Abstract
Pathological ocular manifestations result from a dysregulation in the balance between proinflammatory type 1 cytokines and regulatory type 2 cytokines. Interleukin-10 (IL-10) is an anti-inflammatory cytokine with potent immunosuppressive effects. We have examined the efficiency of viral IL-10 adenovirus (Ad-vIL-10)-mediated gene transfer on experimental autoimmune uveoretinitis (EAU) induced in mice and rats by purified retinal autoantigens, respectively, interphotoreceptor binding protein (IRBP) and S-antigen (S-Ag). B10-A mice that received a single unilateral injection of Ad-vIL-10 in the retro-orbital sinus venosus performed 1 day before immunization with IRBP in the footpads showed high levels of circulating vIL-10 in their sera and a significant reduction in pathological ocular manifestations. Lower levels of IFN-γ and IL-2 were found in cellular supernatants from IRBP-stimulated splenic cells in these treated mice. The local effect on ocular disease of vIL-10 was neutralized completely by injection of a monoclonal anti-vIL-10 antibody, demonstrating the specificity of the treatment. To determine whether the transfer of the vIL-10 gene within the periocular tissues of the eye could prevent acute EAU, a subconjunctival injection of Ad-vIL-10 was performed in Lewis rats simultaneously with S-antigen in the footpads. This injection determined in situ vIL-10 expression with very low circulating vIL-10 and led to a significant reduction of EAU without affecting the systemic immune response. The present results suggest that Ad-mediated gene transfer resulting in systemic and local expression of vIL-10 provide a promising approach for the treatment of uveitis.
Keywords: adenovirus, autoimmunity, cytokines, gene therapy, inflammation
INTRODUCTION
Experimental autoimmune uveoretinitis (EAU) is a CD4+ T cell-mediated disease that targets the neural retina [1]. It serves as a model for several human inflammatory ocular manifestations that affect the posterior segment of the eye and lead to permanent destruction of photoreceptor cells and blindness [2]. EAU is induced in rodents by immunization with a retinal autoantigen purified from the retina, such as the interphotoreceptor retinoid binding protein (IRBP) [3] and S-antigen (S-Ag) [4], or by adoptive transfer of retinal-specific CD4+ T cells to syngeneic recipients [5]. The disease course in rats is acute and monophasic, while the mouse presents a more chronic–relapsing disease course. The pathogenesis of EAU has been related to an elevation in the amount of inflammatory Th1 cytokines in uveitic eyes during the acute phase [6], whereas Th2 cytokines such as IL-4 and IL-10 were observed to increase simultaneously or at later stages of EAU, suggesting an important role for these cytokines in the resolution of the disease [7,8]. Moreover, protection from the development of EAU has been induced by oral or intranasal administration of retinal autoantigens, and by induction of anterior chamber-associated immune deviation through an up-regulation of Th2 cytokines [9–11]. Interestingly, immune privilege of the eye has been attributed to a number of factors including IL-10 production in T cells [12,13].
IL-10 is a 35-kDa homodimeric cytokine synthesized by monocytes, B cells and Th2 lymphocytes [14–17]. This cytokine induces mainly immunosuppressive effects through the down-regulation of macrophage functions [17] and the inhibition of the synthesis of proinflammatory cytokines produced by Th1 lymphocytes or monocytes such as IL-1β, IL-6, IL-8, IFN-γ and GM-CSF [17,18]. However, IL-10 presents some stimulatory properties on B and T cell proliferation [19,20]. Interestingly, murine IL-10 (mIL-10) and human IL-10 cDNA sequences exhibit a strong homology to an open reading frame product from the Epstein–Barr virus (EBV) [15,21]. This viral IL-10 (vIL-10) possesses the immunosuppressive properties of IL-10 but lacks certain immunostimulatory functions such as the up-regulation of MHC class II on B cells, chemotactic activity upon CD8+ T cells and stimulation of thymocytes or mast cells [19,22–24].
