Abstract
To clarify the immune response of CD57+ T cells (most of them are CD8+) in peripheral blood (PB) against alloantigens in order to elucidate the T helper 1 (Th 1) immune response, we assessed the role of CD57+ T cells in IFN-γ (one of the representative Th 1 cytokines) production in a one-way mixed lymphocyte reaction (MLR). In this study, we showed that CD57+ T cells in responder cells were essential for effective IFN-γ production in allogeneic MLR due partly to the augmentation of the alloresponse of regular T cells. Furthermore, IFN-γ production in MLR correlated with the proportions of CD57+ T cells in PB regardless of the responders’ age. We also showed that the extent of the expansion of CD57+ T cells in paediatric patients after haematopoietic stem cell transplantation (HSCT) was markedly lower than that in adult patients. In addition, CD57+ T cells purified and activated with a combination of cytokines showed a greater cytotoxicity than regular T cells against human umbilical vein endothelial cells. Because IFN-γ production in one-way MLR is a useful predictor of graft-versus-host disease (GVHD), especially in the acute phase that occurs after allogeneic HSCT, our findings suggested that CD57+ T cells play a role in the development of GVHD and thus may explain the reason as to why a higher donor age is associated with an increased risk of developing GVHD while, in addition, the incidence of severe GVHD in paediatric patients is lower than that in adult patients.
Keywords: extrathymic, GVHD, NK type T cells, risk factor, stem cell source
INTRODUCTION
In addition to αβ T cells without natural killer (NK) cell markers, αβ T cells with NK cell markers, such as CD56 or CD57, are also present in the peripheral blood (PB) of humans [1–4]. These NK type T (CD56+ T and CD57+ T, most of them are CD8+) cells are relatively rare in peripheral blood mononuclear cells (PBMC), lymph nodes and spleen from healthy individuals [4,5], while these cells are abundant in liver and bone marrow [6]. We reported recently that CD56+ T and CD57+ T cells in PBMC stimulated in vitro with anti-CD3 antibody or T helper (Th) 1 cytokines, such as interleukin (IL)-2, IL-12 and IL-15, produced a large amount of interferon-γ (IFN-γ) and strongly expressed both the cytoplasmic perforin and granzyme B [5] and exerted a potent cytotoxic activity to tumour cells [5,7–9]. In addition, CD57+ T cells increase in older adults and thus seem to play an important role in the immunological changes that take place with ageing [5,10,11]. Therefore, these cells may play a role in the various immune states of the hosts by inducing Th 1 immune responses [5,12].
CD57+ T cells have been suggested to develop extrathymically [11] and have been reported to expand persistently in PB after allogeneic haematopoietic stem cell transplantation (HSCT) in adult patients [13,14]. The expanded CD57+ T cells are suggested to play important roles in the pathophysiology of acute graft-versus-host disease (GVHD) [15] because the CD57+ T cells increased markedly in acute GVHD patients in comparison to those in patients without GVHD after allogeneic HSCT [15]. However, the mechanism by which CD57+ T cells are involved in acute GVHD is largely unknown.
On the other hand, IFN-γ is one of the crucial Th 1 cytokines which are known to cause acute GVHD [16] and it also plays a central role in the cytokine network following allogeneic recognition [17]. The one-way mixed lymphocyte reaction (MLR) is a useful predictor in vitro of acute GVHD that occurs after allogeneic HSCT [18]. The IFN-γ levels in MLR between donors and recipients have been reported recently to correlate with the severity of acute GVHD [19].
In the present study, to clarify the immune response of CD57+ T cells in the PBMC against alloantigens in order to elucidate the Th 1 immune responses, we assessed the role of CD57+ T cells in IFN-γ production in one-way MLR using PBMC as responder cells mainly from healthy volunteers. Furthermore, we investigated the expansion of CD57+ T cells in paediatric patients after HSCT and discuss the possible role of CD57+ T cells in acute GVHD.
