Abstract
C57BL/6 mice were sensitized to Aspergillus fumigatus 1-week culture filtrate, which is rich in the non-glycosylated allergen Asp f1, a major allergen in allergic bronchopulmonary aspergillosis (ABPA). A comparison of the effect of treatment of allergen challenged mice by intranasal administration of a 60-kDa truncated recombinant form of human SP-D (rfhSP-D) or recombinant full length SP-A (rhSP-A) was undertaken. Treatment with rfhSP-D produced significant reduction in IgE, IgG1 and peripheral blood eosinophilia and treatment with rfhSP-D, but not rhSP-A resulted in a significant reduction in airway hyperresponsiveness as measured by whole body plethysmography. Lung histology revealed less peribronchial lymphocytic infiltration in mice treated with rfhSP-D. Intracellular cytokine staining of spleen homogenates showed increases in IL-12 and IFN-γ and decrease in IL-4. The level of endogenous mouse SP-D was elevated sixfold in the lungs of sensitized mice and was not affected by treatment with rfhSP-D. Taken with our previous studies, with a BALB/c mouse model of ABPA using a 3-week A. fumigatus culture filtrate, the present results show that rfhSP-D can suppress the development of allergic symptoms in sensitized mice independent of genetic background and using a different preparation of A. fumigatus allergens.
Keywords: ABPA allergy IL-12 plethysmography SP-D
INTRODUCTION
Aspergillus fumigatus is a ubiquitous fungus of clinical importance that can produce allergic hypersensitivity reactions, referred to as allergic bronchopulmonary aspergillosis (ABPA), characterized by elevated IgE, eosinophilia and bronchial hyperresponsiveness [1,2]. The incidence of ABPA has increased in recent years and presents a threat to patients with pulmonary diseases such as cystic fibrosis and AIDS and severe asthma [3]. The lung surfactant proteins SP-A and SP-D are large multimeric proteins of the collectin family consisting of assemblies of trimeric subunits, each consisting of a short amino-terminal cross-linking domain, a long triple helical collagenous domain and a carbohydrate recognition domain (CRD). These proteins are molecules of innate immunity and play a vital role in pulmonary defence against inhaled microorganisms [4,5]. Both collectins bind carbohydrates in a calcium-dependent way and in one clinically relevant study, SP-A and SP-D bound to glycosylated allergens from house dust mite and were shown to inhibit lymphocyte proliferation and histamine release in asthmatic children [6,7]. A similar study by Madan et al. showed that SP-A and SP-D bound glycosylated allergens from A. fumigatus (Afu) and inhibited allergen-induced histamine release from human basophils [8]. SP-D and SP-A levels increase several-fold in allergic asthma [9] and it seems probable that they are important regulators of allergy. Indeed, Madan et al., in collaboration with our group, went on to establish a model of ABPA in BALB/c mice using a 3-week Afu culture filtrate and demonstrated a protective effect against pulmonary hypersensitivity of exogenously administered human SP-A and SP-D and the recombinant truncated 60 kDa fragment of human SP-D (rfhSP-D) when given intranasally [10]. SP-D is a hydrophilic protein [11] that may be of particular importance in down-regulating allergic responses [12]. The recombinant fragment (rfhSP-D) used in Madan et al.'s study and the present study consists of a trimer of the neck and CRD domain only and it is interesting that this truncated fragment of human SP-D was as effective as the full-length native protein. In the present study the application of rfhSP-D as a down-regulator of allergic hypersensitivity was tested further in an ABPA model established in a different genetic background (C57BL/6), using a different allergen extract (Afu 1-week culture filtrate) and a different sensitization and treatment protocol. In addition to measuring IgE, IgG1 and peripheral blood eosinophilia, airway hyperresponsiveness was assessed and ex vivo cytokines were measured by intracellular cytokine staining. Endogenous SP-D and SP-A levels were also measured to determine if treatment with rfhSP-D might be up-regulating these collectins.
