Abstract
Macrophages play an important role in immune and inflammatory responses, largely through secretion of bioactive molecule such as cytokines. While calcium is known to be an important regulator of this process, less is known about the role of other ions and the ion channels that regulate them. We have previously implicated an outwardly rectifying potassium channel (Kor) in this process and for this reason we have investigated the role of potassium (K+) and K+ channels in the regulation of tumour necrosis factor-α (TNF-α)and interleukin (IL)-8 production by activated human culture-derived macrophages. The effect of blockade of Kor is to inhibit phorbol myristate acetate (PMA)-induced cytokine production by translational or post-translational mechanisms, an effect that is duplicated by increasing extracellular K+. By contrast, the effects of K+ on LPS-stimulated cells are far more complex and are probably mediated through the change of osmolality and occur largely at the mRNA level. This data directly implicates K+, and its regulation through Kor, in early events following PMA stimulation of these cells.
Keywords: cellular activation, cytokines, monocytes/macrophages, potassium ion channels
INTRODUCTION
Macrophages (MACs) play an important role in immune and inflammatory responses, participating in many normal biological processes including wound healing and resistance to tumours and infections. They are also key cells in mediating the pathology of chronic inflammatory diseases such as atherosclerosis and rheumatoid arthritis. This is largely through their capacity to secrete bioactive molecules such as enzymes, lipids and cytokines. The secretion of those molecules is regulated by a variety of stimuli including cytokines, bacterial cell products such as lipopolysaccharide (LPS), and a wide range of other mediators. Some of the responses to these stimuli are mediated through changes in calcium (Ca++) [1], which may be involved in phosphorylation of nucleotides or proteins [2]. However, the role of other ions such as potassium (K+) is much less well understood. For technical reasons the real-time measurement of K+ concentrations in viable cells is difficult. Therefore, much of what is known about K+ and cell function is derived from effects associated with the blockade of K+ channels.
K+ channels are widely expressed on all types of cells, from excitable cells to non-excitable cells. They play important roles in excitable cells, such as neurones and myocytes. In recent years, an increasing number of reports have shown the importance of K+ channels in other cell types, such as β cells and lymphocytes [3–13]. There are four major functional classes of K+ channels: voltage-dependent K+ channels (Kv), which are activated by membrane depolarization; Ca++-activated K+ channels [including large (MaxiK) and small (SK) Ca++-activated K+ channels], which are primarily gated by intracellular Ca++ inwardly rectifying K+ channels (Kir); and sodium (Na+)-activated K+ channels. Each has a somewhat different pattern of sensitivity to a range of K+ ion channel blocking drugs. K+ channels have been identified on lymphocytes, MACs and neutrophils and strong evidence supports their roles in the function of lymphocytes [3–13].
Several different K+ channels have been described on human and mouse monocytes and MACs, such as MaxiK, Kir, Kv and SK [14]. However, due to the great heterogeneity of monocytes and MACs [15], the expression and activity of K+ channels also varies depending on cell origin and culture conditions, such as the presence or absence of serum, time period, adherence or lack of adherence [16–21]. K+ channel expression in MACs can also be modulated by a variety of different factors such as LPS, phorbol myristate acetate (PMA), macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) and Leishmania amazonenisis [22–27].
Evidence from patch-clamp studies has also shown that MAC K+ currents can be triggered immediately by some stimuli, such as platelet activating factor [28], heat shock protein hsp70 [29], G-protein activators (GTPγS, GppNHp, AIF4 and inositol 1,4,5-trisphosphate) [30–32] and zymosan and opsonized zymosan [33]. LPS, a very potent stimulator of MACs, has been reported recently to open MaxiK on human peripheral blood-derived MACs [34,35]. More recently, our group has studied a Ca++ sensitive outwardly rectifying K+ channel (Kor) in 5–7-day-old human monocyte-derived MACs [36]. This channel was stretch-sensitive, being activated immediately on MAC adherence, and was inhibited by 4-amino-pyridine (4AP) and barium chloride (BaCl2), but not tetraethylammonium (TEA). Additionally, it was activated immediately on stimulation by interleukin (IL)-2 or IL-6, but not transforming growth factor (TGF)-β. The open probability of this channel was increased if the stimulated cells were pretreated for 24 h with IFN-γ. This channel was functionally closed when cells were in the non-adherent state with no detectable conductance, even if the cells were stimulated with cytokine.
Largely through strategies involving the use of ion channel blockers, K+ channels have been implicated in the control of MAC function. K+ channel blockers such as quinine, TEA or BaCl2[but not apamin and charybdotoxin (ChTX)] have been shown to inhibit production of TNF-α by IFN-γ primed, LPS-activated MACs [37,38]. Additionally, K+ channel blockers such as 4AP, TEA and apamin have been reported to inhibit LPS-induced tissue factor expression by human monocytes [39]. Conversely, brief K+ depletion (60 min) induced by agents such as nigericin and valinomycin was able to stimulate IL-1 processing [40]. Paxilline, the specific MaxiK channel blocker, was able to block LPS-induced TNF-α release from human MACs by blocking the MaxiK channel [34,35].
From the above findings, it seems clear that K+ channels are involved directly in the activation process of MACs. In this report, we provide further strong evidence in support of the link between K+ and MAC activation. Secretion of TNF-α and IL-8 from human MACs activated by either PMA or LPS is inhibited by 4AP blockade of a Kor channel that we have linked previously to the MAC activation process. In addition, we also elucidate some of the mechanisms underlying this effect and provide further evidence for its specificity by experiments in which we manipulate K+ directly, by raising its extracellular concentration.