MATERIALS AND METHODS
Patients
Twenty-two patients who received HSCT and 11 control patients who had undergone chemotherapy alone due to malignant diseases were studied. All patients were followed at the out-patient clinic of the National Defense Medical College Hospital or Tokyo Metropolitan Kiyose Children's Hospital, Japan. No patients had any underlying complications related to HSCT or had undergone chemotherapy before the start of this study. The characteristics of the patients are summarized in Table 1. Fourteen patients had received bone marrow cells from either HLA-identical siblings or single-antigen mismatched related donors or HLA (A, B, DR)-matched unrelated donors. Cyclosporine and methotrexate were given for acute GVHD prophylaxis [20]. The diagnosis of acute GVHD was recognized clinically and graded according to the criteria described previously [21,22], none of these patients showed severe (grade III and IV) acute GVHD. Seven patients had received autologous stem cells from PB using a cell separator (CS-3000 Plus, Baxter, Chicago, IL, USA) after myeloablative therapy followed by the administration of granulocyte colony stimulating factor. The PB stem cell source contained approximately 1·2% CD34+ cells, 50% CD4+ cells, 40% CD8+ cells and 15% NK cells [23,24]. One patient had received autologous bone marrow cells. None of the HSCT patients showed a clinically overt cytomegalovirus infection. Because the control patients received no HSCT, the ages of the control patients at HSCT were regarded as the ages at which all their chemotherapies were completed. There were no significant differences in the clinical parameters among the allogeneic, autologous HSCT patients and the control groups.
Table 1.
The characteristics of patients who underwent allogeneic (allo-) or autologous (auto-) haematopoietic srem cell transplantation (HSCT) and control pateints
| Allo-HSCT (n =14) | Auto-HSCT (n =8) | Control (n =11) | |
|---|---|---|---|
| Clinical diagnosis* | ALL 5, AML 5, AA 3, CML 1 | ALL 1, ML 2, NB 3, Wilms 1, ES 1 | ALL 8, NB 1, HB 2 |
| Age at HSCT (years) | 7·5 ± 1·0 | 7·8 ± 1·5 | 6·6 ± 1·3† |
| (range) | (3·0–18·0) | (2·2–14·1) | (1·1–15·9) |
| Duration after HSCT (days) | 1603 ± 312 | 1848 ± 442 | 1738 ± 256 |
| (range) | (191–3, 733) | (301–3, 630) | (490–3, 135) |
ALL: acute lymphoblastic leukaemia; AML: acute myelogenous leukaemia; ML: malignant lymphoma; CML: chronic myelogenous leukaemia; NB: neuroblastoma; Wilms: Wilms’ tumour; HB: hepatoblastoma; ES: Ewing's sarcoma; AA: aplastic anaemia.
Age at cessation of all therapies.
Peripheral blood samples
Heparinized PB samples were obtained from both the patients and healthy adult volunteers. Samples of the young patients were obtained from children without any significant clinical features who visited the out-patient clinic of the National Defense Medical College Hospital for routine examination. The study received approval from the ethical committee of our medical school. Informed consent was obtained from all children or their parents.
Cell isolation and flowcytometric analysis
PBL were obtained from the peripheral blood by lymphocyte separation medium (ICN Biomedicals Inc., Aurora, OH, USA) and were then stained with FITC-anti-CD57 antibody (NC1, Beckman Coulter, Miami, FL, USA), PE-antiαβ TCR antibody (Beckman Coulter) and PC5-anti-CD56 antibody (NKH-1, Beckman Coulter) or FITC-anti-CD4 antibody (Beckman Coulter), PE-anti-CD8 antibody (Beckman Coulter) and PC5-anti-CD3 antibody (Beckman Coulter). The surface phenotypes of the PBL were analysed by a flowcytometric analyser (EPICS XL, Beckman Coulter). Human umbilical endothelial cells (HUVEC) were obtained from human umbilical veins and then maintained in culture, as described previously [25]. Such cultures were free from detectable CD45+ contaminating leucocytes and uniformly expressed von Willebrand factor and CD31.
Cell sorting and one-way MLR
CD57+αβ TCR+ (CD57+ T) cells in PBL were depleted by EPICS Elite (Beckman Coulter). PBL or CD57+ T cell-depleted PBL from patients who had received allo-HSCT or adult healthy volunteers were adjusted to a concentration of 4 × 106 cells/ml with RPMI-1640 containing 20% human serum. PBL as allogenic stimulator cells were always isolated from the same healthy volunteer. The stimulator cells were irradiated 40 Gy and adjusted to 4 × 106 cells/ml with RPMI-1640 containing 20% human serum. One hundred µl of either PBL or CD57+ T cell-depleted PBL as responder cells with 100 µl of the stimulator cells were cultured in 96-well round-bottomed plates for 72 or 96 h in a humidified 37°C, 5% CO2 incubator. CD57+ T cells, CD56– CD57– (regular) T cells, regular CD4+ T cells and regular CD8+ T cells were purified by EPICS Elite from PBL of adult healthy volunteers. The purity of each population was more than 95%. The purified CD57+ T cells were added to the CD57+ T cell-depleted PBL and then were adjusted to the indicated proportions (0, 5, 10 and 20%) in whole responder cells and were also added to regular CD4+T cells or regular CD8+ T cells. The adjusted cells, along with either the purified CD57+ T cells, regular T cells, regular CD4+ T cells or regular CD8+ T cells with plastic adherent macrophages were used as responder cells in one-way MLR as described above. Plastic adherent macrophages were obtained by incubating whole PBL (2 × 106 cells/ml) at 37°C in a 5% CO2 incubator for 1 h in 96-well round-bottomed plates and subsequently non-adherent cells were removed.
