by EAST, employing sera from cow's milk allergic patients, not previously exposed to soy proteins. The 30 kD protein was isolated and partially sequenced. It is constituted by two polypeptides (A5 and B3) linked by a disulphide bond. The protein's capacity to bind to the different antibodies relies on the B3 poly-peptide. These results indicate that soy-based formula, which contains the A5-B3 glycinin molecule, could be involved in allergic reactions observed in cow's milk allergic patients exposed to soy-containing foods.
Keywords: cross-reactivity, soybean, caseins, ELISA, immunoblotting
INTRODUCTION
Food allergy is becoming a medical, economical and social problem. Soybean, together with milk, peanuts and eggs, are the major allergenic foods. This pathology affects the infant population, when the gut barrier is immature and the immune system is still refining its ability to tolerate food proteins. In our country, cow's milk allergy (CMA) constitutes the main food allergy in infancy, but soy allergy has become exacerbated because of the increased utilization of soy-based formulas as cow's milk (CM) substitutes and the inclusion of soy-proteins in many processed foods. Once a food allergy is diagnosed, the only proven therapy is the strict elimination of the offending allergens from the diet. Available substitutes for CM include milks from different mammalian animals, soy-based formulas, hydrolysed cow's milk proteins (CMP) and amino acid-based formulas. Furthermore, there is evidence that soy proteins may trigger allergic reactions in CMA patients, as assessed by a double-blind placebo controlled food challenge (DBPCFC) [1].
Milk contains more than 50 proteins and the major ones are implicated in a number of immunologically mediated reactions [2]. Caseins and β-lactoglobulin (β-Lg) have been described as the main antigenic and allergenic components for human beings [3,4], although immunoglobulins, α-lactalbumin (α-La), and bovine serum albumin (BSA) were also found to be reactive with different isotypes of human antibodies [5,6]. Several immunoreactive epitopes have been identified in caseins [7,8] and β-Lg [9]. Most of the epitopes present in the casein molecules are sequential since the high number of proline and hydrophobic residues (45%) in these proteins determine an undefined secondary and tertiary structure [10]. Besides, caseins tend to aggregate as a result of hydrophobic interactions to give quaternary structures [11], and this may create conformational epitopes buried in the hydrophobic interior of the micelle [7]. These epitopes are only exposed and accessible to the immune system after denaturation of the complex by digestion.
A number of soy proteins that bind antibodies, especially IgE, have been identified [12,13]. The main fractions of storage globulins from seed proteins are the complexes 7S or β-conglycinin, and 11S or glycinin. The 7S complex is generally a trimer with MW 150–200 kD, whereas 11S is a hexamer with MW 300–400 kD [14]. In the 7S fraction, or β-conglycinin, several IgE-binding proteins have been identified and some of them have been purified and characterized: Gly m Bd 28K, Gly m Bd 30K, Gly m Bd 60K [15,16]. Sequence homology has been reported between these allergens and the thiol proteinase family [17], and with the allergen Der p1. The 11S fraction, or glycinin, has also been characterized as a major soybean allergen involved in food hypersensitivity reactions [13,18].
Our investigation aimed to analyse a possible in vitro cross-reactivity between CM and soybean proteins. To this end, immunoenzymatic methods have been shown to be excellent tools for the analysis of allergen and cross-reactive component contents of different related or unrelated foods. Specific antisera, monoclonal antibodies, and immunoenzymatic methods of high detectability and specificity were used in this study and a soy protein that cross-reacts with caseins from CM isolated and identified.
Precise identification of cross-reactive epitopes would represent a significant advance in the problem of food intolerance, since it would be possible to develop hypoallergenic substitutes for the treatment and prevention of food allergy.
MATERIALS AND METHODS
Protein extracts
Cow's milk
a protein extract from powdered skimmed CM was prepared by adding phosphate-buffered saline pH 7·4 (PBS) (10 mg/ml) to the milk powder and incubating overnight at 4°C with continuous shaking. Lipids were extracted with chloroform and samples were dialysed overnight against PBS, using a membrane of 2 kD-cutoff (SPECTRA/POR MWCO 2000). Samples were stored at − 20°C until used. Total protein concentration was 3·5 mg/ml.
Caseins
These proteins were obtained according to the isoelectric precipitation technique [19]
Soy
Soybeans were crushed and extracted with 0·01 N NaHCO3 at 90°C (200 mg/ml). The extract was homogenized three times for 1 minute on an Ultraturrax UT 20·000. Then it was centrifuged (5000 g, 20 min at room temperature) and the lipids were extracted with chloroform. The extract was dialysed against Tris buffered saline pH 8·8 and stored at − 20°C until used. Total protein concentration was 38·1 mg/ml.
S30
This 30 kD-fraction was isolated from the soybean extract by electroelution from the proteins fractionated by SDS-PAGE. The Electro-Eluter 422 from Bio-Rad was employed. Dialysis membranes with 12–15 kD cut-off were employed. Samples were then dialysed against PBS overnight using a membrane of 2 kD-cutoff (SPECTRA/POR MWCO 2000). This preparation method rendered a total protein concentration of 0·28 mg/ml.
The total protein content of the preparations was measured by the Lowry's method, with bovine serum albumin as a reference [20].
Monoclonal and polyclonal antibodies
Casein specific monoclonal antibodies were prepared by injecting six-week-old-female Balb/c mice intraperitoneally with 15 μg of total casein proteins emulsified 1 : 1 in complete Freund's adjuvant, followed by three boosts with 15 μg of proteins in Freund's incomplete adjuvant at three-week intervals. Serum specific antibody titres were determined by indirect ELISA. An antigen booster was administered 4 days before removal of the spleen. Hybridomas were obtained according to the Galfre and Milstein technique [21] employing NSO cells as myeloma cells. The casein-specificity of the monoclonal antibodies harvested from the peritoneal fluid was assayed by immunoblotting and indirect ELISA, employing commercial milk proteins and electroeluted casein components from CM extract. Three monoclonal antibodies with high specificity for α-casein (1D5-I), β-casein (2A1-I) and κ-casein (3B5-I) were selected for the study.