MATERIALS AND METHODS
Preparation of rfhSP-D
The cDNA for the neck/CRD, including a short region of the collagen stalk (eight Gly-X-Y triplets) and representing residues 179–355 of the mature protein sequence was cloned from human lung library DNA and inserted into a pET-21d vector (Novagen, Nottingham, UK). The plasmid was transformed into BL21(λDE3) pLysS and a single colony selected and re-plated to give 100–400 colonies/plate. These were scraped and used to inoculate shake-flasks containing 500 ml LB medium supplemented with 100 µg/ml ampicillin and 25 µg/ml chloramphenicol and grown to an O.D. 600 of 0·6–0·8, followed by induction with 0·4 mm IPTG for 2–3 h. Cells were collected by centrifugation and lysed in 20 mm Tris-HCl, 150 mm NaCl, 5 mm EDTA, 0·1% (v/v) Triton X-100, 0·1 mm PMSF, pH 7·5 and sonicated for 3 min. The rfhSP-D is expressed in insoluble inclusion bodies and was collected by centrifugation and washed four times with lysis buffer followed by centrifugation at 10000 g. The pellet was solubilized in 100 ml of 8 m urea, 100 mm 2-mercaptoethanol, pH 7·5 and clarified by centrifugation and refolded by overnight dialysis against 10 l of 20 mm Tris-HCl, 150 mm NaCl, 5 mm CaCl2, pH 7·4 (TCB). Refolded rfhSP-D was separated from denatured rfhSP-D by absorption onto maltose-agarose (Sigma-Aldrich, Poole, UK) and eluted with 20 mm Tris-HCl, 150 mm NaCl, pH 7·4 containing 5 mm EDTA after first washing the column with TCB containing 1 m NaCl to remove impurities. Final purification was by gel filtration column (Superose 12, Amersham Pharmacia, UK) in a running buffer of 20 mm Tris-HCl, 150 mm NaCl, 5 mm EDTA, 0·02% (w/v) sodium azide pH 7·4 (TSE). The rfhSP-D eluted as a single peak corresponding to 60 kDa molecular weight. Endotoxin levels were reduced by passing the purified rfhSP-D, in TSE buffer, through a 10-ml Polymixin B column (Detoxi-Gel, Pierce & Warriner, UK). Endotoxin was measured by the Limulus Amebocyte Lysate Assay (BioWhitaker, UK) and only preparations containing less than 5 pg endotoxin/µg rfhSP-D were used.
A. fumigatus 1-week culture filtrate (Afu 1wcf)
A. fumigatus (Afu) was grown in a synthetic medium (M199, Sigma Chemicals) as a stationary culture for 1 week at 37°C. Arruda et al. [13] demonstrated that the expression of Asp f1, a major allergen, is maximal after 1 week and tends to diminish during longer incubation periods.The 1-week culture was killed by adding 0·1% (w/v) Thimerosal for 12 h at 4°C.The culture was filtered through glass wool and finally through a 0·45-µm membrane to remove all particulates and spores and then dialysed with three buffer changes against water. The dialysate was lyophilized to give a brown powder. SDS-PAGE of the 1wcf revealed a major band at 18 kDa, which corresponds to Asp f1. A band corresponding to Asp f2 (37 kDa) was also evident. The 18 kDa band was N-terminal sequenced giving the sequence ATWTCINQQLNP, corresponding to the N-terminal sequence for Asp f1. It was also demonstrated by ELISA that the 1-week culture filtrate was recognized by human serum from Afu-allergic patients obtained from the National Institute of Biological Standards and Control.
Sensitization
In this study, 6-week-old female C57BL/6 mice were sensitized by intraperitoneal injections of 200 µg Afu 1wcf mixed with alum (1 : 4 v/v) in 100 µl PBS given once a week for 4 weeks.
Allergen challenge and treatment
Sensitized mice were challenged with 50 µl PBS containing 10 µg of Afu 1wcf given intranasally. This was followed by treatment with PBS or 10 µg rfhSP-D in 50 µl PBS given intranasally. Challenge and treatment were performed on a daily basis as described in the Results. In some experiments, a control protein of full-length recombinant human SP-A, purified as described by Voss et al. [14], was used at a concentration of 10 µg/50 µl (kindly provided by Altana Pharmaceuticals, Konstanz, Germany). In a separate experiment the fate of rfhSP-D was monitored by obtaining BAL from different mice at various times after intranasal application rfhSP-D in 50 µl PBS and assaying for rfhSP-D using a polyclonal antibody raised against rfhSP-D that does not recognize mouse SP-D or SP-A. These results showed that at least 50% of rfhSP-D could be accounted for in the BAL taken 30 min after administration and none could be measured after 24 h.
Peripheral blood eosinophils
Blood was collected from the tail vein of the mice (n = 4–8/group) for estimation of eosinophils. Total leucocyte count was measured with an automatic cell counter and the proportion of eosinophils was determined by differential counting of May–Grunwald–Giemsa-stained blood smears. Results are expressed as 106 cells/ml.