MATERIALS AND METHODS
Reagents and solutions
LPS, PMA, K+ aspartate, Na+ selenite, 4AP, TEA, Sotalol, and ChTX were purchased from Sigma (St Louis, USA). Sucrose was from Ajax (Auburn, NSW, Australia). The ELISA kits for measuring human TNF-α and IL-8 were bought from Genzyme (Germany). Iscove's Modified Dulbecco's Medium (IMDM) was from Gibco Life Technology (Grand Island, NY, USA). Nutridoma-SR (100×) was from Boehringer Mannheim (Germany) and l-glutamine was from P.A. Biologicals (Sydney, Australia). The RNeasy kit was purchased from Qiagen (Germany) and the LightCycler-FastStart DNA Master SYBR Green I kit was from Roche Molecular Biochemicals (Germany).
Human peripheral blood mononuclear cells (PBMC) were isolated from buffy coats of normal volunteers by Ficoll/Paque density gradient centrifugation and monocytes were then purified from PBMC by counter-current centrifugal elutriation [41]. Culture-derived MACs developed from these cells were cultured essentially as previously described [42,41]. In brief, cells were cultured at a density of 5 × 105 cells/ml in IMDM supplemented with 1% Nutridoma-SR, 4 mm l-glutamine (P.A. Biologicals, Australia) and 5 ng/ml Na ± selenite (Sigma).
Cytokine assay
Monocytes purified by elutriation were plated into 24-well (5 × 105 cells per well) or 96-well (1 × 105 cells per well) tissue culture plates (Falcon, Becton Dickson, USA). After overnight incubation, cells were washed with PBS twice to remove any nonadherent cells and then fresh culture medium alone or with either blockers, K+, or sucrose was added to cells 15 min prior to activation by LPS (1 or 10 µg/ml) or PMA (40 nm). Cells were then incubated for various periods at 37°C (with 5% carbon dioxide) until the supernatants were harvested for the measurement of TNF-α and IL-8 using DuoSet human TNF-α and IL-8 ELISA kits according to the manufacturer's instructions. The DNA content of cells was measured by CyQuant cell proliferation assay kit, following the company's instruction. All treatments were performed in triplicate and all experiments were repeated at least three times. Data represent the mean ± standard deviation of 15–18 determinations from three or five individual experiments. Significance was determined with anova using StatView software (ABACUS Concepts, USA).
Reverse transcription (RT)-polymerase chain reaction (PCR) analysis by real-time PCR
Cells, 5 × 106 for each treatment, were incubated for 3 h with 1 µg/ml LPS or 40 nm PMA, and total RNA was extracted using an RNeasy kit according to the manufacturer's protocol. For accurate RNA quantification, the concentration of total RNA was measured by SYBR Green II staining (Molecular Probes, the Netherlands) on a Fluoroskan II (Labsystems, Finland). Total RNA, 600 ng, were incubated (10 min, 65°C) and transcribed into cDNA by 50 units ExpandTM reverse transcriptase in a 20-µl reaction that also contained 20 nmol dNTPs, 10 pmol oligo(dT)17, 200 nmol DTT, 4 µl 5x ExpandTM reverse transcriptase buffer (Roche, Germany), and deionized water. The mixtures were incubated for 1 h at 42°C. One µl of the mixture was assayed in each real-time PCR on a LightCycler (Roche), using FastStart DNA master SYBR Green I kit. The forward primer sequences for TNF-α (GeneBank accession no: XM_041847), IL-8 (GeneBank Accession No: Y00787), and GAPDH (GeneBank Accession No: XM_033269) were CTGCCTGCTGCACTTTGGAGTGAT, GAAGGAACCATCTCACTGTGTGTA and CATGGCAAAT TCCATGGCACCGT, respectively, and the reverse primer sequences for TNF-α, IL-8 and GAPDH were GCAGCCTTG GCCCTTGAAGAGGA, CTTCAAAAACTTCTCCACAAC CCT and GCAGTGATGGCATGGACTGTGGT, respectively. The DNA primers were derived from different exons to ensure the PCR products represent the specific mRNA species and not genomic DNA. The reaction mixtures were prepared according to the company's instruction with a slight change. The final concentration of magnesium chloride was 3 mm and the reaction volume was 10 µl only. The PCR was carried out as follows: (1) denaturation: 95°C for 8 min; (2) amplification: 95°C for 15 s, 55°C (IL-8) or 56°C (TNF-α and GAPDH) for 5 s and 72°C for 20 s; (3) melting curve: 66°C for 10 min; (4) cooling 2 min PCR products of selected samples were separated on 1% agarose gels and stained with ethidium bromide to confirm that there was only a specific single band from each sample. Data represents mean values ± standard deviation of 3 PCR reactions for each treatment compared with the control. Significance was determined by anova in StatView.
RESULTS
The effects of K+ channel blockers on MAC TNF-α and IL-8 production
To help determine whether K+ channels are involved in cytokine production by MACs, we analysed the effects of the K+ channel blockers on TNF-α and IL-8 production by LPS or PMA-activated MACs. Four different types of K+ channel blockers (4AP, TEA, Sotalol and ChTX) were used in these experiments because of their different blocking mechanisms and preference for different types of K+ channels. ChTX is a specific blocker for MaxiK and Kv1·3 channels. Sotalol blocks human erg K+ channels. TEA and 4AP inhibit several K+ channels including Kv and Kir. Electrophysiological studies have shown that 4AP, but not TEA, can block the Kor channel activity of MACs [36]. As we had previously implicated this ion channel in the early phases of MAC activation, it was of particular interest to us.