Assays for IFN-γ levels
IFN-γ levels in the supernatants of MLR cultured for 72 or 96 h were assayed using cytokine-specific ELISA (OptEIATM, PharMingen, San Diego, CA, USA).
Cytotoxic assay against HUVEC
HUVEC were resuspended and seeded into 96-well flat-bottomed plates. Achieving confluence, HUVEC monolayers were labelled with 100 µCi Na2 (51Cr) O4 overnight at 37°C in RPMI-1640 containing 10% FCS, washed three times with medium, and subjected to cytotoxic assay. Purified CD57+ T cells (2 × 105), regular CD4+ T cells or regular CD8+ T cells in 200 µl of RPMI containing 20% human serum were cultured with 100 ng/ml of IL-2 (Pepro Tech EC, London, UK), 20 ng/ml of human IL-12 (Pepro Tech EC) and 5 ng/ml of IL-15 (Genzyme). After 4 days of culture, the cells were harvested and then subjected to a cytotoxic assay. Labelled targets (1 × 104/well) were incubated in a total volume of 200 µl with effector cells (E:T ratio =10 : 1) in RPMI-1640 containing 10% FCS. The plate was centrifuged after incubation for 4 h, after which the supernatant was harvested and counted with a gamma counter. The cytotoxicity was calculated as the percentage of releasable counts after subtraction of spontaneous release. The spontaneous release was less than 15% of the maximum release.
Statistical analysis
All values were expressed as mean ± s.e. The Mann–Whitney U-test was used to compare differences between unpaired samples, while Wilcoxson's signed-rank test was used to compare differences between paired samples. Spearman's rank correlation was used to examine the relationship between various parameters, and the deviation was examined by Fisher's Z exchange. Statistical analysis was performed using the Statview software package (version 4·5). Differences were considered to be significant when P < 0·05.
RESULTS
CD57+ T cells in responder cells are essential for effective IFN-γ production in one-way MLR
To explore the role of CD57+ T cells in IFN-γ production in MLR, MLR was performed after a depletion of CD57+ T cells from responder PBL. Control PBMC were also passed through a cell sorter to neutralize the effect of sorting procedure on lymphocytes. The results showed that IFN-γ production in MLR using CD57+ T cell-depleted PBL as responder cells were significantly lower than those using whole PBL from patients (Fig. 1a) as well as adult healthy volunteers (Fig. 1b). Furthermore, the IFN-γ production in MLR rose gradually as the proportions of CD57+ T cells added to responder cells increased, especially when cultured for 96 h (Fig. 2).
Fig. 1.
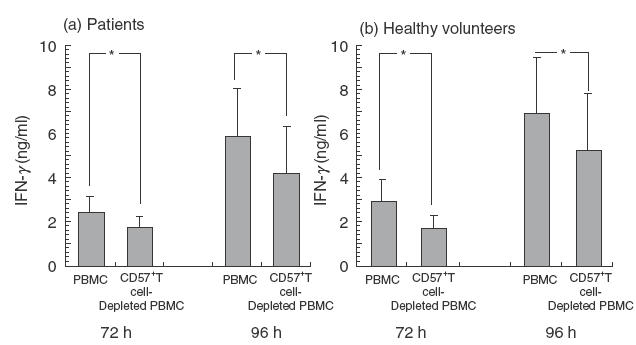
Decrease in IFN-γ production in one-way MLR due to the depletion of CD57+ T cells in responder cells. MLR was performed after the depletion of CD57+ T cells from responder PBL. After a 72- or 96-h culture, the IFN-γ levels of the culture supernatants were analysed using ELISA. (a) patients following allogeneic HSCT. (b) adult healthy volunteers (n =5). All data represented are the mean ± s.e. from five independent experiments. *P < 0·05.
Fig. 2.