Serum IgE and Afu-specific IgG1
Total serum IgE was measured by sandwhich ELISA (BD PharMingen, Cowley, UK) in blood serially diluted from a maximum dilution of 1 : 20 to give values which were linear with respect to a standard curve of mouse IgE. Results are expressed in µg/ml. Afu-specific IgG1 was measured by ELISA using 96-well plates coated with Afu allergen extract. Antibody was detected with HRP-labelled anti-mouse IgG1. Results are expressed as relative absorbance units (O.D. 450).
Endogenous mouse SP-D and SP-A in the lung
Immediately after humane sacrifice by CO2 asphyxiation, bronchoalveolar lavage was performed with 3 × 1ml PBS, which were pooled and the volumes adjusted by addition of PBS to 4 ml for all samples and centrifuged to remove cells. SP-D and SP-A were measured by ELISA using polyclonal antibodies raised against recombinant mouse SP-D or SP-A (kindly provided by Dr P. Lawson). These antibodies were shown not to cross-react with human SP-D or SP-A and were specific for mouse SP-D or SP-A, respectively. Results are expressed as µg/ml of BAL.
Intracellular cytokine staining of spleen cells
To assess the systemic immune response to treatment, cytokines were measured in the spleen by intracellular cytokine staining. After treatment, mice were sacrificed humanely by CO2 asphyxiation and their spleens removed and homogenized in PBS. The homogenate was filtered and red blood cells lysed with ammonium chloride lysing reagent (BD Pharmingen) and fixed with 4% (v/v) paraformaldehyde for 20 min. The cells were washed with PBS supplemented with 3% (v/v) heat inactivated fetal calf serum with 0·1% (w/v) sodium azide (FSB), re-suspended in 10% DMSO (v/v) in FSB and stored at −80°C. Cells were permeabilized with Cytoperm wash buffer (CPB, BD Biosciences, Cowley, UK) for 15 min at 4°C and aliquots of 106 cells were blocked by incubation for 30 min at 4°C with CPB supplemented with 50 µg/ml rat IgG. Intracellular cytokines were stained with 1 µg PE-conjugated anti-mouse cytokine monoclonal antibody (BD Biosciences) incubated for 60 min at 4°C. The cells were washed with CPB followed by FSB and re-suspended in 500 ml FSB. Flow cytometry was performed with a FACScan flow cytometer (Beckton Dickinson, Mountain View, CA, USA) using CellQuest software. Data were collected for 20 000 cells. The average FSC of spleen cells was 100 in all cases. Stained cells (FSC > 100, FL2 > 100) were gated and the proportion of these cells staining intensely for PE (PE > 1000) was calculated. Results are expressed as the percentage intensely stained cells after subtraction of background fluorescence for unstained cells incubated with rat IgG (% PE > 1000).
Lung histology
Immediately after treatment, the lungs of mice from each treatment group were fixed in 10% (v/v) neutral buffered formalin and sent for independent analysis. Lungs were embedded in paraffin, sectioned and stained with haematoxylin and eosin. The slides were evaluated for peribronchial inflammation and scores were assigned on a scale of 0–4, corresponding to a score of normal to severe, respectively [15].
Whole body plethysmography
In this study, airway hyperresponsiveness was measured using unrestrained whole body plethysmography [16] with a four-chamber system (Buxco, Sharon, CT, USA). The parameter measured and used to indicate the severity of airway hyperresponsiveness is the enhanced pause (Penh). Increased constriction of the airways results in a longer expiration time and an elevation in Penh. Mice were first challenged intranasally with antigen and allowed to recover for 2 h before being placed into the chambers and their breathing monitored for 10 min. When acclimatized, their baseline response was measured for 5 mins. The mice were then subjected to 1 min of aerosolized PBS, followed by progressively increasing doses of methacholine (5, 10, 20, 30, 40 mg/ml PBS). Responses are recorded for 5 min in every case with a short interval between to allow return to baseline Penh.
Each group contained 4–8 mice. Results are presented as the average percentage elevation in Penh over baseline after a challenge of methacholine.
Statistics
Results are average for the 4–8 mice/group and error bars are ± s.e.m. Significance was determined by Student's two-tailed t-test. Significance was accepted for P < 0·05.