Due to its short half-life, daily injections of recombinant IL-10 are needed to treat autoimmune diseases. Repeated injections of IL-10 allowed limitation of the expression of EAU [7] and endotoxin-induced uveitis (EIU) [25,26]. The sustained delivery of cytokines obtained by means of gene transfer has shown its efficacy under several experimental conditions. Indeed, the beneficial effect of the gene transfer of IL10 has been shown successfully in transplantation [27,28] in autoimmune diseases [29], such as experimental arthritis [30,31] and in diabetes in NOD mice [32]. Interestingly, local delivery of cytokine after intra-articular adenovirus-gene transfer was shown to be effective in arthritis [33–35].
In the eye, adenovirus-marker gene transfer in ocular structures has been reported after intraocular injection into the vitreous body, the anterior chamber, the peribulbar and subretinal space [36,37]. Moreover, this strategy successfully transferred brain-derived neurotrophic factor, basic fibroblast growth factor or ciliary neurotrophic factor allowing to induce a protection against retinal degenerative diseases [38–40].
In the present study, we have investigated the effect of adenovirus-mediated vIL-10 (Ad-vIL-10) gene transfer by systemic and subconjunctival injection on the incidence and severity of EAU induced in mice and rats.
MATERIALS AND METHODS
Induction of EAU
Mice
Female B10A mice (Harlan, Gannat, France, 6–8 weeks of age) were immunized subcutaneously with 150 µg of IRBP in 150 µl of saline with 150 µl of complete Freund's adjuvant containing 2·5 mg/ml Mycobacterium tuberculosis H37RA (Difco, Detroit, MI, USA). In addition, all mice received simultaneously 1 µg of Bordetella pertussis toxin (RBI, Natick, MA, USA) intraperitoneally. Mice were sacrificed 21 days after immunization. Five separate experiments were carried out using a total of 65 mice.
IRBP was isolated from bovine retinas as described previously [41], with some modifications [42]. Briefly, IRBP was isolated from bovine retinas and purified through two chromatographic steps on ACA 34 (Pharmacia, Uppsala, Sweden) and Concanavalin A (Con A)-Sepharose affinity chromatography (Pharmacia). IRBP was then eluted using Tris-HCl/0·15 mm NaCl/1 mm CaCl2/0·1 mm MnCl2/0·2 mm methyl-d-mannopyranoside pH 7·5 (Sigma, UK). Further purification was obtained using mannose agarose affinity column (Sigma, UK) to remove contaminating Con A.
Rats
Male Lewis rats (Charles River, Saint-Aubin-lès-Elbeuf, France), 8–11 weeks of age were used for immunization. S-antigen (S-Ag) was purified from bovine retinas [1]. The antigen was emulsified (1 : 1) in complete Freund's adjuvant (CFA, Difco, Detroit, MI, USA), supplemented with 250 µg of Mycobacterium tuberculosis H37Ra (Difco). A total of 0·2 ml containing 30 µg of S-Ag was injected into the footpads of each rat. Four separate experiments were carried out using a total of 50 rats.
Recombinant adenovirus constructs
Construction of the replicative defective adenoviral vector expressing vIL-10 (Ad-vIL-10) and adenoviral vector expressing mutated IL-10 (Ad-vIL-10mut) has been described previously (43). Briefly, the BCRF1-coding gene (−16, +625), flanked in 5′ with the promoter of the cytomegalovirus and in 3′ with a SV40 polyadenylation sequence, was inserted in the E1 region of the adenoviral genome. In the Ad-vIL-10mut control, a mutation was inserted in position + 71, just after the signal sequence, leading to a modification of the reading frame.
Ad-GFP (promoter: CMV) was a generous gift of Dr M. Methali (Transgène, Strasbourg, France) and Ad-βGal (promoter: RSV) from Dr P. E. Rakoczy (Centre for Ophthalmology and Visual Science, Perth, Australia).
High titres of recombinant adenoviruses were prepared by amplification in HEK 293 cells, and purified according to established methods [44].