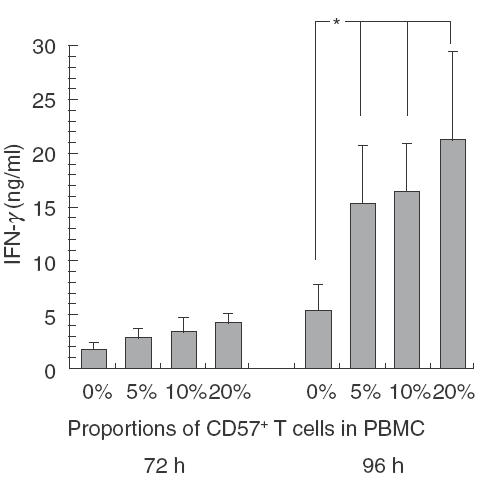
Increase in IFN-γ production in one-way MLR when the proportion of CD57+ T cells in responder cells increases. MLR was performed using the cells to adjust the indicated proportions (0, 5, 10 and 20%) of CD57+ T cells in whole responder cells from healthy volunteers (n =5). After a 72- or 96-h culture, the IFN-γ levels of the culture supernatants were analysed using ELISA. All data represented are the mean ± s.e. from five independent experiments. *P < 0·05.
CD57+ T cells augment IFN-γ production of regular T cells in one-way MLR
IFN-γ production of purified CD57+ T cells in one-way MLR was unexpectedly lower than that of the purified regular T cells (Fig. 3a). However, when 20% of CD57+ T cells in regular CD4+ T cells or CD8+ T cells were present, IFN-γ production in one-way MLR was augmented significantly (Fig. 3b). These results suggest that CD57+ T cells may play an important role as a bystander stimulator to induce IFN-γ production from other T cells.
Fig. 3.
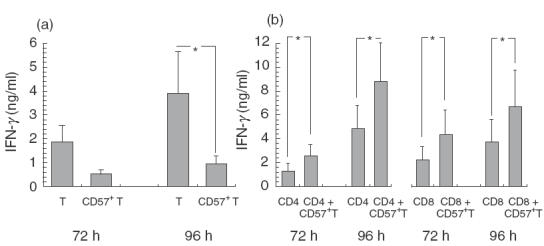
CD57+ T cells augment IFN-γ production of regular T cells in one-way MLR. MLR was performed using the purified CD57+ T cells, regular T cells (a), the purified regular CD4+ T cells, CD8+ T cells or either regular CD4+ T cells or CD8+ T cells added with 20% of CD57+ T cells (b) as responder cells from healthy volunteers (n =5). After a 72- or 96-h culture, IFN-γ levels of the culture supernatants were analysed using ELISA. All data represented are the mean ± s.e. from five independent experiments. *P < 0·05.
IFN-γ production in one-way MLR correlates with the proportion of CD57+ T cells in the PBL of healthy volunteers
Based on the above results and the fact that CD57+ T cells in PBL are known to increase with ageing, using PBMC from healthy volunteers as responder cells, the relationships between IFN-γ production in one-way MLR and the responders’ age or the proportions of CD57+ T cells in PBMC were examined. IFN-γ production in MLR increased constantly with the responders’ age (Fig. 4a). Furthermore, IFN-γ production in MLR also correlated with the proportion of CD57+ T cells in the PBL of the responders regardless of age (Fig. 4b).
Fig. 4.
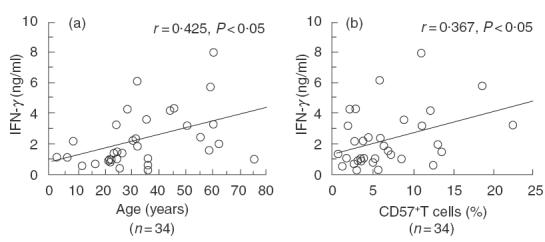
IFN-γ production in one-way MLR correlates with the proportion of CD57+ T cells in the PBMC of healthy volunteers. MLR was performed using PBMC from healthy volunteers as responder cells. After a 72-h culture, the IFN-γ levels of the culture supernatants were analysed using ELISA. The relationships between IFN-γ production and the responders’ age (a) or the proportion of CD57+ T cells in PBMC (b) were examined. The data are based on the findings of several independent experiments from 34 individuals.
CD57+ T cells are cytotoxic against HUVEC
When purified CD57+ T cells from the PBL of healthy adult volunteers were stimulated with a combination of IL-2, IL-12 and IL-15 for 4 days, CD57+ T cells showed a much stronger cytotoxicity against HUVEC than either regular CD4+ T cells or regular CD8+ T cells (Table 2.).