RESULTS
Serum IgE, Afu-specific IgG1 and peripheral blood eosinophilia are reduced by rfhSP-D in a different genetic background
To determine if treatment with rfhSP-D was effective in a different genetic background an ABPA model was established in C57BL/6 mice. To determine if rfhSP-D could modulate allergic hypersensitivity during allergen challenge the sensitized mice were first challenged with 10 µg Afu 1wcf and treated 1 h later. Serum IgE measured 3 days after treatment with five daily doses of 10 µg rfhSP-D given intranasally to allergen challenged mice was significantly reduced (P < 0·001) and this reduction was maintained after re-challenge with three daily doses of 10 µg Afu 1wcf given the following week (Fig. 1a). A similar significant reduction was also measured in Afu-specific IgG1 (Fig. 1b) and peripheral blood eosinophilia measured after treatment of allergen challenged mice and re-challenge with allergen alone the following week (Fig. 2).
Fig. 1.
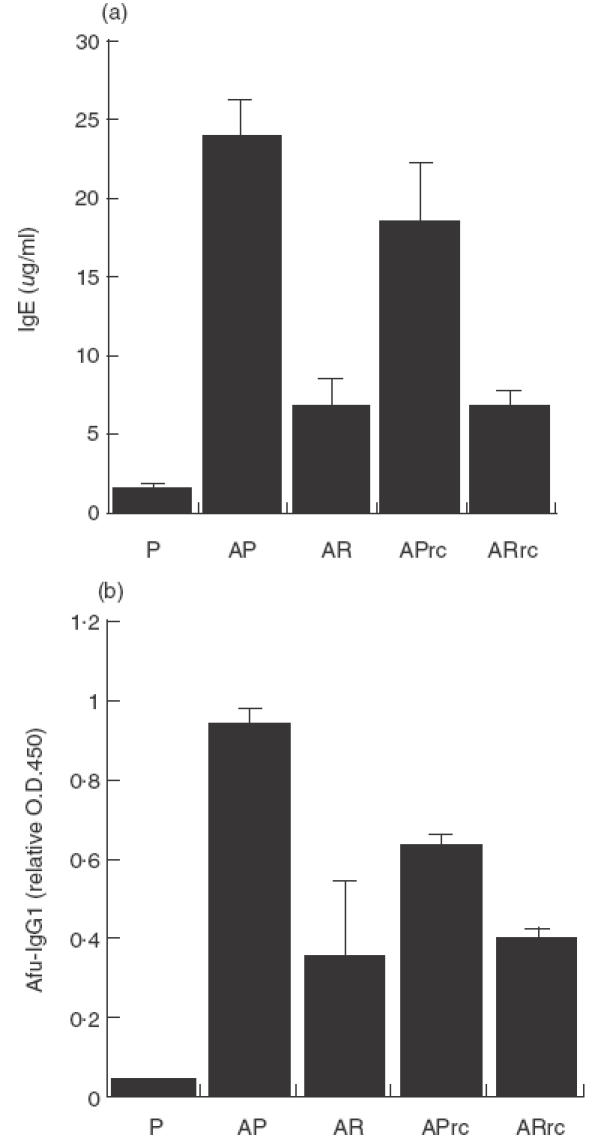
(a) Serum IgE and (b) Afu-specific IgG1 measured 3 days after treatment with five daily doses of 10 µg rfhSP-D or PBS given intranasally after intranasal challenge with Afu 1wcf and after re-challenge 1 week later with Afu alone. P = PBS-treated non-sensitized mice, AP = PBS-treated sensitized mice, AR = rfhSP-D-treated sensitized mice, APrc = Afu re-challenged mice that had been treated with PBS, ARrc = Afu re-challenged mice that had been treated with rfhSP-D.
Fig. 2.
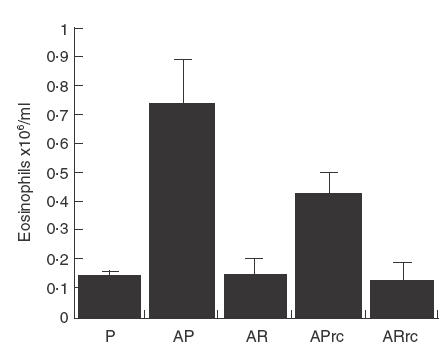
Peripheral blood eosinophilia measured 1 day after treatment with four daily doses of 10 µg rfhSP-D or PBS given intranasally after intranasal challenge with Afu 1wcf and after re-challenge 1 week later with Afu alone. P = PBS-treated non-sensitized mice, AP = PBS-treated sensitized mice, AR = rfhSP-D-treated sensitized mice, APrc = Afu re-challenged mice that had been treated with PBS, ARrc = Afu re-challenged mice that had been treated with rfhSP-D.
