Abstract
The splenic marginal zone (S-MZ) is especially well equipped for rapid humoral responses and is unique in its ability to initiate an immune response to encapsulated bacteria (T-cell independent type 2 (TI-2) antigens). Because of the rapid spreading through the blood, infections with blood-borne bacteria form a major health risk. To cope with blood-borne antigens, a system is needed that can respond rapidly to a great diversity of organisms. Because of a number of unique features, S-MZ B cells can respond rapid and efficient to all sorts of blood-borne antigens. These unique features include a low blood flow microenvironment, low threshold for activation, high expression of complement receptor 2 (CR2, CD21) and multireactivity.
Because of the unique high expression of CD21 in a low flow compartment, S-MZ B cells can bind and respond to TI-2 antigens even with relatively low-avid B cell receptors. Although TI-2 antigens are in general poorly opsonized by classic opsonins, a particular characteristic of these antigens is their ability to bind very rapidly to complement fragment C3d without the necessity of previous immunoglobulin binding. TI-2 primed S-MZ B cells, already by first passage through the germinal centre, will meet antigen-C3d complexes bound to follicular dendritic cells, allowing unique immediate isotype switching. This explains that the primary humoral response to TI-2 antigens is unique in its characterization by a rapid increase in IgM concurrent with IgG antibody levels.
Keywords: splenic marginal zone, B cells, memory, T-cell independent type 2 antigens, pneumococcal polysaccharides
INTRODUCTION
Infections with blood-borne bacteria form a major health risk because of the rapid spreading of the organism through the body and the risk of sepsis. A rapid first line defense, able to produce large amounts of neutralizing antibodies in a short period is necessary to prevent bacteriaemia. To produce this large amount of antibodies a system is needed that can respond instantly to a great diversity of blood-borne organisms. This system needs to be able to react with broad specificity even if this implies to react with low affinity/avidity. The splenic marginal zone (S-MZ) is specifically suited for this job. Martin et al. [1] showed in a recent study the ability of S-MZ B cells to rapidly produce large amounts of IgM within 3–4 days after antigenic stimulation. This splenic marginal zone is a unique compartment in its location and composition. It contains mostly pre activated B cells, the S-MZ B cells, with a high surface density of complement receptor 2 (CD21) and IgM [2]. The architectural structure of the S-MZ results in a strongly reduced blood-flow allowing intimate contact between antigens and effector cells [3,4]. The blood-flow is significantly reduced because of the presence of a marginal sinus in which part of the arterial bloodstream opens [5]. In the human S-MZ, the presence of a sinus has not been demonstrated but others and we like to speculate that the splenic perifollicular zone and marginal zone contain a sort of sinusoidal system in which the arterial blood flow opens, also leading to a strongly reduced blood flow [5–7].
The S-MZ appears to have a dual function. Firstly, it is especially well equipped for rapid humoral immune responses to blood-borne antigens [1,5,8,9]. Because of the central position of the spleen in the blood stream and the sluggish blood flow through the S-MZ, S-MZ B cells are one of the first to come in close contact with blood-borne antigens [2].
The second function of the S-MZ is a more unique one and involves the initiation of an immune response to T cell independent type 2 (TI-2) antigens [3–5,10–12]. Based on immunogenicity in congenitally athymic (nu/nu) mice and mice with and X-linked immune B cell defect (xid-mice), antigens are divided in T-cell dependent (TD), T-cell independent type 1 (TI-1) and T-cell independent type 2 (TI-2) antigens. TD antigens, that fail to induce a response in nu/nu mice, include proteins and hapten-protein conjugates. TI-1 antigens stimulate excellent antibody responses in nu/nu mice and the xid-mice. TI-2 antigens also stimulate excellent responses in nu/nµ mice but fail to initiate a response in the xid-mice [13–15]. The capsular polysaccharide antigens of encapsulated bacteria are in general TI-2 antigens. These antigens stimulate antibody production in the absence of MHC class II restricted T cell help but do need T cell derived factors [13,16]. TI-2 antigens are poor immunogens and the S-MZ B cells in the splenic anatomical context are the main cells that can initiate a rapid, adequate response against these antigens.
Guinamard et al. [4] using Pyk-2-deficient mice, have demonstrated the direct relation between S-MZ B cells and TI-2 antigens. These mice do not develop a S-MZ and were demonstrated to be deficient in their TI-2 antibody responses. The exact mechanism by which Pyk-2 deficiency leads to the loss of S-MZ B cells is unknown but is likely related to defects in lymphocyte migration since Pyk-2 has been linked to migration and adhesion processes [4].
Because of a number of unique features, which will be discussed in this article, S-MZ B cells are capable of responding fast and efficient to all sorts of blood-borne antigens in general, and more specifically to TI-2 antigens even without highly specific B cell receptors and the need of specific T cell contact.