CM polyclonal antiserum was prepared by inoculating NZW rabbits with 100 μg of CMP emulsified with complete Freund′s adjuvant. A series of four injections (50 μg) of the same antigen in incomplete Freund′s adjuvant was administered every 3 weeks. Antibody titres were determined by indirect ELISA.
Sequential competitive ELISA
Polystyrene microtitre plates (NUNC, Maxisorp, Denmark) were coated with 1 μg/well of CMP or 10 μg/well of soy protein extract, and incubated for 12 h at 4°C. After washing with 9 g/l NaCl containing 0·05% (v/v) Tween 20 (NaCl-Tween 20), 200 μl/well of blocking buffer containing 5% horse serum in PBS pH 7·4 was added and incubated for 2 h at 37°C. Then wells were washed three times with NaCl-Tween 20.
Inhibition experiments were performed by preincubating equal volumes of antigen, and rabbit anti-CMP polyclonal antiserum — both diluted in blocking buffer — for 1 h at 37°C. The antiserum dilution used was previously determined from the titration curve: 1 : 300·000 for wells coated with CMP or 1 : 1000 for wells coated with soy proteins.
A volume of 100 μl of each of the above mentioned preincubated samples was transferred to the respective coated wells for competition with antigen in solid phase and incubated for 1 h at 37°C. After washing with NaCl-Tween 20, 100 μl/well of goat antirabbit IgG horseradish peroxidase (HPR) conjugate (BioRad, Richmond, CA, USA) diluted 1 : 3000 in blocking buffer, was added and incubated for 1 h at 37°C. Colour was developed by adding a solution containing ο-phenylenediamine and 30% H2O2 in 0·1 m citrate-phosphate buffer pH 5·0. The reaction was stopped by the addition of 50 μl/well of 4N sulphuric acid, and the reaction product was quantified by reading the optical density at 490 nm.
Electrophoresis and immunoblotting
Sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) of protein extracts was carried out in a 14% polyacrylamide gel with a discontinuous buffer system according to Laemmli [22]. Gels were migrated on a Bio-Rad Mini-Protean III (Bio-Rad) at 100 V through the stacking gel, and at 150 V through the running gel, either with or without 5%β mercaptoethanol. A 10-μl sample of the protein extract was loaded onto each well. The amount of protein loaded was between 8 and 15 μg. Gels were fixed and stained with either Coomasie Blue R250 or silver staining, according to the protein content of the sample. The relative molecular weights of the resolved protein fractions were compared with those of marker proteins.
Tricine-SDS-PAGE was performed as described by Schagger [23]. Electrophoresis was performed initially at constant 30 V for 1 h and then at constant 85 V for 5 h.
The fractionated proteins were electroblotted to 0·45 nm pore size nitrocellulose transfer membranes (SMI) at 300 mA for 45 min [24]. Membranes were stained with Ponceau S to demonstrate successful electrophoresis and transfer of proteins onto the membranes. The blots were blocked for 1 h in blocking buffer containing 5% horse serum in PBS pH 7·4. Then, they were cut into 5 mm strips for immunostaining. Sera from patients and controls (diluted 1 : 5 in blocking buffer), rabbit polyclonal antiserum (diluted 1 : 1000 in blocking buffer) and monoclonal antibodies were added to detect the presence of antigenic and allergenic components in each extract. Hybridoma supernatants were assayed undiluted. Human sera were incubated for 12 h at room temperature, and polyclonal antiserum and monoclonal anti-bodies were incubated for 1 h at 37°C.
After thorough washing (0·05% Tween-PBS), incubation with different conjugate systems were assessed: biotinylated goat antihuman IgE (ɛ-chain specific) antibody (Vector, California, USA), diluted 1 : 2000 in blocking buffer (2 h at 37°C), and then, ExtrAvidin-alkaline phosphatase conjugate (Sigma) 1 : 1000 for 1 h at 37°C, were employed to reveal human serum IgE antibodies; antimouse IgG (whole molecule) horseradish peroxidase conjugate (BioRad) diluted 1 : 2000 was employed to detect the presence of monoclonal antibodies, goat antirabbit IgG (whole molecule) HRP conjugate (BioRad), diluted 1 : 1000 in blocking buffer (2 h at 37°C), or biotin conjugated monoclonal antirabbit IgG (γ-chain specific) (Sigma), diluted 1 : 2000 (overnight at 4°C), and then, ExtrAvidin-alkaline phosphatase (Sigma), diluted 1 : 1000 (1 h at 37°C) were used for detection of rabbit antibodies.
The blots were washed and subsequently stained with 5-bromo-4-chloro-3-indolylphosphate/nitro blue tetrazolium (5-BCIP/NBT, Sigma) or 4-chloro-1-naftol (Sigma)-H2O2.
In-gel digestion
SDS-PAGE electrophoresis was performed with 10 μg/lane of the 30 kD-protein, isolated by electroelution from the soybean extract. The gel was stained with 0·1% Coomasie blue R-250–20% methanol −0·5% acetic acid to visualize the band. Then it was destained (30% methanol) and the excised part was washed with 100 mm ammonium bicarbonate pH 8 for 1 h. Disulphide bonds were reduced with 100 mm ammonium bicarbonate and 45 mm dithiothreitol for 30 min at 60°C. Alkylation of free sulphydryl groups was performed by incubating the gel with 100 mm iodoacetamide. The piece of gel was further washed with 50% acetonitrile and 50% 100 mm ammonium bicarbonate for 1 h. Acetonitrile was added and the gel was dried (Speed Vac). Following that it was incubated with trypsin (Sigma) in 25 mm ammonium bicarbonate overnight at 37°C. Finally, the peptides were extracted from the gel with 60% acetonitrile and 0·1% trifluoroacetic acid, and concentrated by vacuum drying (Speed Vac).
N-terminal sequencing
Separation of peptides from the tryptic digest was carried out by reverse-phase HPLC on a Sephasyl Peptide C18 2·1/220 column (Vydac V1). The mobile phase contained 0·1% trifluoroacetic acid and the peaks were separated by applying a linear gradient with 80% acetonitrile and 0·08% trifluoroacetic acid (0–80% of acetonitrile gradient). The column temperature was maintained at 25°C. Separation was monitored by UV absorbance at 220 nm. Forty-one peaks were obtained and two main peaks (14 and 31) were collected for sequencing.