Table 2.
Cytotoxicities of cytokine-actived CD57+ cells against HUVEC
| Subsets | % cytotoxicities against HUVEC† (E:T ratio =10 : 1) |
|---|---|
| CD57+ T cells | 28·1 ± 10·4 |
| Regular CD4+ T cells | 4·8 ± 0·8* |
| Regular CD8+ T cells | 2·6 ± 0·9* |
All data represented are the mean ± s.e. from four individuals.
P < 0·0.5 versus CD57+ T cells.
Proportions of CD57+ T cells in PBL increase following allogeneic and autologous HSCT in paediatric patients
The proportions of CD57+ T cells in PBMC increased significantly more in the patients following allogeneic HSCT (8·6 ± 1·7%, P < 0·05) and autologous HSCT (5·0 ± 0·6%, P < 0·01) than in control patients (2·6 ± 0·5%) (Fig. 5). However, there were no significant differences in the proportions of regular T cells and CD56+ NK cells in PBMC (not shown).
Fig. 5.
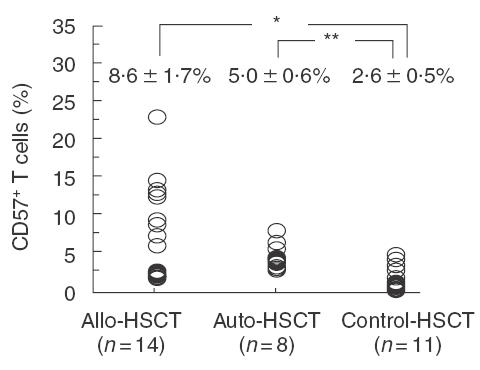
The proportions of CD57+ T cells in PBMC increase following allogeneic and autologous HSCT in paediatric patients. The proportions of peripheral CD57+ T cells in patients following allogeneic HSCT (n =14) or autologous HSCT (n =8), or in control patients (n =11), were analysed by a flowcytometric analyser. *P < 0·05, **P < 0·01.
DISCUSSION
CD57+ T cells have been reported recently to be potent IFN-γ producers [5,26]. IFN-γ is well known to be a representative Th 1 cytokine and acts against bacterial infections and malignant tumours [27,28]. IFN-γ is also one of the important cytokines in controlling and amplifying the immune response against alloantigens or initiating acute GVHD [29]. On the other hand, MLR is a useful model of the immune response between donors and recipients [18] and IFN-γ is reported to play a key role in MLR [19].
As shown in this study, CD57+ T cells in PBMC were shown to induce the production of a larger amount of IFN-γ in MLR. CD57+ T cells are mostly CD8+ and thus are supposed to recognize MHC class I antigens. However, it was noteworthy that, in contrast to CD3 or Th 1 cytokine-stimulated IFN-γ production [5], IFN-γ production of purified CD57+ T cells themselves in MLR was lower than that of the purified regular T cells. CD57+ T cells may thus play an important role as a bystander stimulator which induces IFN-γ production from other T cells in MLR. CD57+ T cells stimulated with alloantigens might produce some cytokines or factors which could stimulate regular T cells to produce Th1 cytokines. To clarify this phenomenon, further investigation will be needed.
The predictive factors for the severity of acute GVHD after allogeneic HSCT have all been well documented. The most powerful factor governing the severity of acute GVHD is the HLA disparity between the allogeneic donor and the transplant recipient [30]. However, other factors such as the advanced age of donors or recipients and gender mismatching may therefore also be associated with the severity of acute GVHD. The incidence of severe acute GVHD in paediatric patients has been reported to be lower than that in adult patients [31]. A steadily increasing age of the donors is also considered to be associated with an increased risk of acute GVHD [32]. However, the exact reason as to why increasing age of either the recipients or donors is associated with an increased risk of developing acute GVHD has yet to be elucidated.
We have shown that the IFN-γ production in MLR increased constantly with the responders’ age and also correlated with the proportion of CD57+ T cells in PBMC, regardless of the responders’ age. Because the levels of the IFN-γ produced in MLR can be used to predict the severity of acute GVHD [19], these findings may explain why an increasing donor's age for HSCT is associated with an increased risk of developing acute GVHD [32]. Several clinical studies have shown that acute GVHD can be prevented by removing T cells from the donor marrow. Conversely, although CD8+ cells in the donor marrow cells are important for preventing rejection of donor marrow cells, the number of CD8+ cells needed to prevent rejection caused severe acute GVHD [33]. CD57+ CD8+ T cells are abundant in the bone marrow [6], and this fact raises the possibility that the depletion of CD57+ T cells from the marrow cells or peripheral stem cell source may prevent acute GVHD.