Treatment of EAU
Mice
B10-A mice were infected using a single unilateral injection in the retro-orbital sinus venosus of 3–6 × 108 plaque-forming units (pfu) of Ad-vIL-10 in 0·2 ml of saline the day before immunization or Ad-vIL-10mut or saline as controls. In a particular experiment, one group of mice received a retro-orbital sinus venosus unilateral injection performed on the same eye as that used for Ad-vIL-10 injection of 0·1 mg of rat antihuman and viral IL-10 MoAb (clone JES3–19F, Pharmingen) (described as not cross-reactive towards mouse IL-10 by ELISA). This injection was performed simultaneously with the IRBP immunization and 1 day later.
Rats
Lewis rats received either 3 × 109 pfu (in 200 µl of saline) of Ad-vIL-10 by intravenous injection in the penis vein, 1 day before immunization, or 2 × 109 pfu (in 20 µl of saline) by subconjunctival injection in the both eyes on the day of immunization; Ad-vIL-10mut or saline were used as controls.
Adenovirus-mediated expression of GFP and βGal in mouse and rat ocular tissues
In mice, an injection of 4·5 × 108 pfu in 200 µl of Ad-GFP was performed into the retro-orbital sinus venosus of one eye and eyes were taken at 4 days. In rats, after a subconjunctival injection of 109 pfu in 10 µl of Ad-GFP or Ad-βGal performed in each eye of the rat, eyes were taken at 4, 7, 15 or 21 days.
For βGal expression, the rat eyes were processed as follows [45]. Briefly, the eyes were fixed in 4% paraformaldehyde and, after washing in PBS at 4°C, the eyes were incubated overnight in 5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside (X-gal). A postfixation was performed in 4% paraformaldehyde for 2 h, then the eyes were washed in PBS and paraffin-embedded. Sections were cut and stained with haematoxylin and eosin.
For GFP expression, the mouse or rat ocular globes were collected, fixed in 2% paraformaldehyde for 4 h at RT and, after washing with PBS, were embedded in OCT (Tissue-tek, Miles, Elkhart, IN, USA) and snap-frozen in melting isopentane. This process was essentially the same as described previously [46]. The transgene expression was characterized on 10 µm sections under UV light [45].
Histopathology and EAU grading
Enucleated mouse and rat eyes collected 21 days after immunization were fixed for 24 h in Bouin's solution, paraffin-embedded, and sections were made through the pupillary–optic nerve plane and stained with haematoxylin–eosin for histological evaluation. These sections were examined by a masked investigator [46].
For mice, the incidence and severity of EAU was scored on a scale of 0–4 according to a semiquantitative grading system [47]. This system takes into account cellular inflammation in the anterior and posterior segments of the eye, the presence of retinal vasculitis, choroidal granuloma, retinal folding and/or detachment and photoreceptor damage. Severity of disease was calculated as the mean of values from all eyes in each group from five separate experiments.
For rats, the incidence and severity of EAU was scored by a masked investigator on a semiquantitative scale of 0–7 [48]: 0, no destruction; no cell infiltration; 1–7: limited or total destruction of the various layers of the retina; grade 1–2: destruction of outer segments of rods and cones; grade 3–4: destruction of the outer nuclear layer; grade 5–6: of the inner nuclear layer and grade 7: destruction of the ganglion cell layer. Severity of disease was calculated as the mean value from of all rats from four separate experiments. From these values, the level of protection was also expressed as:
In some experiments, the degree of Ad-vIL-10 protection was quantified further by measuring the outer nuclear layer (ONL) surface, which serves as an index of photoreceptor loss or protection. To survey the whole retina, sections were made through the optic nerve from the superior to the inferior pole. ONL surface was determined directly from slides using a microscope (Eclipse E800, NIKON) and objective 20×. Sections were mapped on video images digitized with a computerized image analysis system (Visioscan 200, Biocom, France). For each animal, the surface was measured on one slide through the optic nerve. Normal control rat surface was measured (n = 4) and the mean value of surface considered as 100%.