These blockers, in a range of doses, were added 15 min before activation with LPS (1 µg/ml) or PMA (40 nm). After 24 h, the conditioned medium was collected for measurement of TNF-α and IL-8, two important products of activated MACs that are secreted from MAC by different mechanisms. The results of representative experiments are shown in Fig. 1a,b. For statistical comparison, the full datasets have been normalized by assigning the sample activated in the absence of blockers an arbitrary value of 100%. Other samples were then scaled in direct relationship to this value. As shown in Fig. 2, 4AP at 2 mm or 5 mm was the only K+ channel blocker which caused a major decrease in the release of both TNF-α and IL-8. For example, PMA-induced TNF-α and IL-8 secretion was inhibited to 27 ± 11·6% and 18·2 ± 18·9% (P < 0·0001) of maximum, respectively, by 5 mm 4AP (Fig. 2c,d). It is noticeable that the reduction was greater when cells were activated with PMA rather than LPS (Fig. 2a,c). Additionally, there was greater reduction in IL-8 than TNF-α (Fig. 2c,d). As PMA and LPS activate MACs through different signalling pathways [1,43], it seems likely that the greater reduction in cytokine production in PMA-activated cells reflects the greater susceptibility of this pathway to changes induced by 4AP. The greater reduction in release of IL-8 compared with TNF-α may be due to the autocrine/paracrine effects of TNF-α in up-regulating the secretion of other cytokines such as IL-8 [44].
Fig. 1.
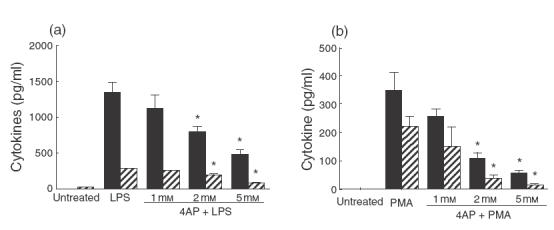
Effects of 4AP on TNF-α and IL-8 production by activated human MACs. Cells were incubated with (a) LPS (1 µg/ml) or (b) PMA (40 nm) in the presence or absence of 4AP (1, 2 and 5 mm) for 24 h. Supernatants were collected and assayed for TNF-α and IL-8 concentration by ELISA and the results plotted as the mean ± 1 standard deviation of triplicate data points. The results of one representative experiment are illustrated. The bar key, IL-8 (×100) indicates that the real concentrations of IL-8 are 100-fold of the bar indicated in the graph. Statistically significant differences (P-value < 0·01) are highlighted with an asterisk. ▪, TNF-α;  , IL-8 (×100)
, IL-8 (×100)
Fig. 2.
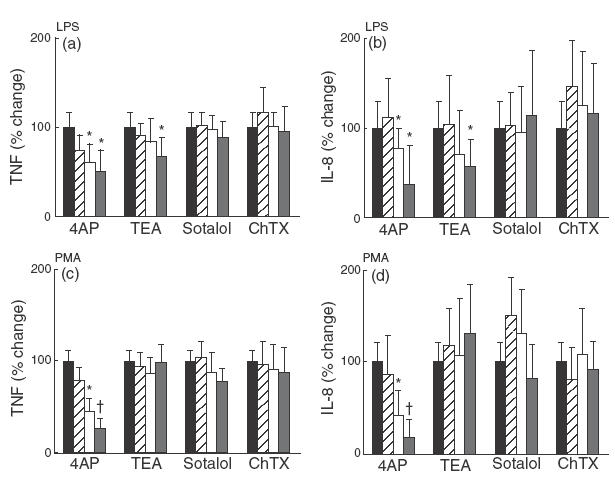
Effect of K+ channel blockers on TNF-α and IL-8 production by activated human MACs. Cells were cultured with LPS (1 µg/ml) (a) and (b) or PMA (40 nm) (c) and (d) in the presence or absence of 4AP, TEA, Sotalol and ChTX. The low, medium and high concentrations in the graph represent 1, 2 and 5 mm of 4AP; 1, 10 and 20 mm of TEA; 10, 50 and 100 µm of Sotalol; 10, 50 and 100 nm of ChTX. After 24 h, the conditioned medium from triplicate data points was collected and assayed for TNF-α (a) and (c) and IL-8 (b) and (d) concentration by ELISA. The mean data was then normalized so that the cytokine concentration in activated, but otherwise untreated samples was 100%. The results in these panels represent the mean value of 12–18 individual samples from four to six different experiments ± standard deviation. Statistically significance differences are marked highlighted with an asterisk (P < 0·05) and dagger (P < 0·01). (a, b): ▪, LPS only;  , low; □, medium;
, low; □, medium; high. (c, d): ▪, PMA only;
high. (c, d): ▪, PMA only;  , low; □, medium;
, low; □, medium;  , high.
, high.
TEA, another commonly used K+ channel blocker, caused a small but significant reduction in TNF-α and IL-8 but only at the highest concentration (20 mm). However, this occurred only where MACs were activated by LPS and not in PMA-stimulated cells (Fig. 2a,b,c,d). Sotalol and ChTX had no effect on TNF-α or IL-8 production induced by LPS or PMA.
