Abstract
Immune dysregulation, polyendocrinopathy and enteropathy with X-linked inheritance (IPEX) is a serious disease arising from mutations in FOXP3. This gene codifies for a transcription factor whose dysfunction results in hyperactivation of T cells. It is not clear, however, why an intermediate phenotype is not seen in heterozygous females, who are completely healthy. In order to address this question, we investigated X-chromosome inactivation in peripheral blood lymphocytes from a heterozygous female with a child affected by IPEX. No preferential inactivation was shown in freshly sorted CD4+, CD8+, CD19+ cells or in IL-2 cultured CD4 and CD8 T cells, indicating that peripheral blood lymphocytes in these women are randomly selected. Moreover, only one single FOXP3 transcript was expressed by CD4 T cell clones analysed by RT-PCR, confirming that this gene is subject to X- inactivation. We hypothesize that hyper-activation of T cell in carriers of FOXP3 mutations is regulated by the presence of normal regulatory T cells.
Keywords: IPEX, X-chromosome inactivation, FOXP3, T lymphocytes
INTRODUCTION
IPEX is a severe disorder characterized by immune dysregulation, polyendocrinopathy, enteropathy and X-linked inheritance [1–4]. The disease is due to mutations in the FOXP3 gene, which is orthologous of the gene mutated in scurfy mice. Recent data suggest that the FOXP3 protein acts as a transcription repressor in T cells, down-regulating cytokine production [5]. This defect in T cell regulation may account for the lymphocytosis, for the activated T cell phenotype and for the allergic-autoimmune response seen in patients with IPEX. Early death is thought to be due to a wasting syndrome, induced by overproduction of proinflammatory cytokines [6,7].
As mutation in FOXP3 seems to favour proliferation, one might expect that female carriers of IPEX would display various degrees of the disease depending on the proportion of T lymphocytes expressing the mutated allele, as observed in some carriers of X-linked chronic granulomatous disease [8].
We performed X chromosome inactivation analysis in a series of different sets of lymphocytes. We also produced some T cell clones so as to further study the expression of FOXP3 by RT-PCR amplification and sequencing of FOXP3 mRNA.
PATIENT
The patient is the mother of a 8-year-old-male who presented aged 4 weeks with a purulent and exudative erythroderma desquamativum, mucous bloody diarrhoea, rising leucocyte count, and a high percentage of lymphocytes with a ‘committed’ phenotype. Chronic interstitial pneumonia, haemolytic anaemia and insulin dependent diabetes mellitus occurred in the second year of life. Genetic analysis of the FOXP3 gene showed a G to A transition at nucleotide 1338 resulting in a putative Ala384Thr substitution at residue 384, confirming the suspected diagnosis of IPEX. This mutation falls within the winged-helix domain of scurfin and is associated with a poor prognosis, as shown in two families previously described [1,2,9]. Although the woman is heterozygous for the same mutation, she is healthy and has no family history of autoimmune disease.
METHODS
Cell isolation and DNA samples
Mononuclear cells and granulocytes were separately collected from peripheral blood by Ficoll gradient. Monocytes were obtained by adhesion to plastic tissue culture plates. Antibody-coated magnetic beads (Dynal, Oslo, Norway) were used to obtain B cells (CD19), CD4 and CD8 T cells, and activated T cells (CD25). T cells obtained by T cell negative isolation kit were activated with PHA (1 μg/ml) and cultured in RPMI medium with 10% FCS and IL-2 (20 IU/ml) in U-bottom 96-well microplates (Costar). The cells were harvested on day 14, and sorted into CD4+and CD4- (CD8). DNA was extracted from all the subsets.
X inactivation study
X inactivation study was performed according to the protocol previously described for the screening of X-linked immunodeficiencies [10].