Peptides were identified by N-terminal sequencing using the Edman's method on a sequencer Applied Biosystems 477 (Laboratorio Nacional de Investigación y Servicios en Péptidos y Proteínas-Lanais Pro, CONICET, UBA).
Total IgE measurements
Total serum IgE levels of both the CM allergic patients and individuals of the control group were measured as previously described [25]. Briefly, paper discs activated with CNBr were coupled with rabbit antihuman IgE (ɛ-chain specific) (Behring) and incubated with serum, diluted 1 : 10, for 12 h at room temperature. After adequate washing, the discs were incubated with monoclonal antihuman IgE (ɛ chain specific) alkaline phosphatase conjugate (Sigma), diluted 1 : 5000 in blocking buffer for 12 h at room temperature. Color was developed with disodium ρ-nitrophenyl phosphate, and DO was measured at 405 nm.
The calibration curve was obtained using a commercial IgE standard (Behring), and checked against an international standard (Immunoglobulin E Reference Preparation 69/204)
Detection of specific IgE
Food-specific IgE antibodies were identified by enzyme-allergo-sorbent-test (EAST), as previously described [3]. Cellulose discs were activated with CNBr according to the method of Ceska et al. [26]. Protein extracts were coupled to CNBr-activated paper discs at 1 mg/ml concentration in 0·1 m carbonate/bicarbonate buffer, pH 8·5.
Different protein concentrations of each extract were used to optimize the coating of the activated paper discs. The concentration leading to no further increase in OD, when analysed with a pool of positive sera (1 mg/ml), was chosen as the working condition. To determine whether this amount of coupled antigen was sufficient to bind all the IgE antibodies present in the serum, and to verify that no interference with specific IgG antibodies was occurring, a pool of positive sera was adsorbed with an anti-human IgG (γ-chain specific) monoclonal antibody and then, specific IgE antibodies were measured as described above. No variations were found in the OD values.
To measure specific IgE classes, a calibration curve was obtained by using a pool of anti-Dermatophagoides sp. sera, as reported by Lundkvist [27].
Sera
Serum samples were taken from 10 children (1–4 years) with a history of immediate reactions to CM (urticaria, dermatitis, gastrointestinal symptoms), as source of specific IgE antibodies. All the children never ingested soy derivatives, and they were selected on the basis of their elevated total serum IgE, positive EAST results, and positive skin prick test to CMP and common environmental allergens.
Thirty sera from subjects (aged between 2 and 6 years old) with no history of atopy and no clinical reactions after ingestion of CM were used as negative control sera.
All sera were obtained from the Hospital San Juan de Dios de La Plata. This study was approved by the Ethical Committee of the Hospital San Juan de Dios de La Plata and the informed consent was obtained from the parents of all children participating in the study.
RESULTS
Cross-reactivity between cow's milk and soy proteins
Cross-reactivity between proteins from CM and soybean was studied by inhibition ELISA, using a polyclonal antiserum specific for CMP. Inhibitory proteins and antiserum were preincubated separately from the coated antigen, to achieve a higher detectability.
The binding of antibodies to immobilized CMP was completely inhibited by preincubation of the antibodies with 1 mg/ml of CMP, while 50% of inhibition was achieved with 3 μg/ml. These results show that antiserum is specific for CMP, and that homologous proteins in the fluid phase are able to compete with immobilized ones. When soy protein extract was used as the competitive antigen, an inhibitory dose–response effect was observed. This result suggests that the CM-specific antiserum contains antibodies that react with components present in the soy extract. The concentration of soy proteins producing 50% inhibition was 18 mg/ml, an amount 6000 times higher than that required for CMP (Fig. 1a). These results agree with indirect ELISA titration assays in which the solid phase was coated with either CM (titre = 1 : 250·000) or soy proteins (titre = 1 : 1000). The antiserum titre was defined as the dilution of antiserum producing 50% of the maximum binding to the antigen-coated plates.
Fig. 1.
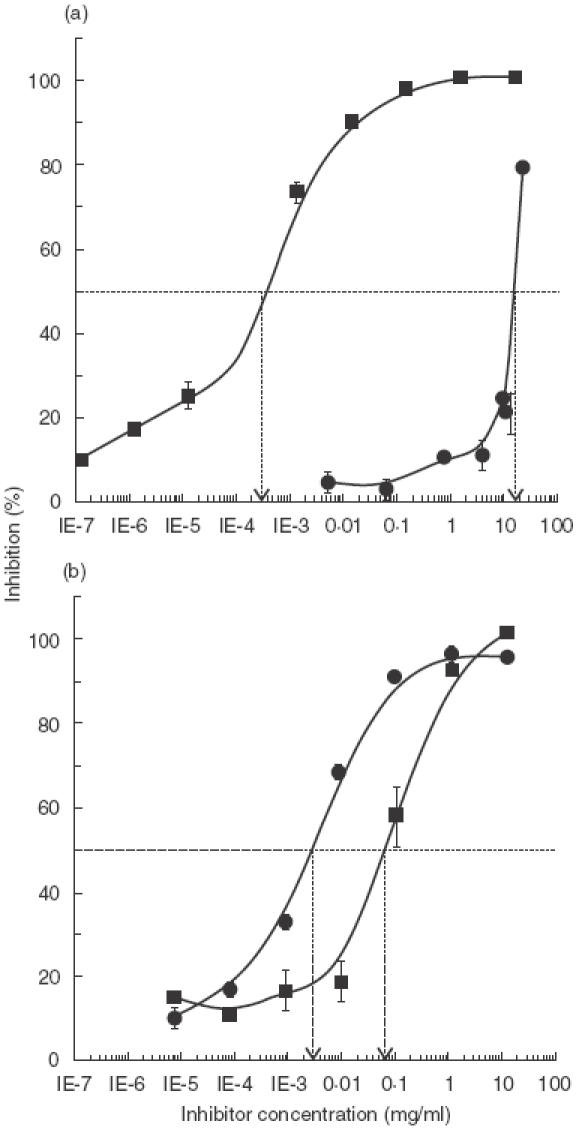
Competitive inhibition ELISA tests Increasing concentrations of CMP (▪) or soybean proteins (•) were preincubated with specific CM polyclonal antiserum in order to inhibit the binding of antibodies to immobilized CMP (a) or soybean proteins (b).