To exclude the possibility that the reduction in cytokine release was due to cell death, cell numbers were quantified by CyQUANT cell proliferation assay kit and viability of cells was examined by the trypan blue exclusion method. There was no reduction in cell number or viability associated with the use of 4AP (data not shown).
Time-course of 4AP effect on MAC TNF-α secretion
To understand more about the action of 4AP, time-course experiments were performed. 4AP at a final concentration of 5 mm was added to MAC cultures at different time-points prior to or after activation by LPS or PMA. After 24 h of culture with the activating agent the conditioned medium was then collected for TNF-α measurement. The inhibition of TNF-α secretion induced by 4AP was equipotent at time points between 15 min before and 60 min after activation (Fig. 3). However, when 4AP was added 2 h after activation, the inhibition was somewhat weaker although still marked.
Fig. 3.
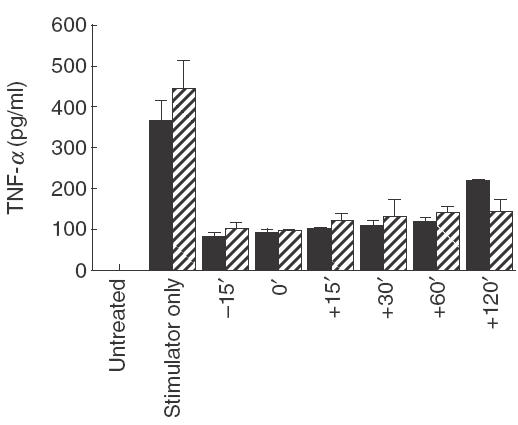
Time-course effect of 4AP on TNF-α production by human MACs. 4AP, at a final concentration of 5 mm, was added to the culture medium at the different time points (15 min before, same time or 15 min, 30 min, 60 min or 120 min after LPS or PMA activation). MACs were then cultured for the remaining time period to 24 h. The media were harvested for TNF-α measurement by ELISA. These data illustrate the results from one of three individual experiments and represent the mean value of triplicate samples ± 1 standard deviation. The bar key, LPS × 10 shows that the real concentrations of TNF-α by LPS-activated MACs are 10-fold of the bar indicated in the graph. ▪, LPS × 10 (1 µg/ml);  , PMA (40 nm).
, PMA (40 nm).
Effects of extracellular K+ on MAC TNF-α production
It is always possible that pharmacological blockers such as the ones used in the experiments described above exert their effects by mechanisms other than ion channel blockade. In order to obtain further supporting evidence for the role of K+ ions in the activation process, we have directly mimicked the altered changes in cellular K+ associated with blockade of Kor by adding differing concentrations of extracellular K+ to MAC culture. Two different types of experiment were performed with each of the activating agents: the short-term exposure of the cells to the changed K+ concentrations (for only the first 90 min of total incubation period) and the prolonged experiments in which MACs were exposed to increased K+ for the whole 24 h period of the cell culture. In both types of experiment, the extracellular K+ concentration in the culture medium was modified through the addition of K+ (in the form of K+ aspartate) at 30 mm, 60 mm or 90 mm concentrations (the final concentration of the K+ being 34·5 mm, 64·5 mm and 94·5 mm, respectively). K+ aspartate was used instead of KCl to exclude any effect due to chloride (Cl) ions. Sucrose was added at equimolar concentrations to some cultures to control for the osmotic changes induced by the added K+.
In the short-term experiments, the modified medium was added to the cells and allowed to equilibrate for 15 min MACs were then activated with LPS or PMA. After 90 min in the presence of the increased osmolyte and the activating agent, the medium was removed from all wells and cells were washed twice with PBS. Fresh culture medium with a normal K+ content was then added to each well. No further LPS or PMA were added. The cells were then cultured to a total of 24 h after activation and the conditioned medium was harvested for analysis. As shown in Fig. 4d, when MACs were activated with PMA the increased K+ inhibited TNF-α production in a dose-dependent manner, giving a reduction from 64·7 ± 53·1% to 30·8 ± 25·9% of the maximum (P < 0·01) (Fig. 4d). Equimolar concentrations of sucrose showed no effect on PMA activated MACs, suggesting that K+ may play a role in the early PMA-induced MAC activation events. However, when MACs were activated by LPS (Fig. 4c) both K+ and sucrose induced statistically significant and similar decreases in TNF-α production in a dose-dependent manner, with complete inhibition of TNF-α secretion at the 90 mm concentration. This result indicated that the effect of increased K+ on TNF-α production by LPS-activated MACs appears to be due to the osmotic change rather than a direct effect of K+ ions. This is in contrast to PMA mediated changes.
Fig. 4.