THE IMPORTANCE OF A FUNCTIONAL SPLEEN
A functional spleen is essential for the immune response to TI-2 antigens [3,10,13]. Absence or dysfunction of the spleen results in an increased risk of infections caused by bacteria that have a polysaccharide capsule [17–19]. Children below the age of 2 years have a poor response to bacterial infections caused by encapsulated bacteria (e.g. Streptococcus pneumoniae, Neisseria meningitides or Haemophilus influenzae) [19–22]. The incidence of invasive pneumococcal disease (US data) is around 25·8 cases per 100000 (0.025%) in children below the age of 2 years compared to 2–7 cases per 100000 (0.002–0.007%) between the age of 2–60 years [22]. We have demonstrated that all cellular compartments of the spleen have completed their maturation to an adult immunophenotype and morphology within the first five months except for the S-MZ. The main characteristic of the immature infant S-MZ is the low or absent expression of complement receptor 2 (CR2, CD21) on S-MZ B cells until the age of 2 years [19].
Splenectomized patients are also at risk for developing severe infections. In a comprehensive review, Holdsworth et al. [17] reported that the incidence of infections after splenectomy in children below the age of 16 years is 4·4% with an overall mortality rate of 2·2%. For adults the incidence for post splenectomy infections is 0·9% with a mortality rate of 0·8%. Infections were predominantly caused by Streptococcus pneumoniae (56.7%). Of the 114 reported deaths due to pneumococcal infections, 78 (68%) died within 24 h after the onset of symptoms [17] indicating the importance of antibodies, preferably pre-existing but in case of first contact rapidly induced. This rapid first-line defense can be initiated in the spleen as discussed in this review.
THE CD19/TAPA-1/LEU-13 COMPLEX ON B CELLS
The initial encounter with antigens is the most difficult phase of an immune response because naïve B cells express unmutated antigen receptors of low affinity [23]. Lowering the threshold for activation is an adaptation of the immune system to cope with new antigens [9,24]. If the threshold for activation is low on B cells, these B cells can respond to a lower concentration of antigens even when the B cell receptor is of low affinity [23]. The complement receptor 2 (CD21) forms a complex with CD19, TAPA-1 and Leu-13 on B cells [23,25,26]. This complex is linked to the B cell antigen receptor (BCR) resulting in a complex capable of lowering the threshold for activation (Fig. 1) [27]. After activation by a TI-2 antigen, small numbers of highly cross-linked clusters of surface immunoglobulin M (sIgM) are critical for the delivery of the first signal to the B-cell [9]. CD21 serves as a complement-binding subunit, binding the C3d degradation fragment of activated C3 [28]. CD21 is directly associated with CD19 [28]. Both the extracellular and transmembrane region of CD21 and CD19 are required for interaction. CD19 mediates intracellular signalling and is involved in the activation and growth regulation of B cells [24]. A ligand for CD19 has not been demonstrated, it is assumed that CD21 binding of its ligands provides the primary recognition site for CD19 [25]. CD19 lowers the amount of sIgM that needs to be ligated to activate PLCg followed by Ca2+ mobilization [23]. TAPA-1 (CD81) is a widely expressed cell-surface protein involved in a broad range of cellular functions and thought to activate adhesion and promote intracellular interactions [27]. For the CD19/TAPA-1 complex only the extracellular domain is required. TAPA-1 is physically associated with the interferon inducible antigen Leu-13, generating a bimolecular complex. The function of Leu-13 is unknown but it is suggested to be involved in responses to viral attacks, growth regulation and signal transduction [9,23,24,28]. The exact function of TAPA-1 and Leu-13 in the CD21/CD19/Leu-13/TAPA-1 complex has not been resolved but the complex expressed on B cells reduces the threshold for B cell activation via the B cell receptor by bridging antigen specific recognition and CD21 mediated complement (C3d) recognition (Fig. 1), reviewed in [23] and [29]. Cross-linking either CD19 or CD21 to limited numbers of sIgM molecules enhances the cellular responses by 10–1000 fold thus the CD19/CD21 complex promotes signalling through the BCR [28]. In this way the amount of antigen required for an adequate response is effectively reduced by 10–100 fold [25]. Activation by antigens induces a complex of intracytoplasmic signalling cascades [9]. A simplified scheme is give in Fig. 1. Btk is a critical enzyme in TI-2 signalling cascade, further depending on PI-3, which can be recruited directly by PTK's or via CD19 resulting in the generation of PIP-3 [9]. Activated Btk induces a variety of downstream processes including calcium signalling via PLC (Fig. 1). The differentiation towards S-MZ B cells is dependent on Btk but also on molecules like CD19, vav, PKC and PIP-3 that are involved in the signalling via the BCR [30,31]. Recently it has been suggested that toll-like receptors play a role in signalling events in S-MZ B cells [1]. Toll-like receptors play an important role in the recognition of components of pathogens and subsequent activation of innate immunity and are essential in the recognition of TI antigens [9,32]. The above described lowering of the threshold for activation combines a broad specificity with a high sensitivity by bridging the innate immune system with the acquired immune system. The CD21/CD19/TAPA-1/Leu-13 complex is generally expressed on most B cells. S-MZ B cells however, express CD21 in higher quantities makes them even more responsive to limited numbers of opsonized antigens.
Fig. 1.