Using soy proteins as immobilized antigens, total binding inhibition was obtained with both CM (10 mg/ml) and soybean proteins (0·9 mg/ml) (Fig. 1b). Inhibitions of 50% were obtained with the soy protein extract at a concentration 20 times lower than that of the CMP extract.
To test the specificity of the antiserum, protein extracts from wheat, barley, rye, oat, peanut, amaranth and sunflower seeds, as well as OVA, were used as inhibitors in 4 concentrations ranging from 1 to 10 mg/ml. Maximal inhibition values were below 10–15%, indicating the specificity of the inhibitions shown in Fig. 1 (results not shown).
Protein composition of extracts and immunoblotting reactivities
Protein composition as analysed by SDS-PAGE is shown in Fig. 2. The protein profile of the CM extract can be seen in lane 1. The main bands correspond to αs1- and β-casein. Some minor bands corresponding to κ-casein and whey components can also be observed. Alpha-lactalbumin (14·2 kD), β-lactoglobulin (18 kD under reducing conditions) and bovine seroalbumin (67 kD) appeared as single bands. The casein components can be observed in lane 2, where isolated total casein fractions were loaded onto the gel under reducing conditions. In the high molecular weight-zone of the gel (upper 94 kD) a broad band could be detected in the CM sample, that corresponds to casein aggregates. In lane 3 the protein profile of the soy extract can be observed. Different soy components could be tentatively identified by comparison with the SDS-PAGE determined relative MWs with known values of MWs of different soy fractions.
Fig. 2.
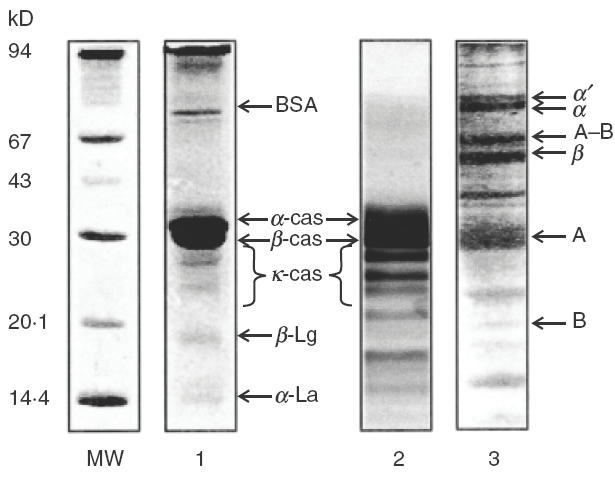
SDS-PAGE analysis of protein extracts. MW: molecular weight markers; 1: CMPextract (under reducing conditions); 2: total casein components (under reducing conditions), 3: soy extract (under non reducing conditions). Gels were stained with Coomasie Blue R 250 (15–25 μg of protein were loaded per lane).
Reactivities of protein components separated by SDS-PAGE were analysed by immunoblotting, employing a specific CM polyclonal antiserum and specific casein monoclonal antibodies (Fig. 3). These antibodies would recognize cross-reactive epitopes present in soy components, since they were obtained using CMP as the immunogen. When separated CMP were incubated with the polyclonal antiserum (lane 1A) the most intense bands were found to correspond to α-casein and β-Lg, while α-La and the glycosylated κ-caseins could also be detected. The highly antigenic casein aggregates were also recognized. When the immunoblots were developed with each specific monoclonal antibody, bands in the regions of casein monomers (α-, β-, and κ-caseins) and high MW aggregates could be observed (lanes 1 B, C, D, respectively). In lane 2 are shown the immunoblots of the soy extract proteins revealed with the different anti-CM antibodies. The polyclonal antiserum revealed an intense band in the 30 kD region, while some minor bands can also be observed (lane 2 A). Casein specific monoclonal antibodies also reacted with the 30 kD fraction (lanes 2 B, C, D). This 30 kD-component was isolated by electroelution and then immunoblotted. The binding of either the polyclonal antiserum or monoclonal antibodies to the 30 kD component is shown in lanes 3 A, B, C, D. In all cases a single band was observed.
Fig. 3.
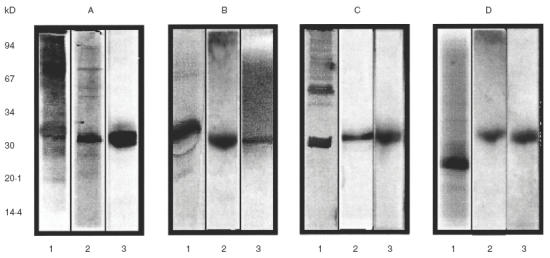
Immunoblottings of the different protein extracts Lanes 1: CMPextract (under reducing conditions); lanes 2: soybean extract, lanes 3: S30 component (under non reducing conditions). Immunoblottings were revealed with rabbit polyclonal antiserum (A) and the monoclonal antibodies 1D5-I (B), 2A1-I (C) or 3B5-I (D).
Isolation and identification of the cross-reactive soy component
Because the amino terminal residue of the isolated soybean cross-reactive component was blocked, in situ digestion with trypsin was performed. The fragments resulting from the treatment were separated by RP-HPLC. Two main peaks were collected for sequencing. Peak 31 was found to contain two peptides (I and II), while peak 14 contained only one peptide (III).
Peptide I had the same amino acid sequence as that of sequence (76–97) of glycinin A5 from soy (theoretical MW: 10·6 kD). Peptide II had a sequence similar to that of peptide (141–154) from soybean glycinin B3 (theoretical MW: 18·4 kD). Moreover, peptide III had a sequence identical to that of sequence (11–27) of soy glycinin B3. The sequences are shown in Fig. 4. The theoretical MW of the dimer A5-B3 is in good agreement with the relative MW of the S30-component estimated from the electrophoresis.
Fig. 4.

Comparison of the amino acid sequences of soybean glycinins A5 and B3, and proteolitic peptides obtained from S30. Similarities in amino acid residues are indicated as *.