CD57+ T cells in PBMC in adult patients expanded not only following HSCT [13,14,34] but also following other organ transplantations [35]. The proportions of CD57+ T cells in the adult patients after allogeneic HSCT have been reported to be remarkably high (15–60%) [14]. We also confirmed that adult patients who had received autologus HSCT showed that the mean proportion of CD57+ T cells in PBMC was more than 20% (our unpublished observation). As shown in this study, the expanded CD57+ T cells in the PB from the patients as well as healthy volunteers were considered to induce a large amount of IFN-γ production in one-way MLR. Fukuda et al. reported that CD57+ T cells but not CD57–CD8+ T cells increased remarkably in patients with acute GVHD compared to patients without acute GVHD after allogeneic HSCT [15]. However, the extent of the expansion of CD57+ T cells in paediatric patients demonstrated herein was substantially lower than that in adult patients. The incidence of severe (grades III–IV) acute GVHD in paediatric patients was reported to be lower than that in adult patients [36]. Consistent with these findings, none of patients in this study showed severe acute GVHD. The differences between adult patients and paediatric patients in the extent of the expansion of CD57+ T cells following HSCT may be one of the mechanisms which can explain this phenomenon. Mackall et al. [37] suggested that CD8+ CD57+ T cells expand rapidly via the extrathymic pathway after completion of the chemotherapeutic regimen. Since the thymuses are already involuted in adult patients and may not regrow after HSCT, CD57+ T cells may expand extrathymically and thus be present continuously in large numbers in the peripheral blood of adult patients. On the other hand, paediatric patients after HCST may have lower CD57+ T cells because they may use their thymuses to reconstitute their peripheral T cells at a greater rate than adults [38].
Endothelial cell injury [39] in the liver [40], lung [41] and skin [42] in patients with GVHD after allo-HSCT has been well reported. We have also demonstrated herein that CD57+ T cells acquired a stronger cytotoxicity than regular T cells against HUVEC. Taken together, these findings suggest the possibility that CD57+ T cells expanded following HSCT may play a role in initiating acute GVHD. However, we cannot rule out the possibility that, in one respect, GVHD itself may result in the generation of CD57+ T cells in peripheral blood after HSCT, rather than CD57+ T cells being the cause of GVHD.
On one hand, although the expanded CD57+ T cells may be involved in initiating acute GVHD, it should be noted that these cells may also be related to a favourable prognosis after HSCT, as the patients in this study were considered to be long-term surviving patients. CD57+ T cells have also been reported to expand in long-term adult survivors after allogeneic HSCT [13] or other organ transplantation. We recently demonstrated that CD57+ T cells have a stronger antitumour effect against various tumour cells than regular T cells [5,12]. The possibility was also raised that CD57+ T cells may be either alloreactive or autoreactive and these cells expanded in the patients subjected to both type of HSCT, thus inducing a graft-versus-leukaemia effect [43,44]. Therefore, although CD57+ T cells and their IFN-γ inducing capacity may indeed be involved in acute GVHD, their role in maintaining successful HSCT should also be considered.
In conclusion, both CD57+ T cells in the stem cell source from donors and expanded CD57+ T cells in the PB in recipients following HSCT are considered to play important roles in the production of IFN-γ against alloantigens.