The CD21, CD19, TAPA-1, and Leu-13membrane complex expressed on B cells modulates the BCR signalling. After binding of complement-opsonized (TI-2) antigen to the BCR, an intracellular signal cascade is activated starting with the activation of several tyrosine kinases like fyn, blk, lyn and syk. These tyrosine kinases can activated Btk. Activation of Btk is also dependent on phosphatidylinositol 3-kinase (PI-3K), which is recruited via phosphorylation of intracytoplasmic residues of CD19. Btk induces a variety of downstream effector mechanisms like calcium signalling via phospholipase Cγ (PLCγ) and inositol 3,4,5-triphosphate (IP-3). Through activation of CD21 by complement fragment C3d, CD19 is activated. CD21 and CD19 interact through their extracellular and transmembrane region. CD19 and TAPA-1/Leu-13 are associated through their extracellular region. This results in the activation of Vav which, via a number of downstream processes and phosphatidylinositol 4-phosphate 5-kinase (PIP-5K), also mediated calcium signalling through IP-3. Based on [23,9,24,29].
C3D, LINKING THE INNATE AND ACQUIRED IMMUNE SYSTEM
As described in the introduction, blood-borne pathogens need to be cleared very rapidly because of the risk of bacteriaemia and subsequent sepsis. The innate immune system is much faster in the first-line defense but the response is not specific. Rapid clearance in general is mainly facilitated by opsonins like specific immunoglobulins and complement. S-MZ B cells are able to bridge the innate and the acquired immune system because of the high CD21 expression in combination with the fact that most TI-2 antigens are able to bind complement fragment C3d without the necessity of specific immunoglobulin-binding (Fig. 1) [33].
We demonstrated the rapid localization of TI-2 antigens in the S-MZ after injection [3]. We immunized rats with pneumococcal polysaccharides (PPS, Pneumovax®) and sacrificed the rats at different time points. Immunohistological examination of the spleens showed extensive localization of TI-2 antigens in the S-MZ already 15 min after immunization(Fig. 2a). Two hours after immunization the antigens could be traced in the primary follicles and lymphocyte corona (Fig. 2b) and after 3–5 days localization on follicular dendritic cells (FDC) in the germinal centres was found (Fig. 2c). The binding of polysaccharides in the spleen is dependent on the presence of complement because no binding was observed in rats de-complemented by injecting cobra venom factor (Fig. 2d). Absence of complement also reduced the primary IgM and IgG response against TI-2 antigens. In rats we found that the TD response was not affected by complement depletion (Breukels et al. unpublished observations). In contrast, de-complementation in mice has been demonstrated to impair the TD response but only when no adjuvant was used [34].
Fig. 2.
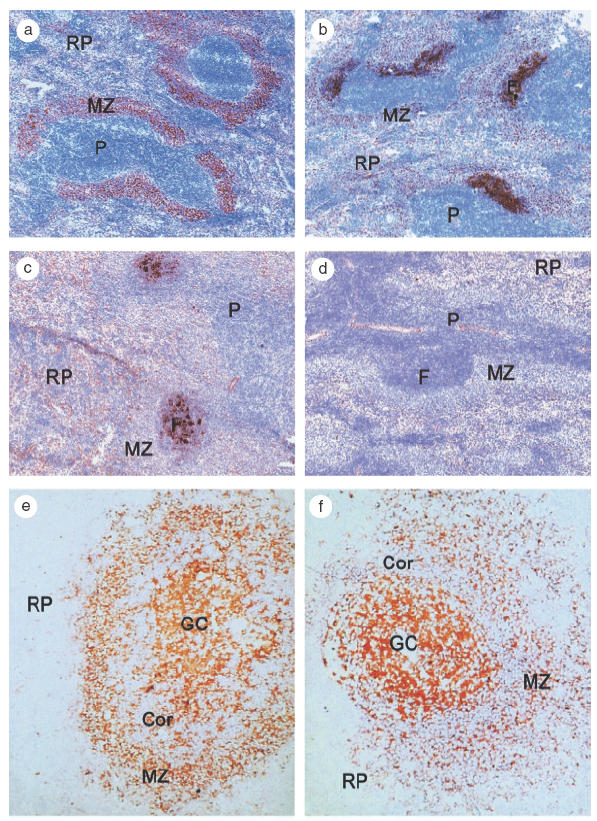
(a–d) Localization pattern of PPS in the rat spleen. Rats were injected intravenously with PPS and sacrificed at (a) 15 min, (b) 1 day, or (c) 5 days after treatment. PPS localization was demonstrated with an immunohistochemical method using a polyclonal antibody directed to PPS type 19. 15 min after treatment, PPS localizes extensively in the S-MZ (a), 1 day after injection, PPS is detected in the primary follicle and 5 days after vaccination, PPS binding is observed in a FDC like pattern in the follicles. (d) No binding is observed in animals, decomplemented by cobra venom factor (CVF) treatment. S-MZ, marginal zone; P, periarteriolar lymphoid sheath; RP, red pulp; F, follicle. (e–f): Localization pattern of PPS (e) type 2 and (f) type 9 N in the human spleen. Spleen sections were incubated with PPS in combination with serum (complement). Sections were subsequently incubated with type specific antibodies against PPS. Strong PPS staining on FDC's in the germinal centres and on B cells in the S-MZ. S-MZ, marginal zone; RP, red pulp; Cor, corona; GC, germinal centre.