Briefly, 500 ng of purified DNA were digested in 100 μl volume of buffered solution with 6U RsaI (New England Biolabs, Beverly, MA, USA) at 37°C overnight and then divided into 2 fractions of 50 μl each. One aliquot was further digested with HpaII (New England Biolabs) and incubated overnight at 37°C. The exon 1 of the polymorphic locus ‘Humara’ was amplified both from RsaI digested and from RsaI-HpaII digested DNA samples, using the forward primer 5′ TGC GCG AAG TGA TCC AGA ACC 3′ and the reverse primer 5′ TGG GCT TGG GGA GAA CCA TCC 3′ The amplification was performed for 38 cycles with a Perkin Elmer 480 thermocycler (95°C 1′, 60°C 30′, 74°C 30′) and Pfu polymerase (Stratagene, Amsterdam, NL).
For the analysis, 4 μl of each amplified sample was run on a 10% polyacrylamide gel with 10% urea The gel was stained with silver nitrate.
T cell clones
CD4 T cells selected by specific magnetic beads were plated at a limiting dilution in 96-well microplates in presence of donor feeder obtained from irradiated buffy coat.
FOXP3 expression analysis
Total RNA was extracted from six CD4 T cell clones following the protocol previously described by Chomczynski & Sacchi [11]. RT-PCR was performed using the GeneAmp RNA PCR Core Kit (Applera Genomics, Foster City, CA, USA) according to the manufacturer's instructions. Primers (Sense: 5′ TCA CCT ACG CCA CGC TCA T 3′, Antisense: 5′ ACT CAG GTT GTG GCG GAT G 3′) were designed by using the software Primer Express 1·5 (Applera Genomics) on the basis of the FOXP3 mRNA sequence identified in GenBank with the accession number AF277993. For PCR, after a denaturation step at 95°C for 10 min, 35 cycles were chosen at 95°C for 30 s, 53°C for 30 s and 72°C for 30 s 40 ng of the PCR product were purified using exonuclease I and shrimp alkaline phosphatase (presequencing kit Amersham LIFE SCIENCE, Amersham, UK). A thermal cycle reaction was carried out with BigDye Terminator Cycle Sequencing Ready Reaction Kit v 2·0 (Applera Genomics) using sense and antisense primers. DNA sequences were detected and analysed on an automated ABI Prism 3100 Genetic Analyser (Applera Genomics).
RESULTS AND DISCUSSION
The X-chromosome inactivation analysis showed no significant skewing in any of the cell lines tested (B cells, monocytes, polymorphonuclear cells, freshly isolated CD25 cells, CD25 neg CD4+, CD25 neg CD8+, IL-2 cultured CD4 and CD8 T cells) (Fig. 1). These results demonstrate that both normal and mutated FOXP3 alleles are expressed to a similar degree in the peripheral blood lymphocytes of IPEX carriers.
Fig. 1.
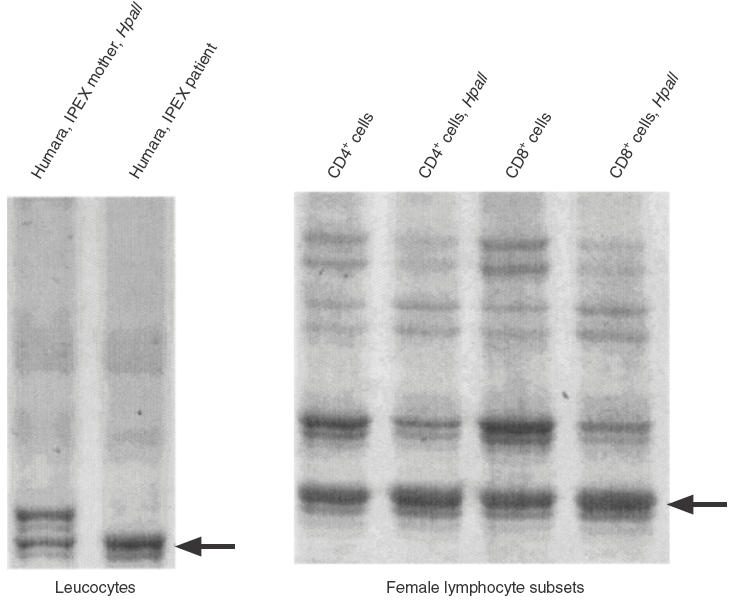
X chromosome inactivation pattern in peripheral CD4 and CD8 T cells. PCR amplification of the highly polymorphic exon 1 of the Humara gene from females give two products which can be separated on gel electrophoresis. After digestion with HpaII, an enzyme which cuts active, nonmethylated DNA, PCR products would represent only inactivated, nondigested, X-chromosome. When preferential X- inactivation occur, one of the two X-chromosome is activated in all the cells and the corresponding Humara band after digestion with HpaII disappears, while the band corresponding to the other X-chromosome, which is always inactive, is unaffected. The gel on the left shows Humara bands in the mother as compared with her son. The gel on the right shows Humara bands from female CD4 and CD8 cells before and after HpaII digestion. The persistence of both bands indicate that there is no preferential X-chromosome inactivation.