The ONL surface of rats injected by subconjunctival or intravenous route with Ad-vIL-10 or Ad-vIL-10mut was measured and a percentage of protection was calculated for each rat as compared with the surface value of normal controls.The mean percentage of protection was then calculated in each series of Ad-vIL-10-treated rats.
T cell responses
To measure IRBP-specific T cell proliferation and cytokine production, spleens were removed from treated and control mice 21 days after immunization and cell preparation was tested as follows.
Lymphocyte proliferation was performed as previously described [42].
Lymphokine production by in vitro Ag-stimulated cells was performed by culture of 2 × 106 cells/1 ml/well in 24-well flat-bottomed plates in the presence of 50 µg/ml IRBP in 1 ml of RPMI-1640–10% fetal calf serum (FCS). Supernatants were collected after 24 h or 48 h for cytokine determination. The use of FCS in culture medium has no implication on the T cell responses as IRBP was free of any contamination with bovine serum albumin. No significant production of cytokines was observed in wells containing medium alone.
In addition, the effect of Ad-vIL-10 treatment on the immune response was evaluated in rats giving a score from 0 to 3 for the footpad reactivity, the site of the S-Ag immunization. Briefly, the scoring was as follows: induration and redness = 1; + oedema and swelling = 2; + necrosis = 3.
To measure specific delayed-type hypersensitivity (DTH), a skin test to S-Ag was performed by ear assay. Forty-eight h before the end of the experiment, the pinna of one rat ear was injected with 10 µg of S-Ag in 10 µl of saline through a 30-gauge needle, the pinna of the other ear was injected similarly with saline. Ear swelling was measured with a metric caliper and results were expressed as the difference in thickness (mm) between the S-Ag-injected and the saline-injected ears.
Cytokines and anti-IRBP antibodies (ELISA)
IFN-γ, IL-2, IL-4, murine and viral IL-10 were determined by ELISA using antibody pairs (Pharmingen) as described previously [43]. In the case of vIL-10 determination, the MoAbs (clones JES3–9D7b and JES3–12G8) used in the test were cross-reactive for viral and human IL-10 but not for mouse, and the human cytokine was used for standard curve determination. The limits in sensitivity of the ELISA test are around 2–5 pg/ml for vIL-10, 5–10 pg/ml for IL-2 and IL-4, 20–50 pg/ml for mouse IL-10 and IFN-γ.
Serum levels of anti-IRBP total IgG, IgG2a and IgG1 subclasses were determined by ELISA as described [42].
RNA isolation and reverse transcriptase polymerase chain reaction (RT-PCR)
Total RNA was isolated from spleens or freshly enucleated eyes by the acid guanidinium thiocyanate–phenol–chloroform method. The protocol for reverse transcription was described previously [42]. In each reverse transcribed sample, the concentration was readjusted according to the intensity of β-actin band as determined by densitometry. The relative band intensity was calculated in comparison to that for β-actin. Viral IL-10, IL-2 and β-actin sense primers and antisense primers were obtained from Clontech Laboratories, Palo Alto, CA, USA and the PCR amplification was performed according to the manufacturer's instructions.
These primers were designed to amplify specifically cDNA fragments representing mature mRNA transcripts of 540 bp for β-actin, 420 bp for vIL-10, 413 bp for IL-2.
Statistical analysis
Data are presented as mean ± s.e.m. Statistical analysis of EAU scoring was performed using the non-parametric Mann–Whitney sum test or by the Kruskal–Wallis non-parametric anova test, followed by the Bonferroni multiple comparison test. A P-value adjusted by the multiple comparison test was calculated in each experiment. Analysis of lymphocyte proliferation and cytokine production was performed using Student's t-test. Values of P < 0·05 were considered significant.