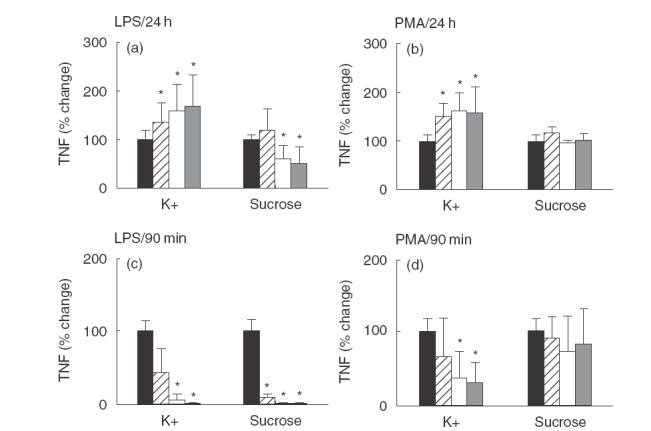
Effects of K+ and sucrose on TNF-α production by human MACs. MACs were cultured under different conditions: (a) LPS (1 µg/ml) ± K+ or sucrose for 24 h; (b) PMA (40 nm) ± K+ or sucrose for 24 h; (c) LPS (10 µg/ml) ± K+ or sucrose for 90 min only. At 90 min the culture medium was changed for normal culture media for the remaining time period up to 24 h; (d) PMA (40 nm) ± K+ or sucrose for 90 min only. At 90 min the culture medium was changed for normal culture media for the remaining period up to 24 h. All the conditioned media were harvested for TNF-α measurement at 24 h after stimulation. The results represent the mean data for the experimental condition divided by the corresponding control, expressed as a percentage. The results in these panels represent the mean value of 12 individual samples from four individual experiments ± standard deviation. Statistically significant differences (P-value < 0·01) are highlighted with an asterisk. (a, c): ▪, LPS only;  , 20 mM; □, 60 mM;
, 20 mM; □, 60 mM;  , 90 mM. (b, d): ▪, PMA only;
, 90 mM. (b, d): ▪, PMA only;  ;, 20 mM; □, 60 mM;
;, 20 mM; □, 60 mM;  , 90 mM.
, 90 mM.
In 24 h experiments, the modified culture medium remained on the cells for the whole 24 h following activation with either LPS or PMA. When MACs were activated with LPS and cultured with sucrose a statistically significant decrease in TNF-α production occurred (Fig. 4a). At 60 mm sucrose only 61·2 ± 27·3% of the maximum TNF-α levelwas produced (P < 0·0034), and at 90 mm only 51·6 ± 33·6% of the maximum was produced (P < 0·0004). This was similar to that in the short-term experiments described above, although the inhibition of TNF-α in the latter was somewhat more effective. Surprisingly, adding K+ to LPS stimulated cells in long-term experiments gave a totally different result to that in the short-term ones. In LPS-stimulated cells, K+ at 30 mm to 90 mm significantly up-regulated TNF-α secretion by 159·4 ± 54·8% to169·0 ± 64·8% of the maximum, respectively (Fig. 4a). In doing this, it also overcame the inhibitory effects due to the increased osmolality.
When PMA was used as an activator, the increased sucrose gave essentially similar results to those observed in short-term exposure, with no effect on TNF-α secretion (Fig. 4b). However, the prolonged incubation of MACs in increased K+ caused a significant increase in TNF-α secretion of more than 150 ± 25% (P < 0·0001) of the maximum when 30 mm extra K+ was added, just as observed in LPS stimulated MACs.
Effect of 4AP, K+ and sucrose on cytokine gene expression by activated MACs
To help understand the mechanisms underlying the effects of 4AP, and K+ on the activation of MACs, TNF-α and IL-8 mRNA was quantified using RT-PCR (Fig. 5). GAPDH mRNA was measured as a control. Gene expression of TNF-α in PMA-activated human MACs was not affected by 4AP, K+ and sucrose (osmolyte) (Fig. 5a,b). However, when cells were stimulated by LPS, TNF-α mRNA was significantly inhibited by 4AP, K+ and sucrose. While extracellular K+ caused more than 50% inhibition (P < 0·05), this is likely to be due to the osmotic effects because of similar changes induced by sucrose. This suggests that in LPS-activated cells, the effects on TNF-α are mediated substantially at the mRNA level, while in PMA activated cells, the effects are largely post-transcriptional.
Fig. 5.
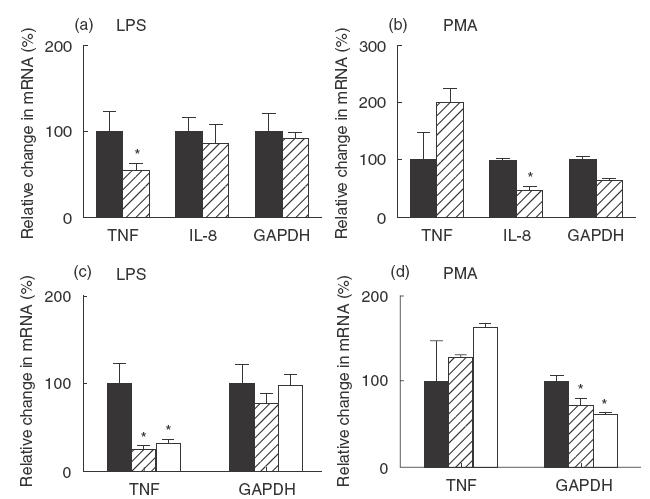
Effects of 4AP, K+ and sucrose on the expression of cytokines by MACs. MACs were incubated with LPS (a, c) or PMA (b, d) in the presence or absence of 4AP (5 mm) (a, b), K+ (90 mm) or sucrose (90 mm) (c, d) for 3 h. Then total RNA was extracted and mRNA of TNF-α, IL-8 and GAPDH was measured by real-time PCR. The data represent one of three individual experiments. Each data point represents the mean value of triplicate samples ± their standard deviation. Statistically significant differences (P-value < 0·05) are highlighted with an asterisk. (a, b): ▪, stimulator only; 4AP stimulator. (c, d): ▪, stimulator only;
4AP stimulator. (c, d): ▪, stimulator only;  K+/stimulator; □, sucrose/stimulator.