At a theoretical dilution of 6 cells per well, 6 clones were created. The wild type FOXP3 mRNA was amplified in one out of six clones; in the remaining five clones, only the mutated allele was found (Fig. 2).
Fig. 2.
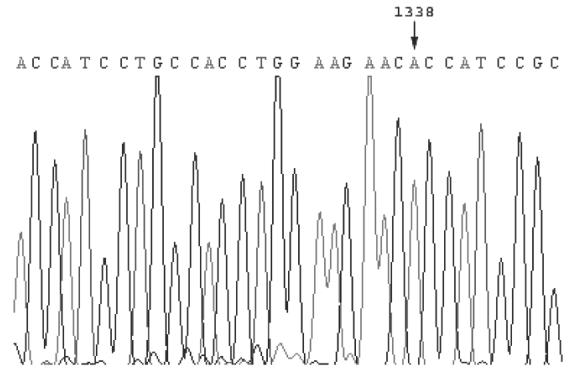
Sequence of RT-PCR product from the CD4 T cell clone no. 6/1. The sequence show the expression of a single allele, bearing a G to A transition at position 1338.
Recessive X-linked immunodeficiencies such as X-SCID and WAS are characterized by severe disorders in the affected males, while female carriers have a normal immunological phenotype because of selective inactivation of haematopoietic precursors expressing the mutated allele. Indeed, the possible X-linked nature of an immunodeficiency in a male is often evaluated by means of X-chromosome inactivation analysis in their mothers.
IPEX is a newly discovered X-recessive immunological disease characterized by immunodeficiency and hyperactivation due to T-cell dysfunction. Female carriers are completely healthy, and all the immunological parameters analysed were normal, including lymphocyte subpopulations. Thereby, we wondered whether the normal clinical and immunological phenotype observed in the female carriers is due (as in other immunodeficiencies), to prevalence of lymphocytes bearing active wild type gene X-chromosome. To answer this question, we analysed the X-chromosome inactivation pattern in a woman heterozygous for FOXP3 mutation, who is the mother of a child affected by IPEX. We found both X-chromosomes expressed in peripheral blood lymphocytes from this woman. Our attention focused particularly on the CD4 T cells, as FOXP3 expression is described in these cells and their dysfunction seems to be responsible for the classic IPEX phenotype. There was no significant inbalance in X- chromosome inactivation in CD4+ or CD8+ T cells. These results indicate that T lymphocytes expressing the mutated FOXP3 allele are present in a similar proportion to normal T-cells, and they do not show any immunological evidence of hyperactivation. This is in contrast with the notion that FOXP3 protein acts as a repressor of T-cell transcription by down-regulating cytokine production [5] and that T cells have a diminished capacity to undergo activation-induced apoptosis in the animal model of the disease [12]. Indeed, we observed a similar pattern of X-chromosome inactivation in CD25+ T cells, suggesting that among activated cells, the percentages of cells expressing wild type and mutated FOXP3 were similar. Although the test is not quantitative, these results lead to speculate that heterozygous FOXP3 mutation does not affect the chances of the single cell surviving or undergoing activation in the peripheral blood compartment.