Treatment with rfhSP-D results in elevation in IL-12 and IFN-γ and a reduction in IL-4
The reduction in IgE and peripheral blood eosinophilia suggests a systemic modulation at the cytokine level and these cytokines were measured by intracellular staining. IL-12 (Fig. 3a) measured in the spleen, 1 day after treatment for 2 days with 10 µg rfhSP-D, given intranasally to allergen challenged mice was significantly reduced (P < 0·05), as was IFN-γ (Fig. 3b). Measurement of IL-4 showed a decrease to the level measured in non-sensitized mice (Fig. 3c). The same treatment with rhSP-A did not produce these effects.
Fig. 3.
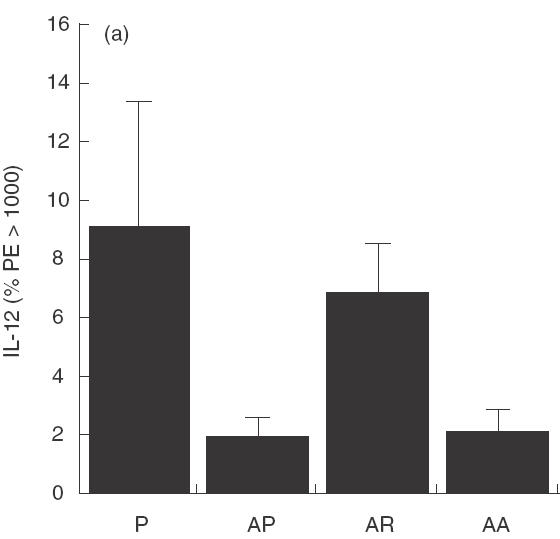
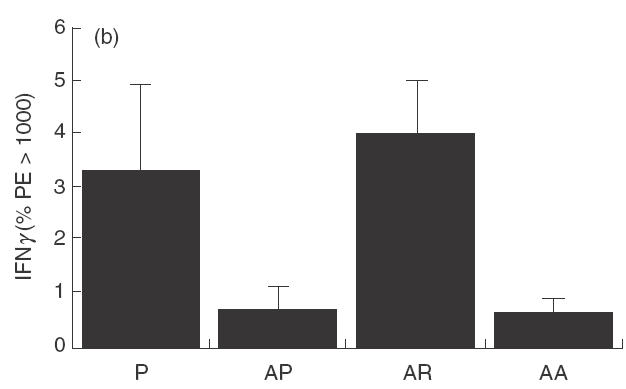
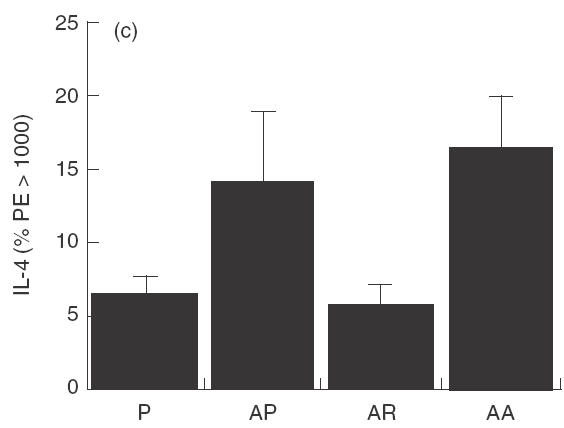
Cytokines measured 1 day after treatment with two daily doses of 10 µg rfhSP-D given intranasally after intranasal challenge with Afu 1wcf. (a) IL-12 measured in the spleen, (b) IFN-γ measured in the spleen and (c) IL-4 measured in the spleen. P = PBS-treated non-sensitized mice, AP = PBS-treated sensitized mice, AR = rfhSP-D-treated sensitized mice, AA = rhSP-A treated sensitized mice.
Treatment with rfhSP-D results in reduced airway hyperresponsiveness
Mice treated with four daily doses of 10 µg rfhSP-D given 1–2 h after allergen challenge showed a significant reduction (P < 0·05) in airway hyperresponsiveness on re-challenge with allergen 3 days after completion of treatment (day 7) in all methacholine doses tested (Fig. 4a). Mice treated with 10 µg rhSP-A in the same way did not show a significant reduction in AHR (Fig. 4b).
Fig. 4.