In previous studies [33,35], we found strong indications that C3d is the most important complement component in the binding of PPS and subsequent binding of the complex to CD21 expressed on B cells. First, the localization is complement and CD21 dependent. To demonstrate this, anti-PPS antibodies were preincubated with normal human serum or heat-inactivated serum. Human spleen frozen sections were subsequently incubated with these sera. PPS incubated with normal human serum showed a localization pattern identical to the staining pattern with antibodies directed to CD21, with strong expression on FDC and weak expression on small mantle zone B cells, and intermediate to strong expression on S-MZ B cells (Fig. 2e, f). This pattern was clearly different from the pattern observed with antibodies directed to the C3b receptor (CD35) [33,35]. Incubation of PPS with heat inactivated human serum showed no staining. Second, staining otherwise untreated spleen sections with anti-C3d monoclonal antibodies only showed positive FDC staining, suggesting the presence of C3d epitopes, presumably as a general component of immune complexes localized at FDC’s. Sections preincubated with PPS and normal human serum and subsequently incubated with anti-C3d monoclonal antibodies not only showed FDC staining in the germinal centre but also in the mantle zone and S-MZ B cells indicating that C3d formed a complex with PPS which can localize on S-MZ B cells [33,35]. We concluded that the PPS-C3d complex localized exclusively in areas with cells with (high) CD21 density like the S-MZ and FDC's in germinal centres.
Opsonization of polysaccharides by complement fragment C3d seems to be a powerful mechanism to elicit an immune reaction to these antigens that, because of their poor biodegrability, are poor immunogens [36]. Dempsey et al. demonstrated this for a protein, hen egg lysozyme (HEL). Fusion of HEL to 2 or 3 copies of C3d increased the immunogenicity of the protein with about 1000–10000 fold compared to the antigen alone [37]. Whether fusion of polysaccharides to C3d also result in such an impressive increase in immunogenicity needs elucidation. Experiments using TI-2 antigens opsonized with different numbers of C3d have not been done yet. It has been shown that C3d can increase the immunogenicity of proteins as well as polysaccharides [36,38]. The first study however, uses a PPS-C3d conjugate which elicits B cell memory in a TD like fashion, possibly due to contamination of the used conjugate.
TI-2 ANTIGENS SHOW UNIQUE IMMUNE COMPLEX INDEPENDENT ISOTYPE SWITCHING
In immunization procedures with TI-2 antigens like pneumococcal polysaccharides (Pneumovax®), beside IgM also IgG antibodies are found in the same early phase [39–41]. Normally, for isotype switching and affinity maturation of antibodies, the presence of immune complexes in germinal centres and therefore of specific immunoglobulins is required [42]. However, rats immunized with Pneumovax® show a simultaneous IgG and IgM response already at 4–5 days after immunization [39,41] (Fig. 3). Because of the observation of the presence of high levels of IgG already at 4–5 days after immunization, only limited affinity maturation may be expected generating ‘primitive’ memory B cells, suggesting an alternative to the classical germinal centre reaction [25]. The mechanism of this rapid isotype switching of IgM antibodies in absence of specific immunoglobulins is largely unknown. As described in the previous section, accumulating evidence led us to the suggestion that the specific ability of TI-2 antigens to bind complement fragment C3d, even without the presence of specific immunoglobulins, plays an important role in this specific immunoglobulin independent isotype switching [3,33,35,39].
Fig. 3.

Immune responses in rats after Tetanus Toxoid (TT) or PPS vaccination. Rats were intravenously vaccinated with either TT (Tetavax®) or PPS (Pneumovax ®) and blood was sampled at different time points after vaccination. A simultaneous, rapid IgM (▪) and IgG (d) response was observed after PPS vaccination, which declined to almost prevaccination values at day 24. Rats vaccinated with TT showed a late induction of only IgG antibodies (▴). IgM antibodies after TT vaccination were hardly detected. Values represent the average of 6–9 rats. Antibody titres are depicted in U/ml.
Switching from IgM to IgG in general occurs in a germinal centre reaction where affinity maturation and isotype switching takes place. To enable this, localization of antigen-antibody-complement complexes at FDC in germinal centres is essential. Most (TD and TI-1) antigens require the presence of specific immunoglobulin for this localization. TI-2 antigens can also localize at FDC in germinal centres but this process can take place independently of specific immunoglobulins because of the interaction with complement fragment C3d. In contrast to C3b binding, C3d binding to (TI-2) antigens can occur without the need of specific immunoglobulins [25,33], and takes place immediately after entering the blood. This implies that after entering of TI-2 antigens (already complexed to C3d) in the spleen and other lymphoid tissues immediate localization at FDC will take place, because of the local high density of CD21 on FDC. At the same time, after first contact with TI-2 antigens (S-MZ) B-cells will migrate to the germinal centre [3]. Already at that time point these B-cells will then meet locally bound antigen-C3d complexes enabling isotype switching and likely a first step to affinity maturation. This can explain the finding of specific IgG in an early stage after antigenic challenge.
MEMORY VERSUS NAÏVE MARGINAL ZONE B CELLS
A number of studies in antigen-challenged rats as well as in human adults have demonstrated that the S-MZ can contain large numbers of memory B cells [43–47]. In contrast, the S-MZ of rats not challenged with antigen appears to contain mostly naïve cells [48].