When the S30 protein was analysed by Trycine SDS-PAGE under non reducing conditions a single band was observed at 30 kD, as expected (Fig. 5, lane 1). This immunoblotted fraction was also recognized as a single band with the CM specific antiserum (lane 3). When the sample was analysed under reducing conditions, two additional bands appeared, with relative MWs 11·6 kD and 21·5 kD (Fig. 5, lane 2). This result suggests that this cross-reactive component is composed of two polypeptides linked by a disulphide bridge. The separated components migrated with relative MWs similar to A5 and B3, as determined by sequencing. In Fig. 5, immunoblots of S30 treated with β-mercaptoetanol and revealed with polyclonal and monoclonal antibodies were also included. In lane 4 the bands corresponding to S30 and B3 contain components that could be bound by antibodies from polyclonal antiserum. As can be seen, S30 was not completely reduced. Lanes 5, 6 and 7 show that the B3 polypeptide was recognized by the casein-specific monoclonal antibodies.
Fig. 5.
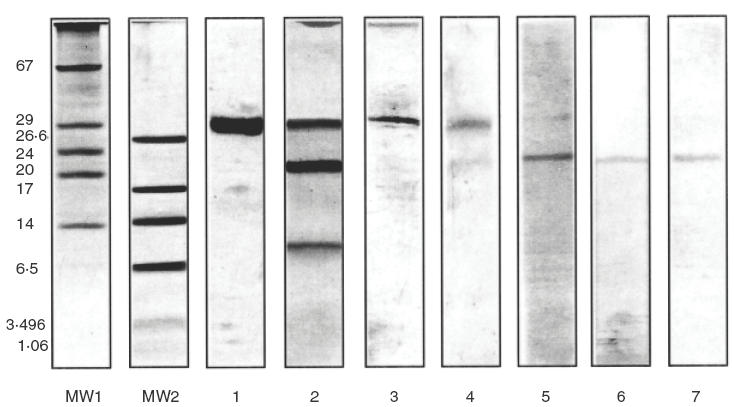
Analysis by Tricine SDS-PAGE and immunoblotting of the S30 component. MW 1 and 2: different molecular weight markers, lanes 1 and 2: Tricine SDS-PAGE electrophoresis of the S30 protein under nonreducing (lane 1) or reducing conditions (lane 2), stained with silver staining (2 μg of protein per lane), lane 3–7: immunoblots of the S30 component under nonreducing (lane 3) or reducing conditions (lanes 4–7), revealed with the polyclonal antiserum (lanes 3 and 4) or the monoclonal antibodies ID5-I (lane 5), 2A1-I (lane 6) and 3B5-I (lane 7).
Allergenicity of the cross-reactive soy protein
The capacity of the proteins to be bound by serum specific IgE was studied by EAST. Results expressed as class are shown in Table 1. When sera from the control group were analysed by EAST DO values were similar to those obtained with the dilution of the pool serum used in the standard curve, corresponding to class 0. Sera 1–10 were obtained from CM allergic patients that had not been exposed to soy products. These sera contain casein specific IgE antibodies that also recognize soybean proteins, including the S30 component.
Table 1.
Total and specific IgE levels determined by EAST of CMA patients
| EAST (class) | ||||||
|---|---|---|---|---|---|---|
| Sera number | Age (years) | Total IgE (UI/ml) | CM | Casein | Soybean | S30 |
| 1 | 2 | 260 | 2 | 2 | 3 | 3 |
| 2 | 3 | 6100 | 2 | 3 | 2 | 3 |
| 3 | 3 | 760 | 2 | 2 | 2 | 3 |
| 4 | 2 | 326 | 2 | 2 | 3 | 3 |
| 5 | 1 | 160 | 2 | 3 | 1 | 2 |
| 6 | 2 | 890 | 2 | 2 | 2 | 2 |
| 7 | 3 | 831 | 2 | 2 | 2 | 2 |
| 8 | 4 | 955 | 2 | 2 | 2 | 2 |
| 9 | 2 | 857 | 3 | 2 | 2 | 2 |
| 10 | 2 | 87 | 2 | 2 | 2 | 2 |
| 11* | 2 | 12 | 0 | 0 | 0 | 0 |
| 12* | 3 | 41 | 0 | 0 | 0 | 0 |
| 13* | 1 | 38 | 0 | 0 | 0 | 0 |
Sera from subjects from the healthy control group.
Figure 6, shows the immunoblots revealed with a pool of sera obtained from the CM allergic subjects. Lane 1 shows the pattern of IgE reactivities when CMP were used as antigen. Bands corresponding to caseins can be seen. Reactivity in the region of 30 kD (lanes 2 and 3) or 21 kD (lane 4) were detected in the soy component, indicating that S30 and B3 are able to be bound by IgE as well. When a pool of sera obtained from the healthy control group was used to develop the immunoblots no bands were observed (data not shown).
Fig. 6.
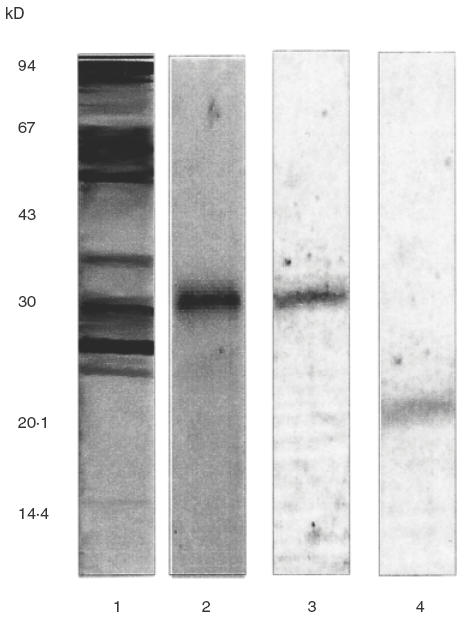
IgE immunoblots revealed with a pooled serum obtained from CMA patients. Lane 1: CM immunoblotting under reducing conditions, lane 2: soybean immunoblotting (under non reducing conditions), lane 3: S30 immunoblotting under nonreducing conditions, lane 4: S30 immunoblotting under reducing conditions.
DISCUSSION
Cow's milk allergy constitutes the main food allergy in infancy in our country. At present one of the most widely used formulas for feeding babies with CMA are soy-based formulas. This substitute is nutritionally adequate, palatable and less expensive than CMP-based formulas [28]. There is evidence that 10–35% of the children with CMA do not tolerate soy derivatives, even those children not previously exposed to soy proteins [1,29,30].