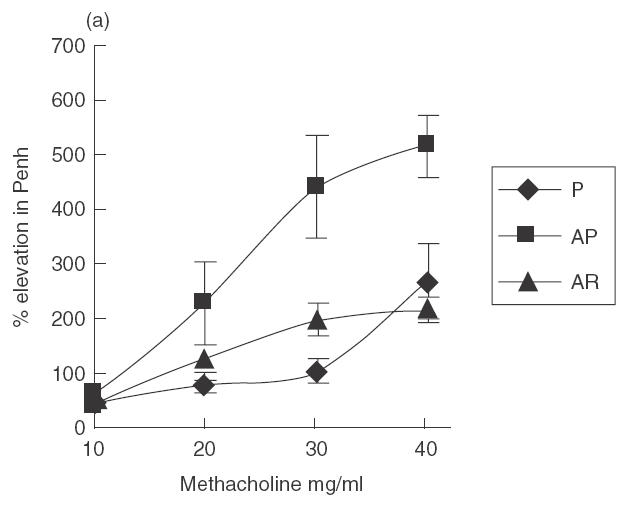
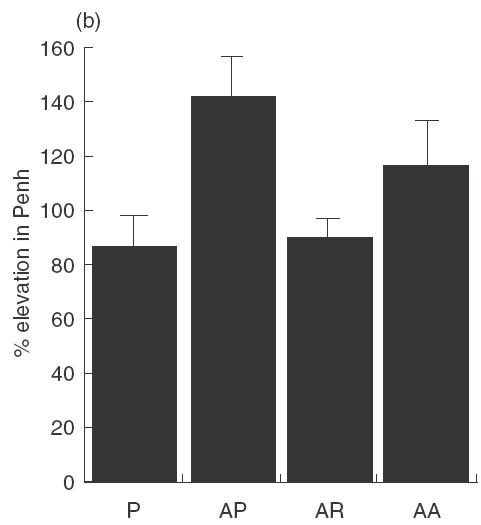
Mice were treated with four daily doses of 10 µg rfhSP-D given intranasally after intranasal challenge with Afu 1wcf. Plethysmography was measured 2 h after an intranasal re-challenge with Afu 1wcf alone given 3 days after completion of treatment. (a) Dose–response to increasing doses of methacholine. ♦, P; ▪, AP; ▴, AR. (b) Response to 30 mg/ml methacholine after treatment with four daily doses of 10 µg rfhSP-D or rhSP-A given intranasally after intranasal challenge with Afu 1wcf. P = PBS-treated non-sensitized mice, AP = PBS-treated sensitized mice, AR = rfhSP-D-treated sensitized mice, AA = rfhSP-A-treated sensitized mice.
Treatment with rfhSP-D results in reduced lung inflammation
Of the sensitized mice in this study four of the 6 PBS treated mice had a score of 2+ and of the five in the rfhSP-D treatment group four had a score of 1. The score for non-sensitized mice was 0.
Endogenous mouse SP-D and SP-A
Endogenous levels of SP-D measured in the BAL of allergic mice were elevated sixfold from a level of 0·25 ± 0·015 µg/ml in BAL from normal mice to 1·4 ± 0·15 µg/ml, while no difference was found for the level of endogenous SP-A which was measured at 1·3 ± 0·1 µg/ml in normal, sensitized and treated mice BAL. Treatment with five daily doses of 10 µg rfhSP-D did not produce any change in the level of endogenous SP-D or SP-A levels.