In rats, after immunization with TNP-LPS, hapten-binding cells are induced in the S-MZ. The appearance of these hapten-binding (memory) cells in the S-MZ correlates with the capacity of these antigens to induce a secondary response [43]. In human adults, two markers, CD27 and CD148, that identify memory B cells, are found on most S-MZ B cells indicating that these are memory B cells [44,45]. This is confirmed by the fact that microdissected S-MZ B cells of normal human adult spleens have somatic mutations in the heavy chain (Vh) gene of immunoglobulins, a hallmark for memory B cells [46,47]. These genes showed characteristics of antigen selection [46]. Unmutated Vh genes were also found but in a low frequency of about 1 per 15 analysed genes [46,47].
Because of our interest in the development of the human S-MZ, we studied the presence of memory B cells in the infant S-MZ at different time points after birth. We analysed CD27 expression in infant spleen sections ranging in age from 6 days to 15 years. We found that the infant S-MZ initially is populated by CD27 negative, presumably naive B cells which are replaced by CD27 positive, memory B cell in a time frame of approximately 2–5 years after birth probably driven by ongoing antigenic stimulation [49].
The presence of memory B cells in the S-MZ indicates the capacity to respond directly to an infection with blood-borne antigens and give rise to the production of a large amount of antibodies in a short period.
The memory pool in the S-MZ seems to represent mainly IgM memory B cells derived from TD or TI-1 responses. After immunization with TI-2 antigens, memory B cells are not found in the S-MZ [50,51]. TNP-LPS, a TI-1 antigen, elicits an adequate secondary immune response in rodents and hapten binding B cells are present in the S-MZ after the primary immunization. Immunization with the TI-2 antigens, DNP-HES or NP-Ficoll, although leading to IgG producing B-cells (see above) does not elicit classic B cell memory, nor do they elicit hapten specific B cells in the S-MZ [43,52,53].
Based on animal models the following kinetics can be hypothesized. After antigenic stimulation, the S-MZ B cells can migrate to the outer PALS and red pulp were they proliferate and differentiate into antibody producing plasma cells [54](Fig. 4). Antigen specific B cell blasts accumulate in extra follicular zones in the red pulp adjacent to the PALS exit zones. After TD or TI-1 antigenic stimulation, activated B cells can migrate to the primary follicle to initiate a germinal centre process. In TI-2 antigenic stimulation, probably only a few cells can migrate to the primary follicle to start a germinal centre reaction because follicular B cell proliferation on TI-2 antigens has been shown to be moderate [54]. Memory B cells generated in a germinal centre after TD antigenic stimulation are supposed to be able to directly colonize the adjacent marginal zone, a finding that was demonstrated already in 1974 by Nieuwenhuis and Keuning [55]. The appearance of memory B cells in the marginal zone correlates with the capacity to induce a secondary antibody response [43,51]. Memory B cells can reside in the marginal zone for a couple of weeks [43,52], recirculating B cells however, enter the marginal zone through the marginal sinus and subsequently migrate into a primary follicle. After some hours, they leave the follicle and migrate along the outer PALS towards the red pulp [54].
Fig. 4.

Migration pattern of antigen activated marginal zone B cells. Naïve B cells enter the marginal zone through the marginal sinus. After antigenic stimulation, activated B cells can migrate directly to the red pulp (A) to accumulate in extra follicular zones and differentiate to antibody producing plasma cells (B) or migrate to the PALS (C) were they can accumulate in the outer PALS (D) followed by clonal expansion. Activated cells can migrate to the red pulp through bridging channels (exit zones) (E). Activated B cells can also migrate to the primary follicle (F) to initiate a germinal centre process with clonal expansion and affinity maturation (G). In the germinal centre, memory B cell are generated which can directly colonize the marginal zone (H). After TI-2 antigenic stimulation, only few activated cells will initiate a germinal centre process. S-MZ, splenic marginal zone; PALS, periarteriolar lymphoid sheath; Cor, lymphocyte corona; GC, germinal centre; RP, red pulp.
ARE MARGINAL ZONE B CELLS MULTIREACTIVE?
Recent studies have demonstrated that, at least for the naive B cells, only a limited number of B cell clones colonize the S-MZ [48,56]. Chen et al. [56] demonstrated that B cells from the low affinity self reactive B cell repertoire may populate the S-MZ. They used transgenic mice that express a germline rearranged µ heavy chain transgene, Vh81X, from the preimmune repertoire, known to lead to generation of multireactive IgM antibodies. They followed the development of B cells expressing this germline µ heavy chain. These self-reactive Vh81X B cells localize mainly in the S-MZ. It was proposed that these self-reactive B cells in the S-MZ form the ‘core’ of a low-affinity antiself B cell repertoire which functions to clear self-antigens and to react rapidly with low affinity IgM to a large array of bacterial associated antigens [56].
In another transgene mouse model, it was demonstrated that only a limited number of B cell clones colonize the S-MZ and that this selection is dependent on CD19 expression [30]. These findings suggest that the S-MZ B cell repertoire is positively selected based on multi reactivity and presence of the CD19 coreceptor. It is not known whether a specific ligand is required for this BCR/coreceptor dependent selection [30,56].