The change of the dietary habits in patients with food allergy may constitute a difficult task. Some foods cannot be easily substituted (milk in newborns for instance), some others may contain components with cross-reactive epitopes that are not denatured during digestion. The intensive use of new substitutes (soy formulas in CMA patients) may sensitize patients to newly ingested proteins. Moreover, some proteins (caseins, soy proteins) used as ingredients in newly available foods are not always detailed on the labelling, etc. Small amounts of food ingredients may be added without the need to be listed, and it is well known that small quantities of allergenic components may trigger allergic reactions in sensitized patients.
We aimed to characterize the immunochemical cross- reactivity detected between CM and soy proteins. The CM specific antiserum employed contains specific antibodies with both different specificities and relative affinities for the epitopes present in the casein components. Depending on whether the antigen is coated to the solid phase or in fluid phase, different antibodies of the antiserum, with different affinities, may be measured. It is therefore impossible to predict the molecular behaviour of the system, since two complex antigenic systems in different physical states are competing with different antibodies.
In the experiment depicted in Fig. 1a, the amount of CMP necessary to achieve 50% inhibition of antibody binding to the immobilized homologueous antigens was lower than the amount of soy proteins necessary to produce the same effect. This dif-ference is most likely due to differences in the number of antigen–antibody interactions involved in each reaction. CM contains all the antigens necessary to inhibit each of the multiple interactions between CM and anti-CM antibodies. In contrast, the soy extract probably contains only a few components able to react with CM specific antibodies; in other words, a restricted population of antibodies are being bound by soy proteins and therefore a higher amount of soy proteins needs to be added to diminish the binding of antibodies.
The experiment shown in Fig. 1b only detects the restricted population of antibodies with the capacity to recognize the cross-reactive soy component attached to the solid phase. Interactions that do not concern cross-reaction are not detected in this experiment. Hence, the amount of CMP needed to abrogate these interactions is higher than in experiment 1a. In conclusion, these experiments reflect the interaction of a restricted population of CM specific antibodies with a prevalent soy antigenic component. Similar results can be found with other cross-reacting complex antigenic systems, when this type of competitive solid-phase immunoassays is used (e.g. wheat, barley, rye).
We identified, by immunoblotting, the cross-reactive component from soy as a 30-kD fraction (S30) that could be observed as a single band by both polyclonal antiserum and monoclonal antibodies (Fig. 3). Since these monoclonal antibodies were specific for CM caseins, it can be concluded that the S30 component shares common epitopes with α-, β-, and κ-caseins. To demonstrate the protein nature of the S30 fraction, it was excised from the SDS-PAGE gels, treated with trypsin, and then analysed further by SDS-PAGE. No band was detected (data not shown) confirming S30 was proteinic. Furthermore, this component was stained either with silver staining or Coomasie dye after electrophoresis also demonstrating its protein nature. In addition, it is known that globulin 11S, in particular the A5-B3 subunit, lacks sugar [31], and that κ-casein is the only glycosylated casein fraction [11]. These results suggest that this in vitro cross-reactivity is not the result of carbohydrate recognition among protein components.
It was found by N-terminal sequencing that the cross-reactive component is composed of two polypeptides. The certainty that additional polypeptides were not included in the excised bands was provided by the unambiguous N-terminal sequence data obtained. N-terminal sequences of the fragments were found to correspond to the A5 and B3 polypeptides from soybean by comparison with sequences from a data-base (Fig. 4). This dimer may derive from the glycinin A5-A4-B3 during in vitro processing, since A5-B3 has not been described as a storage seed subunit. A5-A4-B3 is synthesized as a single polypeptide precursor, similarly to the other subunits, but in this case the acidic polypeptide is cleaved to produce the A5 (97 residues) and A4 (257 residues) polypeptides during transportation within the cell. One disulphide bond is involved in linking A5 and B3, while the A4 polypeptide interacts noncovalently [32]. Peptide I was found to contain the Cys (85) residue that form the disulphide bond between the two polypeptides. The subunits then interact to form hexamers that are deposited as protein bodies during endosperm development [33].
Immunoblotting under reducing conditions with the S30 component showed that B3 had the ability to bind the different antibodies tested (Fig. 5), even the serum IgE antibodies obtained from CM allergic individuals not sensitized to soy proteins (Fig. 6). A possible explanation for this result may lie in the presence of specific IgE antibodies to CM caseins that recognize cross- reactive epitopes in the B3-component from soy.
Comparison of the A5 and B3 aminoacid sequences with that of CMP showed many spots of high similarity (up to 66% homologuey) with several protein components of CM, including caseins and β-Lg among others. This suggests that sequential epitopes in the S30 component and α-, β-, κ-casein could be involved in the observed cross-reactivity. Nevertheless, shared conformational epitopes cannot be ruled out from our results. A 30-kD allergen has been described as a protein strongly recognized by the IgE antibodies in sera from soybean-sensitive patients. However, it was isolated from the 7S globulin fraction [16].
Bruno et al. [34] have reported that soy allergy is uncommon in atopic infants suffering from food allergy. It has been demonstrated that soy proteins are less immunogenic and allergenic than CMP [35]; no fatal anaphylactic reactions have been triggered in animal models sensitized with soy proteins. Only a few cases of soy anaphylaxis have been reported in human subjects [36], and it appears to be a rare phenomenon [37]. In spite of this, some authors advise avoidance of soy-based formulas in infants, because it is as antigenic as CM formulas [13,38,39].
Cross-reactivities have been found among proteins included in the same food group, or even between food and non food proteins. Some cross-reactive proteins are phylogenetically closely related (legumes, cereals, fish, crustaceans, fruits, pollens, etc.), whereas some others have been described among unrelated proteins (birch pollens and apple, carrot and hazelnut, ragweed pollen and banana and melon, etc.) [40–42]. Some in vitro cross-reactivities may have clinical relevance (i.e. between crustaceans) [43,44], while other cross reactivities may be of no clinical importance (for example between legumes) [45,46]. Different factors may contribute to in vivo reactions: the nature of the food, the structure and conformation of the cross-reactive proteins, the type of epitopes involved, the genetical constitution of the patient, sensitization of the patient, etc.