RESULTS
Effect of vil-10 gene transfer on murine Eau
Detection of vIL-10 in mouse sera following retro-orbital sinus venosus injection
To determine whether administration of Ad-vIL-10 in the retro-orbital sinus venosus resulted in detectable amounts of vIL-10 in the serum, B10A mice were injected 1 day before IRBP immunization with 3–6 × 108 pfu Ad-vIL-10. Administration of 3 (n = 2), 4·5 (n = 8) or 6 (n = 3) × 108 of Ad-vIL-10 resulted, respectively, in 23 ± 2·2, and 28·8 ± 13·8 and up to 100 ng/ml of vIL-10 in the serum at day 3. Serum levels of vIL-10 were detected as early as day 1 after administration and peaked at day 3–4. Then they dropped in titre such that by day 10 postinjection, vIL-10 was undetectable by ELISA.
Detection of a marker gene (GFP) in ocular tissues after retro-orbital sinus venosus injection
We evaluated the possibility of local Ad-mediated gene transfer after injection in the retro-orbital sinus venosus of Ad-GFP (4·5 × 108 pfu) performed in one eye by analysing GFP expression 4 days after injection. As shown in Fig. 1, a low expression of GFP was detected in the injected eye at the periphery of the cornea, the conjunctiva and the periocular muscles without any expression in the retina, the choroid and the retinal pigmented epithelium. In the controlateral uninjected eye, only a very slight GFP expression was detected in the iris vessels.
Fig. 1.
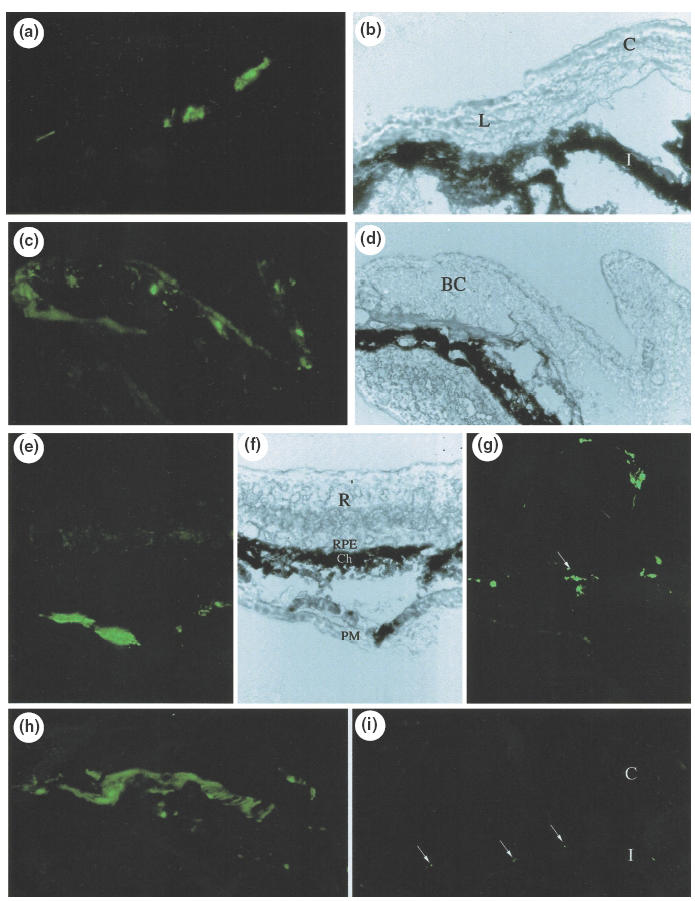
Histochemical examination of mouse ocular tissues 4 days after injection of Ad-CMVGFP (4·5 × 108 pfu) in the retro-orbital sinus venosus on fluorescence micrographs of cryostat sections of ocular tissues from the injected eye (a, c, e, g, h) and from the uninjected eye (i); Corresponding phase contrast micrographs: b, d, f. (a) Low expression of GFP was detected in the injected eye at the periphery of the cornea (limbus), (c): in the bulbar conjunctiva, (e, h): in periocular muscles, (g): in the arachnoid (arrow) of the optic nerve without any expression in the retina, the retinal pigmented epithelium and the choroid; (i): very low level of GFP expression was detected in the iris vessels of the controlateral non injected eye (C = cornea; L = limbus; I = iris; BC = bulbar conjunctiva; R = retina; RPE: retinal pigmented epithelium; Ch: choroid; PM = periocular muscles) (magnification: a–d, g–i: 400; e, f: 140).