REFERENCES
- 1.Abo T, Balch CM. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J Immunol. 1981;127:1024–9. [PubMed] [Google Scholar]
- 2.Lanier LL, Le AM, Civin CI, et al. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–6. [PubMed] [Google Scholar]
- 3.Lanier LL, Testi R, Bindle J, et al. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J Exp Med. 1989;169:2233–8. doi: 10.1084/jem.169.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abo T, Watanabe H, Iiai M, et al. Extrathymic pathways of T-cell differentiation in the liver and other organs. Int Rev Immunol. 1994;11:61–102. doi: 10.3109/08830189409061717. [DOI] [PubMed] [Google Scholar]
- 5.Ohkawa T, Seki S, Dobashi H, et al. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103:281–90. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada T, Iiai T, Kawachi Y, et al. Origin of CD57+ T cells which increase at tumor sites in patients with colorectal cancer. Clin Exp Immunol. 1995;102:159–66. doi: 10.1111/j.1365-2249.1995.tb06650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh M, Seki S, Hashimoto W, et al. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. J Immunol. 1996;157:3886–92. [PubMed] [Google Scholar]
- 8.Kawarabayashi N, Seki S, Hatsuse K, et al. Decrease of CD56+T cells and natural killer cells in cirrhotic livers with hepatitis C may be involved in their susceptibility to hepatocellular carcinoma. Hepatology. 2000;32:962–9. doi: 10.1053/jhep.2000.19362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doherty DG, Norris S, Madrigal-Estebas L, et al. The human liver contains multiple populations of NK cells, T cells, and CD3+CD56+ natural T cells with distinct cytotoxic activities and Th1, Th2, and Th0 cytokine secretion patterns. J Immunol. 1999;163:2314–21. [PubMed] [Google Scholar]
- 10.Miyaji C, Watanabe H, Minagawa M, et al. Numerical and functional characteristics of lymphocyte subsets in centenarians. J Clin Immunol. 1997;17:420–9. doi: 10.1023/a:1027324626199. [DOI] [PubMed] [Google Scholar]
- 11.Abo T, Kawamura T, Watanabe H. Physiological responses of extrathymic T cells in the liver. Immunol Rev. 2000;174:135–49. doi: 10.1034/j.1600-0528.2002.017415.x. [DOI] [PubMed] [Google Scholar]
- 12.Seki S, Habu Y, Kawamura T, et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 13.Leroy E, Calvo CF, Divine M, et al. Persistence of T8+/HNK-1+ suppressor lymphocytes in the blood of long-term surviving patients after allogeneic bone marrow transplantation. J Immunol. 1986;137:2180–9. [PubMed] [Google Scholar]
- 14.Gorochov G, Debre P, Leblond V, et al. Oligoclonal expansion of CD8+ CD57+ T cells with restricted T-cell receptor beta chain variability after bone marrow transplantation. Blood. 1994;83:587–95. [PubMed] [Google Scholar]
- 15.Fukuda H, Nakamura H, Tominaga N, et al. Marked increase of CD8+S6F1+ and CD8+CD57+ cells in patients with graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1994;13:181–5. [PubMed] [Google Scholar]
- 16.Hu HZ, Li GL, Lim YK, et al. Kinetics of interferon-gamma secretion and its regulatory factors in the early phase of acute graft-versus-host disease. Immunology. 1999;98:379–85. doi: 10.1046/j.1365-2567.1999.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danzer SG, aCampo C, Rink L. Interferon-gamma plays a key role in the human mixed lymphocyte culture. Bone Marrow Transplant. 1996;18:991–6. [PubMed] [Google Scholar]
- 18.Jordan WJ, Ritter MA. Optimal analysis of composite cytokine responses during alloreactivity. J Immunol Meth. 2002;260:1–14. doi: 10.1016/s0022-1759(01)00490-2. [DOI] [PubMed] [Google Scholar]
- 19.van der Meer A, Wissink WM, Schattenberg AV, et al. Interferon-gamma-based mixed lymphocyte culture as a selection tool for allogeneic bone marrow donors other than identical siblings. Br J Haematol. 1999;105:340–8. [PubMed] [Google Scholar]
- 20.Storb R, Deeg HJ, Whitehead J, et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–35. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 21.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:832–43. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 22.Thomas ED, Storb R, Clift RA, et al. Bone-marrow transplantation. N Engl J Med. 1975;292:895–902. doi: 10.1056/NEJM197504242921706. [DOI] [PubMed] [Google Scholar]
- 23.Hillyer CD, Lackey DA, III, Hart KK, et al. CD34+ progenitors and colony-forming units-granulocyte macrophage are recruited during large-volume leukapheresis and concentrated by counterflow centrifugal elutriation. Transfusion. 1993;33:316–21. doi: 10.1046/j.1537-2995.1993.33493242639.x. [DOI] [PubMed] [Google Scholar]