DISCUSSION
In the previous study by Madan et al. [10], the effect of treatment of Afu allergic mice with native human SP-D, SP-A and rfhSP-D demonstrated the importance of these proteins in down-regulating allergic hypersensitivity in a mouse model of allergic hypersensitivity to Afu allergens, with decreases in IgE, peripheral blood eosinophilia and lung eosinophil peroxidase levels and decreases in IL-2, IL-4, IL-5 and an increase in IFN-γ. This study indicated that the truncated fragment composed of the trimer of the neck and CRD regions was as effective as full-length human SP-D. The genetic background of mice can influence the response to allergen challenge, with strains such as BALB/c, BALB/cBy and BALB.B being described as high Th2 responders where as C57BL/6, C57BL/10 and B10.D2/nSn favour a Th1 response [17]. Madan et al.'s study used BALB/c mice while C57BL/6 mice were used in the present study. The results demonstrate that the immunomodulation by rfhSP-D is independent of genetic background, with significant decreases in IgE, Afu-specific IgG1 and peripheral blood eosinophilia in the C57BL/6 mouse model of Afu allergy. The present study used a well-characterized Afu 1-week culture filtrate, which is rich in the major allergen Asp f1. This allergen, along with Asp f3, is detected during the early phase of the disease and is therefore of clinical significance [18,19]. In this study it is also demonstrated that treatment with rfhSP-D modifies the underlying responsiveness to allergen challenge as mice were challenged with allergen and treated daily, whereas Madan et al. demonstrated that treatment increased the rate of recovery in the weeks following treatment in the absence of continued allergen challenge. Also, treated mice produce less IgE, IgG1 and eosinophils when re-challenged with allergen in the week following treatment. The present study also shows a shift in the cytokine profile from Th2 to Th1, with increased Th1 cytokines IL-12 and IFN-γ and decreased Th2 cytokine IL-4. The up-regulation of IL-12 suggests the involvement of macrophages and dendritic cells, which are the sole producers of IL-12, and it is possible that rfhSP-D is stimulating macrophages in the lungs and upper respiratory tract directly. Il-12 has a major effect in stimulating the proliferation of Th1 lymphocytes and an antiproliferative effect on Th2 lymphocytes and several studies have shown that Il-12 inhibits airway hyperresponsiveness [20,21], which is consistent with our findings. IL-12 stimulates the production of IFN-γ by Th1 lymphocytes and natural killer cells and the two cytokines have a synergistic effect on reducing allergic hypersensitivity and reducing Th2 cytokines, including IL-4 [22]. IL-4 acts upon B cells, promoting class switching to IgG1 in mice (IgG4 in humans) and IgE [23,24] and IL-4 has been directly linked to the immunopathogenesis of asthma [25–27] including airway hyperresponsiveness [28]. The results from this study, demonstrating a reduction in the level of IL-4 during allergen challenge, are of major importance in the treatment of seasonal allergy and allergic asthma where subjects are continually exposed to allergen. The role of SP-D in Afu-induced allergic inflammation is of interest because it is up-regulated in response to Afu allergen, as shown by Haczku et al., while SP-A levels are unaffected [29]. It is possible that treatment with rfhSP-D might affect endogenous levels of either of these collectins. We observed a similar increase in SP-D and no increase in SP-A in sensitized mice and that treatment did not affect either endogenous SP-D or SP-A levels. The suggestion is that rfhSP-D is producing an effect independently of, or in addition to the endogenous SP-D and may also explain the relative ineffectiveness of rhSP-A in modulating allergy in this model.
Acknowledgments
Many thanks to P. Townsend, R. Embury, R. Parsons and A. Willis for their technical assistance. This work was funded partially by the EU (contract number QLK-CT-2000–00325). Dr Howard Clark holds a Beit Fellowship for Medical Research. Dr W. Steinhilber is thanked for kindly donating the rhSP-A used in the study. (Altana Pharmaceuticals)
REFERENCES
- 1.Kauffman HF, Tomee JF, van der Werf TS, et al. Review of fungus-induced asthmatic reactions. Am J Respir Crit Care Med. 1995;151:2109–15. doi: 10.1164/ajrccm.151.6.7767565. discussion 16. [DOI] [PubMed] [Google Scholar]
- 2.Elstad MR. Aspergillosis and lung defenses. Semin Respir Infect. 1991;6:27–36. [PubMed] [Google Scholar]
- 3.Varkey B. Beware of the fungus [editorial] Wisconsin Medical Journal. 1998;97:48–9. [PubMed] [Google Scholar]
- 4.Eggleton P, Reid KB. Lung surfactant proteins involved in innate immunity. Curr Opin Immunol. 1999;11:28–33. doi: 10.1016/s0952-7915(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 5.Crouch EC. Collectins and pulmonary host defense. Am J Respir Cell Mol Biol. 1998;19:177–201. doi: 10.1165/ajrcmb.19.2.140. [DOI] [PubMed] [Google Scholar]