K+/stimulator; □, sucrose/stimulator.
The mRNA expression of IL-8 followed a slightly different pattern (Fig. 5a). 4AP had no effects on IL-8 mRNA when the cells were either LPS- or PMA-activated. This suggests that when MACs are activated by LPS or PMA, 4AP exerts its effects largely by post-transcriptional mechanisms.
DISCUSSION
Our previous observations that the stretch-sensitive Kor channel [36] is activated almost immediately on exposure of MACs to cytokines suggested that this particular K+ channel may be involved in the activation process of these cells. To explore this hypothesis further, we examined the effects of a range of K+ channel blockers on cytokine production by activated MACs. A number of channel blockers were tested and 4AP and TEA were selected specifically because the Kor channel can be blocked by the former but not the latter. We have demonstrated that only 4AP, but not other blockers can inhibit cytokine production by PMA-activated human MACs. This suggests that the effect of 4AP on MACs is due to specific blocking of one or more potassium channels, and not due to non-specific inhibition. This has been supported further by the results from our 90-min experiments and is discussed in more detail in the next section. In addition, because ChTX, even at 300 nm concentration (data not shown), has not shown the same inhibitory effect, the MaxiK and Kv1·3 channels are less likely to be involved. In tissue culture systems it is possible that there was insufficient bio-available ChTX to exert an effect. We have previously reported a MAC Kor channel sensitive to 4AP, but not TEA [36]. On the basis of these observations it is reasonable to suppose that this ion channel is involved in the effects we have observed. Also involved may be other Kv or Kir channels which can also be sensitive to 4AP, but not TEA.
That the effect of 4AP on the K+ channel is specific and is further supported by the results of experiments involving an increase in the extracellular K+, a strategy that mimics the effect of channel blockade with 4AP. In short exposure experiments (increased K+ for the first 90 min), our results indicate that the increased extracellular K+ concentration inhibited TNF-α production by PMA activated MACs. K+ channels play an essential role in maintaining and resetting the membrane potential of cells [45]. Modification of extracellular K+ concentration is able to regulate the opening and closing of membrane K+ channel at least under some circumstances. It is possible, therefore, that in activated MACs, when the extracellular K+ concentration is increased, the outward K+ currents will be reduced. The consequence of increasing extracellular K+ concentration may be the same as 4AP which blocks the Kor channel.
The result of exposure to increased K+ for the whole 24-h culture period is diametrically opposite to that of short-term exposure. While the reasons for this are not clear, we suggest that long-term exposure to the increased K+ results in a range of compensatory changes to other ions, ion channels and transporters, such as Cl− and Na+ K+ pumps on the cell membrane, which then cause complex intracellular changes resulting in decreased cytokine production.
There are significant differences between results from LPS- and PMA-stimulated MACs. This is not surprising, as they mimic two different biological responses (reaction to gram negative bacteria and the stress response, respectively), and are known to activate different subsets of genes in these cells. Both 4AP and TEA reduced TNF-α and IL-8 production by LPS-stimulated cells, whereas only 4AP acted on PMA-stimulated cells. This suggests that a wider range of K+ channels, such as MaxiK, Kir, Kv and Kor may be involved in activation induced by LPS. While the increasing extracellular K+ affected LPS stimulated cells, as it did PMA-activated MACs, this effect was mediated by increased osmolality rather the ion itself, as sucrose exhibited an identical effect. The effects of increased osmolality are consistent with previous reports [46–48]. Furthermore, the effect exerted by 4AP was at least in part at mRNA level as TNF-α mRNA was decreased by 4AP, unlike the situation with PMA-stimulated cells.
Recently, Blunck et al. have reported that the TNF-α production by LPS activated human peripheral blood derived MACs can be inhibited by Paxilline, a specific MaxiK channel blocker [34]. This effect could be observed on MACs only after 7 days culture with M-CSF, and not on freshly isolated monocytes. They concluded that a Ca++-sensitive and voltage-dependent K+ channel is involved in the LPS-induced activation process in human MACs, but not in human monocytes [34]. In our study, we used freshly isolated monocytes cultured without M-CSF and demonstrated similar results. That is 4AP and TEA, but not ChTX, were able to inhibit TNF-α and IL-8 production by LPS-activated cells.
Overall, the effects of 4AP on LPS or PMA-stimulated TNF-α and IL-8 were similar except that the degree of inhibition of IL-8 was somewhat greater than that of TNF-α. This is likely to be due to the participation of TNF-α in IL-8 release [44]. However, as they are regulated largely by different post-transcriptional mechanisms, other factors may also be involved.
Based on our result and previous reports, we suggest that the K+ channel involved in the PMA activation process of freshly isolated monocytes is more likely to be the Kor channel, although the possibility of other Kv or Kir channels which are sensitive only to 4AP cannot be excluded.
Acknowledgments
This work has been funded in part by grants from St Vincent's Hospital and by Meriton Apartments Pty Ltd, through an R&D syndicate arranged by Macquarie Bank Limited. In addition, this project was partially supported by a New South Wales Health Research and Development infrastructure grant. Min Ru Qiu was funded by an Australian Postgraduate Award. We thank A/Professor William Sewell and Dr Kristina Warton for critical reading of the manuscript.