Dammers et al. [48] analysed Vh genes of S-MZ B cells in nonvaccinated adult rats. The Ig PC7183 Vh gene repertoire was studied in different B cell subsets. They showed that the naive B cell population in the S-MZ is a selected population based on the Vh gene repertoire, which differed from the repertoire expressed in follicular B cells. A striking feature of rat S-MZ B cell immunoglobulins was the length of the CDR3 region in the VhDJh segments compared to that seen in follicular B cells. S-MZ B cells exhibited on average 2–3 amino acids shorter CDR3 region [48]. As it has been demonstrated that a shorter CDR3 region is associated with multireactivity and facilitates the binding of polysaccharides, this suggests that S-MZ B cells are more multireactive for polysaccharides [48].
The above-described studies indicate that the S-MZ, at least for the naïve B cells, maybe populated by a selected, multireactive B cell population resulting in a B-cell compartment that is able to respond rapidly to a broad spectrum of antigens.
Whether the IgM memory B cells present in the S-MZ are also multireactive is not known. The high expression of CD21 facilitates the binding of polysaccharides that are opsonized by complement fragment C3d, overcoming the need for high affinity B cells in the S-MZ. The naive B cells prone to become S-MZ B cells are maybe selected for multireactivity, resulting in a system that is especially well equipped for the first-line defense against blood borne antigens, including polysaccharides.
CONCLUSIONS
Because of the central position of the spleen in the blood stream, the S-MZ is the first lymphoid compartment where B cells meet and can respond to blood-borne antigens. The S-MZ appears to have two main functions for which it is especially well equipped. One function, the rapid response to blood borne antigens in general is facilitated by the reduced blood flow, the lowering of the B cell activation threshold by coreceptors (a.o. high CD21 expression) and the presence of (naïve) multireactive B cells. This way the S-MZ contains a B cell population that can react to antigens in low concentration even in the absence of high affinity B cell receptors. The presence of memory B cells in the S-MZ enables a rapid secondary immune response.
High CD21 expression is in particular important for the other function of the S-MZ, the response to TI-2 antigens. Polysaccharides complexed to C3d can easily be bound by S-MZ B cells because of the high expression of CD21 and the strongly reduced blood flow that facilitates the binding because of the intimate contact. The reduced blood flow, the presence of the TAPA-1 and CD19 complex, and the opsonization of polysaccharides by C3d overcomes the necessity of specific immunoglobulins and actual presence of T cells and results in an easy activation of B-cells with a rapid immune response against TI-2 antigens. After the first antigenic contact, an alternative affinity maturation and isotype switching takes place enabled by polysaccharide-C3d complexes localized on germinal centre FDC’s. This most likely explains the finding of IgG immunoglobulins already at an early time point after first antigenic stimulation. In due time, following further antigenic contact, B cells are generated with higher affinity.
Because of the high expression of CD21, S-MZ B cells can use complement C3d as a component of the innate immune system to enable more rapid involvement of the generally slower, but specific acquired immune system. In addition, the large production of natural antibodies by S-MZ B-cells is another association with the innate immune system [12]. S-MZ B cells appear to be able to respond to all three major classes of antigen, TD, TI-1 and TI-2 [50] but the rapid response to TI-2 antigens is unique for these cells.
In conclusion, the S-MZ appears to be a compartment totally dedicated to rapid humoral responses to (blood-borne) antigens. Because of their abilities and the central position in the blood stream, S-MZ B cells are crucial in the first line defense against life threatening infections by blood-borne organisms.
Acknowledgments
This review is partly based on studies from our group, supported by grants from ‘The Groningen Foundation for Paediatric Oncology Research (SKOG)’ and the ‘Jan Kornelis de Cock Foundation’. The authors would like to thank Monique Lodewijk for critical reading of the manuscript.