To establish the clinical relevance of this in vitro cross- reactivity, both in vitro tests must be combined with food challenge. Since we have not characterized the in vivo allergenic properties of the isolated S30 protein, and as casein fractions from CM behave as major allergens [3,4,47], we did not perform DBPCFC with this component. Besides, no correlation between the total IgE level and the quantity of specific IgE was observed, as is shown in Table 1. Similarly, no correlation was found among the classes of CM-specific IgE and soy- or S30-specific IgE.
The results from in vitro cross-reactivity have important implications for the selection of effective hypoallergenic substitutes for food allergic patients. The precise identification of the cross-reactive components would represent a significant advance in the problem of food intolerance, since this information is critical to avoid exposure to the allergen. As far as we know, this is the first report of cross reactivity between caseins from CM and globulin 11S from soybean. Our findings may be of interest in the study of the relationship between structure, IgE binding capacity, and features of the antigenic and allergenic epitopes of components from different origins that constitute food allergens.
Altogether our results suggest that the highly allergenic caseins cross-react with the B3 polypeptide from the 11S globulin of soybean. This in vitro cross-reactivity should be considered when CMA patients are treated with soy-derivatives.
Acknowledgments
This project was supported by the Agencia Nacional de Promoción Científica y Tecnológica PICT 1177 and the CONICET PIP 0012/98 (Argentina).
REFERENCES
- 1.Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr. 1990;117:561–7. doi: 10.1016/s0022-3476(05)80689-4. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–28. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 3.Docena G, Fernandez R, Chirdo F, Fossati C. Identification of casein as the major allergenic and antigenic protein of cow’smilk. Allergy. 1996;51:412–6. doi: 10.1111/j.1398-9995.1996.tb04639.x. [DOI] [PubMed] [Google Scholar]
- 4.Restani P, Gaiaschi A, Plebani A, et al. Cross-reactivity between milk proteins from different animal species. Clin Exp Allergy. 1999;29:997–1004. doi: 10.1046/j.1365-2222.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- 5.Gjesing B, Osterballe O, Schwartz B, Wahn U, Lowenstein H. Allergen-specific IgE antibodies against antigenic components in cow's milk and milk substitutes. Allergy. 1986;41:51–6. doi: 10.1111/j.1398-9995.1986.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 6.Maynard F, Chatel JM, Wal Jm. Immunological IgE cross-reactions of bovine and human α-lactalbumins in cow's milk allergic patients. Food Agric Immunol. 1999;11:179–89. [Google Scholar]
- 7.Spuergin P, Mueller H, Walter M, Schiltz E, Forster J. Allergenic epitopes of bovine alpha S1-casein recognized by human IgE and IgG. Allergy. 1996;51:306–12. doi: 10.1111/j.1398-9995.1996.tb04614.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernard H, Creminon C, Yvon M, Wal JM. Specificity of the human IgE response to the different purified caseins in allergy to cow's milk proteins. Int Arch Allergy Immunol. 1998;115:235–44. doi: 10.1159/000023906. [DOI] [PubMed] [Google Scholar]
- 9.Ball G, Shelton MJ, Walsh BJ, Hill DJ, Hosking CS, Howden ME. A major continuous allergenic epitope of bovine beta-lactoglobulin recognized by human IgE binding. Clin Exp Allergy. 1994;24:758–64. doi: 10.1111/j.1365-2222.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 10.Mercier JC, Grosclaude F, Ribadeau-Dumas B. Primary structure of bovine s1 casein. Complete sequence] Eur J Biochem. 1971;11:41–51. doi: 10.1111/j.1432-1033.1971.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 11.Dalgleish D. Structure-function relationships of caseins. In: Damodaran S, Paraf A, editors. Food Proteins and Their Applications. New York: Marcel Decker; 1997. pp. 199–223. [Google Scholar]
- 12.Bock SA, Lee WY, Remigio LK, May CD. Studies of hypersensitivity reactions to foods in infants and children. J Allergy Clin Immunol. 1978;62:327–34. doi: 10.1016/0091-6749(78)90132-x. [DOI] [PubMed] [Google Scholar]
- 13.Burks AW, Jr, Brooks JR, Sampson HA. Allergenicity of major component proteins of soybean determined by enzyme-linked immunosorbent assay (ELISA) and immunoblotting in children with atopic dermatitis and positive soy challenges. J Allergy Clin Immunol. 1988;81:1135–42. doi: 10.1016/0091-6749(88)90881-0. [DOI] [PubMed] [Google Scholar]
- 14.Fukushima D. Structures of plant storage proteins and their functions. Food Rev Int. 1991;7:353–81. [Google Scholar]
- 15.Tsuji H, Bando N, Hiemori M, Yamanishi R, Kimoto M, Nishikawa K, Ogawa T. Purification of characterization of soybean allergen Gly m Bd 28K. Biosci Biotechnol Biochem. 1997;61:942–7. doi: 10.1271/bbb.61.942. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa T, Tsuji H, Bando N, Kitamura K, Zhu YL, Hirano H, Nishikawa K. Identification of the soybean allergenic protein, Gly m Bd 30K, with the soybean seed 34-kD oil-body-associated protein. Biosci Biotechnol Biochem. 1993;57:1030–3. doi: 10.1271/bbb.57.1030. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa T, Bando N, Tsuji H, Okajima H, Nishikawa K, Sasaoka K. Investigation of the IgE-binding proteins in soybeans by immunoblotting with the sera of the soybean-sensitive patients with atopic dermatitis. J Nutr Sci Vitaminol. 1991;37:555–65. doi: 10.3177/jnsv.37.555. [DOI] [PubMed] [Google Scholar]