- 6.Wang JY, Shieh CC, You PF, et al. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–8. doi: 10.1164/ajrccm.158.2.9709111. [DOI] [PubMed] [Google Scholar]
- 7.Wang JY, Kishore U, Lim BL, et al. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin Exp Immunol. 1996;106:367–73. doi: 10.1046/j.1365-2249.1996.d01-838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madan T, Kishore U, Shah A, et al. Lung surfactant proteins A and D can inhibit specific IgE binding to the allergens of Aspergillus fumigatus and block allergen-induced histamine release from human basophils. Clin Exp Immunol. 1997;110:241–9. doi: 10.1111/j.1365-2249.1997.tb08323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng G, Ueda T, Numao T, et al. Increased levels of surfactant protein A and D in bronchoalveolar lavage fluids in patients with bronchial asthma. Eur Respir J. 2000;16:831–5. doi: 10.1183/09031936.00.16583100. [DOI] [PubMed] [Google Scholar]
- 10.Madan T, Kishore U, Singh M, et al. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J Clin Invest. 2001;107:467–75. doi: 10.1172/JCI10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crouch EC. Structure, biologic properties, and expression of surfactant protein D (SP-D) Biochim Biophys Acta. 1998;1408:278–89. doi: 10.1016/s0925-4439(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 12.Reid KB. Interactions of surfactant protein D with pathogens, allergens and phagocytes. Biochim Biophys Acta. 1998;1408:290–5. doi: 10.1016/s0925-4439(98)00074-x. [DOI] [PubMed] [Google Scholar]
- 13.Arruda LK, Mann BJ, Chapman MD. Selective expression of a major allergen and cytotoxin, Asp fI, in Aspergillus fumigatus. Implications for the immunopathogenesis of Aspergillus-related diseases. J Immunol. 1992;149:3354–9. [PubMed] [Google Scholar]
- 14.Voss T, Eistetter H, Schafer KP, et al. Macromolecular organization of natural and recombinant lung surfactant protein SP 28–36. Structural homology with the complement factor C1q. J Mol Biol. 1988;201:219–27. doi: 10.1016/0022-2836(88)90448-2. [DOI] [PubMed] [Google Scholar]
- 15.Sur S, Wild JS, Choudhury BK, et al. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J Immunol. 1999;162:6284–93. [PubMed] [Google Scholar]
- 16.Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 17.Hazlett LD, McClellan S, Kwon B, et al. Increased severity of Pseudomonas aeruginosa corneal infection in strains of mice designated as Th1 versus Th2 responsive. Invest Ophthalmol Vis Sci. 2000;41:805–10. [PubMed] [Google Scholar]
- 18.Hemmann S, Nikolaizik WH, Schoni MH, et al. Differential IgE recognition of recombinant Aspergillus fumigatus allergens by cystic fibrosis patients with allergic bronchopulmonary aspergillosis or Aspergillus allergy. Eur J Immunol. 1998;28:1155–60. doi: 10.1002/(SICI)1521-4141(199804)28:04<1155::AID-IMMU1155>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Hutcheson PS, Rejent AJ, Slavin RG. Variability in parameters of allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. J Allergy Clin Immunol. 1991;88:390–4. doi: 10.1016/0091-6749(91)90102-t. [DOI] [PubMed] [Google Scholar]
- 20.Bryan SA, O'Connor BJ, Matti S, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2149–53. doi: 10.1016/S0140-6736(00)03497-8. [DOI] [PubMed] [Google Scholar]
- 21.Gavett SH, O'Hearn DJ, Li X, et al. Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–36. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–9. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pene J, Rousset F, Briere F, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons gamma and alpha and prostaglandin E2. Proc Natl Acad Sci USA. 1988;85:6880–4. doi: 10.1073/pnas.85.18.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopf M, Le Gros G, Coyle AJ, et al. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 25.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 26.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 27.Walker C, Kaegi MK, Braun P, et al. Activated T cells and eosinophilia in bronchoalveolar lavages from subjects with asthma correlated with disease severity. J Allergy Clin Immunol. 1991;88:935–42. doi: 10.1016/0091-6749(91)90251-i. [DOI] [PubMed] [Google Scholar]
- 28.Venkayya R, Lam M, Willkom M, et al. The Th2 lymphocyte products IL-4 and IL-13 rapidly induce airway hyperresponsiveness through direct effects on resident airway cells. Am J Respir Cell Mol Biol. 2002;26:202–8. doi: 10.1165/ajrcmb.26.2.4600. [DOI] [PubMed] [Google Scholar]
- 29.Haczku A, Atochina EN, Tomer Y, et al. Aspergillus fumigatus-induced allergic airway inflammation alters surfactant homeostasis and lung function in BALB/c mice. Am J Respir Cell Mol Biol. 2001;25:45–50. doi: 10.1165/ajrcmb.25.1.4391. [DOI] [PubMed] [Google Scholar]