REFERENCES
- 1.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 2.Timens W, Boes A, Poppema S. Human marginal zone B cells are not an activated B cell subset. strong expression of CD21 as a putative mediator for rapid B cell activation. Eur J Immunol. 1989;19:2163–6. doi: 10.1002/eji.1830191129. [DOI] [PubMed] [Google Scholar]
- 3.Harms G, Hardonk MJ, Timens W. In vitro complement-dependent binding and in vivo kinetics of pneumococcal polysaccharide TI-2 antigens in the rat spleen marginal zone and follicle. Infect Immun. 1996;64:4220–5. doi: 10.1128/iai.64.10.4220-4225.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–6. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 5.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 6.Timens W, Poppema S. Lymphocyte compartments in human spleen. An immunohistologic study in normal spleens and uninvolved spleens in Hodgkin's disease. Am J Pathol. 1985;120:443–54. [PMC free article] [PubMed] [Google Scholar]
- 7.Steiniger B, Barth P, Hellinger A. The perifollicular and marginal zones of the human splenic white pulp: do fibroblasts guide lymphocyte immigration? Am J Pathol. 2001;159:501–12. doi: 10.1016/S0002-9440(10)61722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a ‘natural immune memory’. Immunol Rev. 2000;175:70–9. [PubMed] [Google Scholar]
- 9.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–70. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Amlot PL, Grennan D, Humphrey JH. Splenic dependence of the antibody response to thymus-independent (TI-2) antigens. Eur J Immunol. 1985;15:508–12. doi: 10.1002/eji.1830150516. [DOI] [PubMed] [Google Scholar]
- 11.Spencer J, Perry ME, Dunn-Walters DK. Human marginal-zone B cells. Immunol Today. 1998;19:421–6. doi: 10.1016/s0167-5699(98)01308-5. [DOI] [PubMed] [Google Scholar]
- 12.Ochsenbein AF, Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624–30. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 13.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–92. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 14.Mond JJ, Farrar J, Paul WE, Fuller-Farrar J, Schaefer M, Howard M. T cell dependence and factor reconstitution of in vitro antibody responses to TNP-B. Abortus and TNP-Ficoll: restoration of depleted responses with chromatographed fractions of a T cell-derived factor. J Immunol. 1983;131:633–7. [PubMed] [Google Scholar]
- 15.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr Opin Immunol. 1995;7:349–54. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 16.Mosier DE, Subbarao B. Thymus independent antigens. complexity of B lymphocyte activation revealed. Immunol Today. 1982;3:217–22. doi: 10.1016/0167-5699(82)90095-0. [DOI] [PubMed] [Google Scholar]
- 17.Holdsworth RJ, Irving AD, Cuschieri A. Postsplenectomy sepsis and its mortality rate: Actual versus perceived risks. Br J Surg. 1991;78:1031–8. doi: 10.1002/bjs.1800780904. [DOI] [PubMed] [Google Scholar]
- 18.Timens W, Leemans R. Splenic autotransplantation and the immune system, adequate testing required for evaluation of effect. Ann Surg. 1992;215:256–60. doi: 10.1097/00000658-199203000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Timens W, Boes A, Rozeboom-Uiterwijk T, Poppema S. Immaturity of the human splenic marginal zone in infants: Possible contribution to the deficient infant immune response. J Immunol. 1989;143:3200–6. [PubMed] [Google Scholar]
- 20.Cowan MJ, Ammann AJ, Wara DW, et al. Pneumococcal polysaccharide immunization in infants and children. Pediatrics. 1978;62:721–7. [PubMed] [Google Scholar]
- 21.Totapally BR, Walsh WT. Pneumococcal bacteremia in childhood: a 6-year experience in a community hospital. Chest. 1998;113:1207–14. doi: 10.1378/chest.113.5.1207. [DOI] [PubMed] [Google Scholar]
- 22.Burman LA, Norrby R, Trollfors B. Invasive pneumococcal infections. incidence, predisposing factors, and prognosis. Rev Infect Dis. 1985;7:133–42. doi: 10.1093/clinids/7.2.133. [DOI] [PubMed] [Google Scholar]
- 23.Fearon DT, Carter RH. The CD19/CR2/TAPA-1 complex of B lymphocytes: linking natural to acquired immunity. Annu Rev Immunol. 1995;13:127–49. doi: 10.1146/annurev.iy.13.040195.001015. [DOI] [PubMed] [Google Scholar]
- 24.Tedder TF, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–18. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 25.Carroll MC. The role of complement and complement receptors in induction and regulation of immunity. Annu Rev Immunol. 1998;16:545–68. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- 26.Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841–50. [PubMed] [Google Scholar]
- 27.Levy S, Todd SC, Maecker HT. CD81 (TAPA-1). a molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- 28.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu Rev Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 29.Tsubata T, Co-receptors on B. lymphocytes. Curr Opin Immunol. 1999;11:249–55. doi: 10.1016/s0952-7915(99)80041-7. [DOI] [PubMed] [Google Scholar]
- 30.Martin F, Kearney JF. Positive selection from newly formed to marginal zone B cells depends on the rate of clonal production, CD19, and btk. Immunity. 2000;12:39–49. doi: 10.1016/s1074-7613(00)80157-0. [DOI] [PubMed] [Google Scholar]
- 31.Cariappa A, Tang M, Parng C, et al. The follicular versus marginal zone B lymphocyte cell fate decision is regulated by Aiolos, Btk, and CD21. Immunity. 2001;14:603–15. doi: 10.1016/s1074-7613(01)00135-2. [DOI] [PubMed] [Google Scholar]
- 32.Takeda K, Akira S. Roles of Toll-like receptors in innate immune responses. Genes Cells. 2001;6:733–42. doi: 10.1046/j.1365-2443.2001.00458.x. [DOI] [PubMed] [Google Scholar]