Viral IL-10 gene transfer reduces the severity of EAU
We decided to investigate next the effect of vIL-10 on the development of EAU. In a preliminary experiment, we first evaluated the dose required to obtain an inhibitory effect on EAU: a group of four mice received a retrobulbar injection of recombinant Ad-vIL-10 1 day before IRBP immunization at a dose of 6 × 108 pfu and another group of two mice received 3 × 108 pfu. Compared to the EAU developed in control mice injected with the nonvIL-10-expressing recombinant adenovirus (Ad-vIL-10mut), the treatment resulted in EAU reduction in groups treated with 6 × 108 pfu (score values: Ad-vIL-10: 0·25 ± 0·1 versus Ad-vIL-10mut: 2·38 ± 0·4) or 3 × 108 pfu (score values: Ad-vIL-10: 1 ± 0·5 versus Ad-vIL-10mut: 2 ± 0·5). When using the 6 × 108 pfu dose, the EAU inhibition was more effective (two of four mice did not develop EAU and the other two mice developed EAU of low severity) compared to the 3 × 108 pfu dose; the two mice injected with Ad-vIL-10 developed EAU, although less severe than the control, suggesting a trend to a dose-dependence of the inhibitory effect. Considering the very high amount of vIL-10 induced by injection of the 6 × 108 pfu, we choose for the subsequent experiments to inject Ad-vIL-10 at the intermediate dose of 4·5 × 108 pfu. Under these conditions, treatment with Adv-IL-10 appeared very effective in four independent experiments, causing a significant inhibition of EAU in Ad-vIL-10-treated animals compared to control mice injected with saline (P = 0·003) or with Ad-vIL-10mut (P = 0·02)(Figs 2 and 3). Indeed, five of 13 mice (38%) treated with Ad-vIL-10 did not present any disease (score = 0) and the mean score of the others was low, compared to controls injected with Ad-vIL-10mut in which EAU developed in all 10 mice. A similar pathology (P = 0·32) was developed in both types of controls (saline and Ad-vIL-10mut) discarding the involvement of the vector in the observed protection. In Ad-vIL-10-treated mice, as shown in Fig. 3, a protection of the photoreceptor cell layer was observed compared to the extensive destruction of this layer in Ad-vIL-10mut injected mice. To assess the specific involvement of vIL-10 in the inhibition of EAU, anti-vIL-10 specific monoclonal antibodies were injected at day 0 and day +1 after immunization. As illustrated in Figs 2 and 3 this treatment completely reversed the protective effect obtained with Ad-vIL-10 administration (P = 0·003).
Fig. 2.
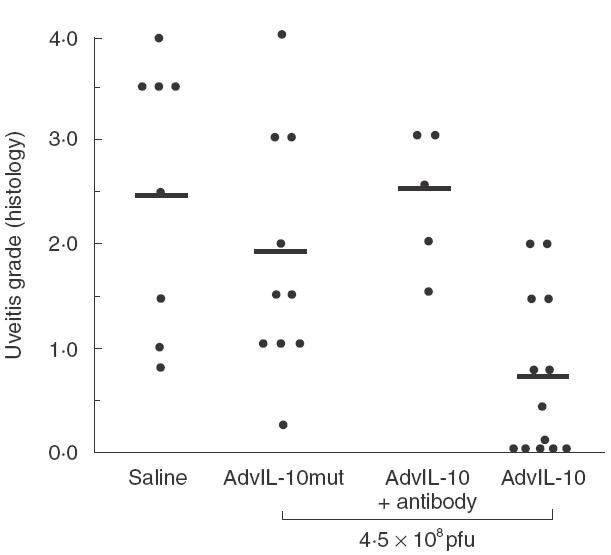
The expression of vIL-10 inhibits EAU. B10A mice were immunized with 150 µg of IRBP in adjuvants and received one unilateral injection in the retro-orbital sinus venosus of 4·5 × 108 pfu of Adv-IL-10, the day before immunization. Eyes were collected on day 21 after IRBP-immunization. The data represents the severity of EAU by histological examination of treated and control mice and EAU was scored on a scale of 0–4. Mice (four separate experiments) were treated as follows: control groups received one injection in the retro-orbital sinus venosus of saline (n = 8) or Ad-vIL-10mut (n = 10); mice were treated with one injection of Ad-vIL-10 (n = 13). One group received one unilateral injection in the retro-orbital sinus venosus of 0·1 mg of rat anti-vIL-10 MoAb performed simultaneously with the IRBP immunization and one day later (n = 5). Each point is one mouse (average of both eyes). The average of each group is shown by a horizontal bar.