REFERENCES
- 1.Sweet MJ, Hume DA. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Gordon S, Keshav S, Chung LP. Mononuclear phagocytes: tissue distribution and functional heterogeneity. Curr Opin Immunol. 1988;1:26–35. doi: 10.1016/0952-7915(88)90047-7. [DOI] [PubMed] [Google Scholar]
- 3.Premack BA, Gardner P. Role of ion channels in lymphocytes. J Clin Immunol. 1991;11:225–38. doi: 10.1007/BF00918180. [DOI] [PubMed] [Google Scholar]
- 4.Sutro JB, Vayuvegula BS, Gupta S, et al. Voltage-sensitive ion channels in human B lymphocytes. Adv Exp Med Biol. 1989;254:113–22. doi: 10.1007/978-1-4757-5803-0_14. [DOI] [PubMed] [Google Scholar]
- 5.Amigorena S, Choquet D, Teillaud JL, et al. Ion channel blockers inhibit B cell activation at a precise stage of the G1 phase of the cell cycle. Possible involvement of K+ channels. J Immunol. 1990;144:2038–45. [PubMed] [Google Scholar]
- 6.Cahalan MD, Chandy KG. Ion channels in the immune system as targets for immunosuppression. Curr Opin Biotechnol. 1997;8:749–56. doi: 10.1016/s0958-1669(97)80130-9. [DOI] [PubMed] [Google Scholar]
- 7.Lin CS, Boltz RC, Blake JT, et al. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med. 1993;177:637–45. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo GC, Blake JT, Talento A, et al. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J Immunol. 1997;158:5120–8. [PubMed] [Google Scholar]
- 9.Cahalan MD, Lewis RS. Role of potassium and chloride channels in volume regulation by T lymphocytes. Soc Gen Physiol Series. 1988;43:281–301. [PubMed] [Google Scholar]
- 10.McKinnon D, Ceredig R. Changes in the expression of potassium channels during mouse T cell development. J Exp Med. 1986;164:1846–61. doi: 10.1084/jem.164.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Decoursey TE, Chandy KG, Gupta S, et al. Two types of potassium channels in murine T lymphocytes. J Gen Physiol. 1987;89:379–404. doi: 10.1085/jgp.89.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahalan MD, Lewis RS. Functional roles of ion channels in lymphocytes. Semin Immunol. 1990;2:107–17. [PubMed] [Google Scholar]
- 13.Freedman BD, Price MA, Deutsch CJ. Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes. J Immunol. 1992;149:3784–94. [PubMed] [Google Scholar]
- 14.Gallin EK. Ion channels in leukocytes. Physiol Rev. 1991;71:775–811. doi: 10.1152/physrev.1991.71.3.775. [DOI] [PubMed] [Google Scholar]
- 15.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–15. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 16.Gallin EK, Sheehy PA. Differential expression of inward and outward potassium currents in the macrophage-like cell line J774.1. J Physiol (Lond) 1985;369:475–99. doi: 10.1113/jphysiol.1985.sp015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallin EK, McKinney LC. Patch-clamp studies in human macrophages: single-channel and whole-cell characterization of two K+ conductances. J Membr Biol. 1988;103:55–66. doi: 10.1007/BF01871932. [DOI] [PubMed] [Google Scholar]
- 18.Randriamampita C, Trautmann A. Ionic channels in murine macrophages. J Cell Biol. 1987;105:761–9. doi: 10.1083/jcb.105.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ypey DL, Clapham DE. Development of a delayed outward-rectifying K+ conductance in cultured mouse peritoneal macrophages. Proc Natl Acad Sci USA. 1984;81:3083–7. doi: 10.1073/pnas.81.10.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson DJ, Jow B, Jow F. Whole-cell currents in macrophages: I. Human monocyte-derived macrophages. J Membr Biol. 1990;117:29–44. doi: 10.1007/BF01871563. [DOI] [PubMed] [Google Scholar]
- 21.Brown H, Kozlowski R, Perry H. The importance of ion channels for macrophage and microglial activation in vitro. Glia. 1998;22:94–7. [PubMed] [Google Scholar]
- 22.McKinney LC, Gallin EK. Effect of adherence, cell morphology, and lipopolysaccharide on potassium conductance and passive membrane properties of murine macrophage J774.1 cells. J Membr Biol. 1990;116:47–56. doi: 10.1007/BF01871671. [DOI] [PubMed] [Google Scholar]
- 23.Nelson DJ, Jow B, Jow F. Lipopolysaccharide induction of outward potassium current expression in human monocyte-derived macrophages: lack of correlation with secretion. J Membr Biol. 1992;125:207–18. doi: 10.1007/BF00236434. [DOI] [PubMed] [Google Scholar]
- 24.Kim SY, Silver MR, DeCoursey TE. Ion channels in human THP-1 monocytes. J Membr Biol. 1996;152:117–30. doi: 10.1007/s002329900091. [DOI] [PubMed] [Google Scholar]
- 25.DeCoursey TE, Kim SY, Silver MR, et al. Ion channel expression in PMA-differentiated human THP-1 macrophages. J Membr Biol. 1996;152:141–57. doi: 10.1007/s002329900093. [DOI] [PubMed] [Google Scholar]
- 26.Eder C, Fischer HG. Effects of colony-stimulating factors on voltage-gated K+ currents of bone marrow-derived macrophages. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:198–202. doi: 10.1007/pl00004932. [DOI] [PubMed] [Google Scholar]