- 18.Shibasaki M, Suzuki S, Tajima S, Nemoto H, Kuroume T. Allergenicity of major component proteins of soybean. Int Arch Allergy Appl Immunol. 1980;61:441–8. doi: 10.1159/000232472. [DOI] [PubMed] [Google Scholar]
- 19.Whitney R. McL. Proteins of Milk. In: Wing NP, editor. Fundamentals of Dairy Chemistry. New York: Van Nostrand Reinhold; 1988. pp. 81–169. [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall LJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Galfré GA, Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Meth Enzymol. 1981;73:3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Schagger H, Von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamida gel electrophoresis for the separation of proteins in the region from 1 to 100 kD. Annal Biochem. 1987;166:368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 24.Tsang VCW, Peralta JN, Simons AR. Enzyme linked immunoelectrotransfer blot techniques (EITB) for studying by gel electrophoresis. Meth Enzymol. 1983;92:377–91. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- 25.Docena G, Fernandez R, Ocampo M, Fossati C. Serological investigation of latex allergy in Argentina. Allergy Asthma Proc. 1999;20:99–106. doi: 10.2500/108854199778612617. [DOI] [PubMed] [Google Scholar]
- 26.Ceska M, Eriksson R, Varga JM. Radioimmunosorbent assay of allergens. J Allergy Clin Immunol. 1972;49:1–9. doi: 10.1016/0091-6749(72)90117-0. [DOI] [PubMed] [Google Scholar]
- 27.Lundkvist U. Research and development of the RAST technology. In: Evans R, editor. Advances in Diagnosis of Allergy: RAST. Miami: Symposia Specialists; 1975. p. 85. [Google Scholar]
- 28.Kahn A, Mozin MJ, Rebuffat E, Sottiaux M, Muller MF. Milk intolerance in children with persistent sleeplessness: a prospective double-blind crossover evaluation. Pediatrics. 1989;84:595–603. [PubMed] [Google Scholar]
- 29.Hill DJ, Ford RP, Shelton MJ, Hosking CS. A study of 100 infants and young children with cow's milk allergy. Clin Rev Allergy. 1984;2:125–42. doi: 10.1007/BF02991061. [DOI] [PubMed] [Google Scholar]
- 30.Perkkio M, Savilahti E, Kuitunen P. Morphometric and immunohistochemical study of jejunal biopsies from children with intestinal soy allergy. Eur J Pediatr. 1981;137:63–9. doi: 10.1007/BF00441172. [DOI] [PubMed] [Google Scholar]
- 31.Utsumi S, Matsumura Y, Mori T. Structure-function relationships ofsoy proteins. In: Damodaran S, Paraf A, editors. Food Proteins and Their Applications. New York: Marcel Decker; 1997. pp. 257–91. [Google Scholar]
- 32.Staswick PE, Hermodson MA, Nielsen NC. Identification of the cystines which link the acidic and basic components of the glycinin subunits. J Biol Chem. 1984;259:13431–5. [PubMed] [Google Scholar]
- 33.Nielsen NC. The structure and complexity of the 11S polypeptides in soybean. J Am Oil Chem Soc. 1985;62:1680. [Google Scholar]
- 34.Bruno G, Giampietro PG, Del Guercio MJ, et al. Soy allergy is not common in atopic children: a multicenter study. Pediatr Allergy Immunol. 1997;8:190–3. doi: 10.1111/j.1399-3038.1997.tb00159.x. [DOI] [PubMed] [Google Scholar]
- 35.Piacentini GL, Benedetti M, Spezia E, Boner AL, Bellanti JA. Anaphylactic sensitizing power of selected infant formulas. Ann Allergy. 1991;67:400–2. [PubMed] [Google Scholar]
- 36.David TJ. Anaphylactic shock during elimination diets for severe atopic eczema. Arch Dis Child. 1984;59:983–6. doi: 10.1136/adc.59.10.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magnolfi CF, Zani G, Lacava L, Patria MF, Bardare M. Soy allergy in atopic children. Ann Allergy Asthma Immunol. 1996;77:197–201. doi: 10.1016/S1081-1206(10)63255-3. [DOI] [PubMed] [Google Scholar]
- 38.Committee on Nutrition. Soy protein formulas: recommendation for use in infant feeding. Pediatrics. 1983;72:359–63. [PubMed] [Google Scholar]
- 39.ESPGAN Committee on Nutrition. comment on the composition on soy-protein based infant and follow-up formulas. Acta Pediatr Scand. 1990;79:1001–5. doi: 10.1111/j.1651-2227.1990.tb11373.x. [DOI] [PubMed] [Google Scholar]
- 40.Enberg RN, Leickly FE, Mccullough J, Bailey J, Ownby DR. Watermelon and ragweed share allergens. J Allergy Clin Immunol. 1987;79:867–75. doi: 10.1016/0091-6749(87)90234-x. [DOI] [PubMed] [Google Scholar]
- 41.Ebner C, Hirschwehr R, Bauer L, et al. Identification of allergens in apple, pear, celery, carrot and potato: cross-reactivity with pollen allergens. Monogr Allergy. 1996;32:73–7. [PubMed] [Google Scholar]
- 42.Caballero T, Martin-Esteban M. Association between pollen hypersensitivity and edible vegetable allergy: a review. J Invest Allergol Clin Immunol. 1998;8:6–16. [PubMed] [Google Scholar]
- 43.Lehrer SB. The complex nature of food antigens: studies of cross- reacting crustacea allergens. Ann Allergy. 1986;57:267–72. [PubMed] [Google Scholar]
- 44.Halmepuro L, Salvaggio JE, Lehrer SB. Crawfish and lobster allergens: identification and structural similarities with other crustacea. Int Arch Allergy Appl Immunol. 1987;84:165–72. doi: 10.1159/000234418. [DOI] [PubMed] [Google Scholar]
- 45.Taylor SL, Lemanske RF, Bush RK, Busse WW. Food allergens. structure and immunologic properties. Ann Allergy. 1987;59:93–9. [PubMed] [Google Scholar]
- 46.Barnett D, Bonhan B, Howden ME. Alergenic cross-reactions among legume foods-in vitro study. J Allergy Clin Immunol. 1987;79:443–38. doi: 10.1016/0091-6749(87)90359-9. [DOI] [PubMed] [Google Scholar]
- 47.Spuergin P, Walter M, Chiltz E, Deichmann K, Forster J, Mueller H. Allergenicity of alpha-casein from cow, sheep and goat. Eur J Allergy Clin Immunol. 1997;52:293–8. doi: 10.1111/j.1398-9995.1997.tb00993.x. [DOI] [PubMed] [Google Scholar]