Fig. 3.
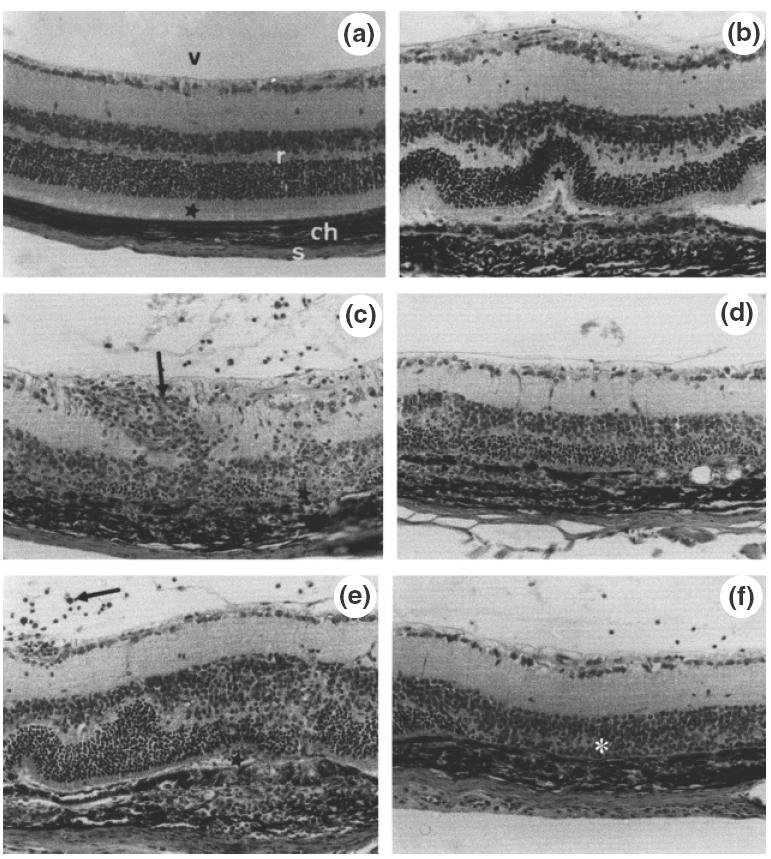
EAU pathology in mice treated with AdvIL-10. Mice treated with AdvIL-10 show either normal ocular tissues (a): retina (r), choroid (ch), sclera (s), vitreous (v) and normal photoreceptor outer segments (asterisk) or (b): limited alterations in the posterior segment: localized retinal fold (asterisk) facing a choroidal infiltration. In contrast, in Ad-vIL-10mut-injected mice (c, d), extensive photoreceptor cell layer destruction (asterisks) with choroidal inflammatory cell infiltration, retinal vasculitis and perivasculitis (arrow), inflammatory cells in the vitreous. In mice injected with AdvIL-10 + anti-vIL-10 antibody (e, f): vitritis (arrow), folds in the retina with focal destruction of the photoreceptor rods (asterisk) facing a choroidal granuloma (e), large destruction of the photoreceptor cell layer (asterisk) (f) (magnification: × 560).