- 27.Forero ME, Marin M, Corrales A, et al. Leishmania amazonensis infection induces changes in the electrophysiological properties of macrophage-like cells. J Membr Biol. 1999;170:173–80. doi: 10.1007/s002329900547. [DOI] [PubMed] [Google Scholar]
- 28.Ichinose M, Hara N, Sawada M, et al. Activation of K+ current in macrophages by platelet activating factor. Biochem Biophys Res Commun. 1992;182:372–8. doi: 10.1016/s0006-291x(05)80155-x. [DOI] [PubMed] [Google Scholar]
- 29.Negulyaev YA, Vedernikova EA, Kinev AV, et al. Exogenous heat shock protein hsp70 activates potassium channels in U937 cells. Biochim Biophys Acta. 1996;1282:156–62. doi: 10.1016/0005-2736(96)00055-7. [DOI] [PubMed] [Google Scholar]
- 30.McKinney LC, Gallin EK. G-protein activators induce a potassium conductance in murine macrophages. J Membr Biol. 1992;130:265–76. doi: 10.1007/BF00240483. [DOI] [PubMed] [Google Scholar]
- 31.Hara N, Ichinose M, Sawada M, et al. Inositol 1,4,5-trisphosphate mediates adrenaline activation of K+ conductance in mouse peritoneal macrophages. Pflugers Arch. 1993;423:140–8. doi: 10.1007/BF00374971. [DOI] [PubMed] [Google Scholar]
- 32.Takao K, Yoshii M, Kanda A, et al. A region of the muscarinic-gated atrial K+ channel critical for activation by G protein beta gamma subunits. Neuron. 1994;13:747–55. doi: 10.1016/0896-6273(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 33.Berger F, Borchard U, Hafner D, et al. Activation of a potassium outward current by zymosan and opsonized zymosan in mouse peritoneal macrophages. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:594–601. doi: 10.1007/BF01258465. [DOI] [PubMed] [Google Scholar]
- 34.Blunck R, Scheel O, Muller M, et al. New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J Immunol. 2001;166:1009–15. doi: 10.4049/jimmunol.166.2.1009. [DOI] [PubMed] [Google Scholar]
- 35.Seydel U, Scheel O, Muller M, et al. A K+ channel is involved in LPS signaling. J Endotoxin Res. 2001;7:243–7. [PubMed] [Google Scholar]
- 36.Martin DK, Bootcov MR, Campbell TJ, French PW, Breit SN. Human macrophages contain a stretch-sensitive potassium channel that is activated by adherence and cytokines. J Membr Biol. 1995;147:305–15. doi: 10.1007/BF00234528. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama N, Kakuta Y, Yamauchi K, et al. Quinine inhibits production of tumor necrosis factor-alpha from human alveolar macrophages. Am J Respir Cell Mol Biol. 1994;10:514–20. doi: 10.1165/ajrcmb.10.5.8179913. [DOI] [PubMed] [Google Scholar]
- 38.Haslberger A, Romanin C, Koerber R. Membrane potential modulates release of tumor necrosis factor in lipopolysaccharide-stimulated mouse macrophages. Mol Biol Cell. 1992;3:451–60. doi: 10.1091/mbc.3.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crutchley DJ, Conanan LB, Que BG. K+ channel blockers inhibit tissue factor expression by human monocytic cells. Circ Res. 1995;76:16–20. doi: 10.1161/01.res.76.1.16. [DOI] [PubMed] [Google Scholar]
- 40.Walev I, Reske K, Palmer M, et al. Potassium-inhibited processing of IL-1 beta in human monocytes. Embo J. 1995;14:1607–14. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci USA. 1997;94:11514–9. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett S, Por SB, Stanley ER, et al. Monocyte proliferation in a cytokine-free, serum-free system. J Immunol Meth. 1992;153:201–12. doi: 10.1016/0022-1759(92)90323-l. [DOI] [PubMed] [Google Scholar]
- 43.Ways DK, Messer BR, Garris TO, et al. Modulation of protein kinase C-epsilon by phorbol esters in the monoblastoid U937 cell. Cancer Res. 1992;52:5604–9. [PubMed] [Google Scholar]
- 44.De AK, Kodys K, Puyana JC, et al. Elevated IL-8 production by trauma patients’ monocytes is associated with elevated secretion of TNF alpha. Shock. 1995;4:171–7. doi: 10.1097/00024382-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Ince C, Thio B, van Duijn B, et al. Intracellular K+, Na+ and Cl- concentrations and membrane potential in human monocytes. Biochim Biophys Acta. 1987;905:195–204. doi: 10.1016/0005-2736(87)90023-x. [DOI] [PubMed] [Google Scholar]
- 46.Burg MB, Kwon ED, Kultz D. Osmotic regulation of gene expression. Faseb J. 1996;10:1598–606. doi: 10.1096/fasebj.10.14.9002551. [DOI] [PubMed] [Google Scholar]
- 47.Plum J, Schoenicke G, Grabensee B. Osmotic agents and buffers in peritoneal dialysis solution: monocyte cytokine release and in vitro cytotoxicity. Am J Kidney Dis. 1997;30:413–22. doi: 10.1016/s0272-6386(97)90287-0. [DOI] [PubMed] [Google Scholar]
- 48.Cendoroglo M, Sundaram S, Jaber BL, et al. Effect of glucose concentration, osmolality, and sterilization process of peritoneal dialysis fluids on cytokine production by peripheral blood mononuclear cells and polymorphonuclear cell functions in vitro. Am J Kidney Dis. 1998;31:273–82. doi: 10.1053/ajkd.1998.v31.pm9469498. [DOI] [PubMed] [Google Scholar]