- 24.Stroncek DF, Clay ME, Smith J, et al. Composition of peripheral blood progenitor cell components collected from healthy donors. Transfusion. 1997;37:411–7. doi: 10.1046/j.1537-2995.1997.37497265342.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani K, Takeshita S, Tsujimoto H, et al. Inhibitory effect of serine protease inhibitors on neutrophil- mediated endothelial injury. J Leukoc Biol. 2001;69:241–7. [PubMed] [Google Scholar]
- 26.Van den Hove LE, Van Gool SW, Vandenberghe P, et al. CD57+/CD28– T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia. 1998;12:1573–82. doi: 10.1038/sj.leu.2401146. [DOI] [PubMed] [Google Scholar]
- 27.Trinchieri G. Proinflammatory and immunoregulatory functions of interleukin-12. Int Rev Immunol. 1998;16:365–96. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- 28.Bancroft GJ, Schreiber RD, Unanue ER. Natural immunity: a T-cell-independent pathway of macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 29.Klingebiel T, Schlegel PG. GVHD: overview on pathophysiology, incidence clinical and biological features. Bone Marrow Transplant. 1998;21(Suppl. 2):S45–9. [PubMed] [Google Scholar]
- 30.Flowers ME, Kansu E, Sullivan KM. Pathophysiology and treatment of graft-versus-host disease. Hematol Oncol Clin North Am. 1999;13:1091–112. doi: 10.1016/s0889-8588(05)70111-8. [DOI] [PubMed] [Google Scholar]
- 31.Bross DS, Tutschka PJ, Farmer ER, et al. Predictive factors for acute graft-versus-host disease in patients transplanted with HLA-identical bone marrow. Blood. 1984;63:1265–70. [PubMed] [Google Scholar]
- 32.Eisner MD, August CS. Impact of donor and recipient characteristics on the development of acute and chronic graft-versus-host disease following pediatric bone marrow transplantation. Bone Marrow Transplant. 1995;15:663–8. [PubMed] [Google Scholar]
- 33.Martin PJ, Rowley SD, Anasetti C, et al. A phase I–II clinical trial to evaluate removal of CD4 cells and partial depletion of CD8 cells from donor marrow for HLA-mismatched unrelated recipients. Blood. 1999;94:2192–9. [PubMed] [Google Scholar]
- 34.Izquierdo M, Balboa MA, Fernandez-Ranada JM, et al. Relation between the increase of circulating CD3+ CD57+ lymphocytes and T cell dysfunction in recipients of bone marrow transplantation. Clin Exp Immunol. 1990;82:145–50. doi: 10.1111/j.1365-2249.1990.tb05418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fregona I, Guttmann RD, Jean R. HNK-1+ (Leu-7) and other lymphocyte subsets in long-term survivors with renal allotransplants. Transplantation. 1985;39:25–9. [PubMed] [Google Scholar]
- 36.Klingemann HG, Storb R, Fefer A, et al. Bone marrow transplantation in patients aged 45 years and older. Blood. 1986;67:770–6. [PubMed] [Google Scholar]
- 37.Mackall CL, Fleisher TA, Brown MR, et al. Distinctions between CD8+ and CD4+ T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood. 1997;89:3700–7. [PubMed] [Google Scholar]
- 38.Mackall CL, Fleisher TA, Brown MR, et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–9. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 39.Salat C, Holler E, Kolb HJ, et al. Endothelial cell markers in bone marrow transplant recipients with and without acute graft-versus-host disease. Bone Marrow Transplant. 1997;19:909–14. doi: 10.1038/sj.bmt.1700767. [DOI] [PubMed] [Google Scholar]
- 40.Snover DC, Weisdorf SA, Ramsay NK, et al. Hepatic graft versus host disease: a study of the predictive value of liver biopsy in diagnosis. Hepatology. 1984;4:123–30. doi: 10.1002/hep.1840040122. [DOI] [PubMed] [Google Scholar]
- 41.Woodard JP, Gulbahce E, Shreve M, et al. Pulmonary cytolytic thrombi. a newly recognized complication of stem cell transplantation. Bone Marrow Transplant. 2000;25:293–300. doi: 10.1038/sj.bmt.1702137. [DOI] [PubMed] [Google Scholar]
- 42.Dumler JS, Beschorner WE, Farmer ER, et al. Endothelial-cell injury in cutaneous acute graft-versus-host disease. Am J Pathol. 1989;135:1097–103. [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson BD, Truitt RL. Delayed infusion of immunocompetent donor cells after bone marrow transplantation breaks graft-host tolerance allows for persistent antileukemic reactivity without severe graft-versus-host disease. Blood. 1995;85:3302–12. [PubMed] [Google Scholar]
- 44.Johnson BD, Truitt RL. A decrease in graft-vs.-host disease without loss of graft-vs.-leukemia reactivity after MHC-matched bone marrow transplantation by selective depletion of donor NK cells in vivo. Transplantation. 1992;54:104–12. doi: 10.1097/00007890-199207000-00019. [DOI] [PubMed] [Google Scholar]