One possible explanation of these results is that the FOXP3 gene is not subject to X-inactivation, and that half-dose expression of the gene is enough for normal function. This hypothesis is not supported by the results of the analysis that we conducted on CD4 T cell clones. In fact, each clone expressed only one transcript, suggesting that FOXP3 gene is subject to x-inactivation. The alternative explanation is that cells with the mutated FOXP3 display normal development in the presence of normal T cells. This hypothesis is in agreement with results obtained by bone marrow transplantation of IPEX patients [13] or scurfy mice [6] showing that mixed chimerism may be able to control the disease. Recently, a subset of CD4 lymphocytes has been described which expresses IL-10 and which display potent immunosuppressive properties on T cell activation even when a low proportion of these cells is present in the culture [14–16]. It may be hypothesized that the transcription factor FOXP3 is required for the functional activity of these suppressor CD4 T cells. The fact that these cells are generated in the thymus [17], an organ shown to be involved in the disease of scurfy mice [18], would seem to support our theory; however, further studies are needed on regulatory T cell populations in children affected by IPEX and in their mothers.
Our conclusions may be important for therapeutical choices in children affected by IPEX. Bone marrow transplantation has been proposed as the only effective treatment for these children, and the possibility of controlling the disease with a mixed chimerism must be borne in mind when choosing the conditioning regimen in these cases.
Acknowledgments
This work was funded by the Italian Ministry of Health, grant no. ICS 2001/136.
REFERENCES
- 1.Wildin RS, Ramsdell F, Peake J, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 2.Bennett CL, Christie J, Ramsdell F, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 3.Brunkow ME, Jeffery EW, Hjerrild KA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 4.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity–allergic dysregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activationsyndrome. J Biol Chem. 2001;276:37672–9. doi: 10.1074/jbc.M104521200. [DOI] [PubMed] [Google Scholar]
- 6.Patel DD. Escape from tolerance in the human X-linked autoimmunity–allergic dysregulation syndrome and the scurfy mouse. J Clin Invest. 2001;107:155–7. doi: 10.1172/JCI11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13:533–8. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Rosen-Wolff A, Soldan W, Heyne K, Bickhardt J, Gahr M, Roesler J. Increased susceptibility of a carrier of X-linked chronic granulomatous disease (CGD) to Aspergillus fumigatus infection associated with age-related skewing of lyonization. Ann Hematol. 2001;80:113–5. doi: 10.1007/s002770000230. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson PJ, Blanton SH, Saulsbury FT, McDuffie MJ, Lemahieu V, et al. Manifestations and linkage analysis in X-linked autoimmune–immunodeficiency syndrome. Am J Med Genet. 2000;90:390–7. doi: 10.1002/(sici)1096-8628(20000228)90:5<390::aid-ajmg9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Wengler GS, Parolini O, Fiorini M, et al. A PCR-based non-radioactive X-chromosome inactivation assay for genetic counselling in X-linked primary immunodeficiencies. Life Sci. 1997;61:1405–11. doi: 10.1016/s0024-3205(97)00686-3. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Clark LB, Appleby MW, Brunkow ME, Wilkinson JE, Ziegler SF, Ramsdell F. Cellular and molecular characterization of the scurfy mouse mutant. J Immunol. 1999;162:2546–54. [PubMed] [Google Scholar]
- 13.Baud O, Goulet O, Canioni D, et al. Treatment of the immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) by allogeneic bone marrow transplantation. N Engl J Med. 2001;344:1758–62. doi: 10.1056/NEJM200106073442304. [DOI] [PubMed] [Google Scholar]
- 14.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 15.Levings MK, Sangregorio R, Roncarolo MG. Related Articles Human cd25 (+) cd4 (+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Annacker O, Pimenta-Araujo R, Burlen-Defranoux O, Barbosa TC, Cumano A, Bandeira A. CD25+ CD4+ T cells regulate the expansion of peripheral CD4 T cells through the production of IL-10. J Immunol. 2001;166:3008–18. doi: 10.4049/jimmunol.166.5.3008. [DOI] [PubMed] [Google Scholar]
- 17.Jordan MS, Boesteanu A, Reed AJ, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey VL, Wilkinson JE, Rinchik EM, Russell LB. Fatal lymphoreticular disease in the scurfy (sf) mouse requires T cells that mature in a sf thymic environment: potential model for thymic education. Proc Natl Acad Sci USA. 1991;88:5528–32. doi: 10.1073/pnas.88.13.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]