- 33.Peset Llopis MJ, Harms G, Hardonk MJ, Timens W. Human immune response to pneumococcal polysaccharides. Complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. J Allergy Clin Immunol. 1996;97:1015–24. doi: 10.1016/s0091-6749(96)80078-9. [DOI] [PubMed] [Google Scholar]
- 34.Pepys MB. Role of complement in induction of antibody production in vivo. Effect of cobra factor and other C3-reactive agents on thymus-dependent and thymus-independent antibody responses. J Exp Med. 1974;140:126–45. doi: 10.1084/jem.140.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Timens W, Harms G, Peset Llopis MJ, Hardonk MJ. Pneumococcal polysaccharides localize on splenic marginal zone B cells and follicular dendritic cells, together with complement fragment C3d, without relation to known CD21 epitopes. In: Schlossman SF, Boumsell L, Gilks W, et al., editors. Leukocyte Typing VWhite Cell Differentiation Antigens. Oxford: Oxford University Press; 1994. pp. 39–40. [Google Scholar]
- 36.Test ST, Mitsuyoshi J, Connolly CC, Lucas AH. Increased immunogenicity and induction of class switching by conjugation of complement C3d to pneumococcal serotype 14 capsular polysaccharide. Infect Immun. 2001;69:3031–40. doi: 10.1128/IAI.69.5.3031-3040.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 38.Griffioen AW, Rijkers GT, Janssens KP, Zegers BJ. Pneumococcal polysaccharides complexed with C3d bind to human B lymphocytes via complement receptor type 2. Infect Immun. 1991;59:1839–45. doi: 10.1128/iai.59.5.1839-1845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leemans R, Harms G, Rijkers GT, Timens W. Spleen autotransplantation provides restoration of functional splenic lymphoid compartments and improves the humoral immune response to pneumococcal polysaccharide vaccine. Clin Exp Immunol. 1999;117:596–604. doi: 10.1046/j.1365-2249.1999.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee CJ, Banks SD, Li JP. Virulence, immunity, and vaccine related to Streptococcus pneumoniae. Crit Rev Microbiol. 1991;18:89–114. doi: 10.3109/10408419109113510. [DOI] [PubMed] [Google Scholar]
- 41.Breukels MA, Zandvoort A, van den Dobbelsteen GP, et al. Pneumococcal conjugate vaccines overcome splenic dependency of antibody response to pneumococcal polysaccharides. Infect Immun. 2001;69:7583–7. doi: 10.1128/IAI.69.12.7583-7587.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song H, Nie X, Basu S, Cerny J. Antibody feedback and somatic mutation in B cells: regulation of mutation by immune complexes with IgG antibody. Immunol Rev. 1998;162:211–8. doi: 10.1111/j.1600-065x.1998.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Liu YJ, MacLennan IC, Gray D, Lane PJ. B cell memory to thymus-independent antigens type 1 and type 2: the role of lipopolysaccharide in B memory induction. Eur J Immunol. 1988;18:1417–24. doi: 10.1002/eji.1830180918. [DOI] [PubMed] [Google Scholar]
- 44.Tangye SG, Liu YJ, Aversa G, de Phillips JH, VJ. Identification of functional human splenic memory B cells by expression of CD148 and CD27. J Exp Med. 1998;188:1691–703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig) M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188:1679–89. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med. 1995;182:559–66. doi: 10.1084/jem.182.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tierens A, Delabie J, Michiels L, Vandenberghe P, de Wolf Peeters C. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood. 1999;93:226–34. [PubMed] [Google Scholar]
- 48.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FGM. Most marginal zone B cells in rat express germline encoded Ig V-H genes and are ligand selected. J Immunol. 2000;165:6156–69. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 49.Zandvoort A, Lodewijk ME, de Boer NK, Dammers PM, Kroese FGM, Timens W. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells. Tissue Antigens. 2001;58:234–42. doi: 10.1034/j.1399-0039.2001.580403.x. [DOI] [PubMed] [Google Scholar]
- 50.MacLennan IC, Liu YJ. Marginal zone B cells respond both to polysaccharide antigens and protein antigens. Res Immunol. 1991;142:346–51. doi: 10.1016/0923-2494(91)90089-2. [DOI] [PubMed] [Google Scholar]
- 51.Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165(Suppl. 1):S49–S52. doi: 10.1093/infdis/165-supplement_1-s49. [DOI] [PubMed] [Google Scholar]
- 52.Oldfield S, Liu YJ, Beaman M, MacLennan CM. Memory B cells generated in T cell-dependent antibody responses colonise the splenic marginal zone. Adv Exp Med Biol. 1988;237:93–8. doi: 10.1007/978-1-4684-5535-9_13. [DOI] [PubMed] [Google Scholar]
- 53.Garcia de Vinuesa C, O'Leary P, Sze DM, Toellner KM, MacLennan IC. T-independent type 2 antigens induce B cell proliferation in multiple splenic sites, but exponential growth is confined to extrafollicular foci. Eur J Immunol. 1999;29:1314–23. doi: 10.1002/(SICI)1521-4141(199904)29:04<1314::AID-IMMU1314>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Liu YJ. Sites of B lymphocyte selection, activation, and tolerance in spleen. J Exp Med. 1997;186:625–9. doi: 10.1084/jem.186.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nieuwenhuis P, Keuning FJ. Germinal centres and the origin of the B-cell system. II. Germinal centres in the rabbit spleen and popliteal lymph nodes. Immunology. 1974;26:509–19. [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Martin F, Forbush KA, Perlmutter RM, Kearney JF. Evidence for selection of a population of multi-reactive B cells into the splenic marginal zone. Int Immunol. 1997;9:27–41. doi: 10.1093/intimm/9.1.27. [DOI] [PubMed] [Google Scholar]