Ad-vIL-10-treated mice show a decrease in type 1 response
Spleens were collected from the different groups of animals at day 21 after immunization and cells were stimulated with IRBP as described in M §M section. A significant decrease in IFN-γ(Fig. 4) and to a lesser extent of IL-2, was obtained upon 48 h stimulation with antigen in the Ad-vIL-10-treated group compared to controls. No type 2 cytokines (IL-4, IL-10) were detected in the supernatants. Moreover, experiment of RT-PCR at day 21 on spleen of these animals showed a decrease in the IL-2 signal in the Ad-vIL-10 groups confirming the general immunosuppression (mRNA expression of IL-2 relative to β-actin: saline: 0·77; Ad-vIL-10mut: 0·80; Ad-vIL-10: 0·5).
Fig. 4.
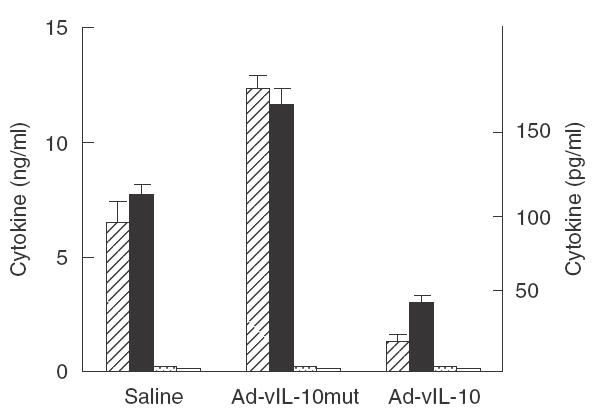
Viral IL-10 gene transfer at day − 1 inhibits proinflammatory cytokine production to IRBP by IRBP-stimulated spleen cells. Cytokine production to IRBP in culture was measured by ELISA. No production of IL-10 or IL-4 was detected in the limits of sensitivity of the ELISA test (respectively, 20–50 pg/ml and 5–10 pg/ml), but small bars were represented to illustrate these data. No production of cytokine was obtained with unstimulated cells in the different tests.  , IFN-γ; □, IL-10; ▪, IL-2;
, IFN-γ; □, IL-10; ▪, IL-2;  , IL-4.
, IL-4.
In the T cell lymphoproliferative assay, a slight but not significant decrease in [3H]-thymidine was observed in the Ad-vIL-10 group compared to the controls (data not shown).
Effect of Ad-vIL-10 treatment on anti-IRBP IgG isotype antibodies
Sera were collected at day 21 and analysed for their specific antibody reactivity in separate experiments. In four of five experiments, we observed a decrease in total IgG antibody response in the Ad-vIL-10-treated mice (20–40%) compared to the controls, affecting both type 1 (IgG2a) and 2 (IgG1) isotypes (data not shown). However, the difference between the 2 groups did not reach significance.
Effect of subconjunctival vIL-10 gene transfer on rat EAU
Taking into account that a systemic treatment with vIL-10 could lead to a general immunosuppression with undesirable effects, we chose to test the effect on EAU of a local IL-10 gene transfer in the rats, whose eye size is more convenient for local injection. This approach was undertaken by comparing the effect of vIL-10 expression either by intravenous (systemic) or subconjunctival (local) administration of the virus on the development of the EAU pathology.
Detection of a marker gene (GFP and βgal) in ocular tissues after subconjunctival injection in rats
We first evaluated the possibility of local Ad-mediated gene transfer by using subconjunctival injections of Ad-βgal or Ad-GFP (109 pfu in 10 µl) performed on Lewis rats. The expression of βgal or GFP activity was monitored at regular intervals.
As shown in Fig. 5, expression of GFP and βgal signals was detected 3 days after gene marker injection in the bulbar conjunctiva, conjunctival epithelium, oculomotor muscle cells, at the limbus of the cornea, in the sclera and the epi-sclera and the arachnoid of the optic nerve. No transduction of the choroid, the retinal pigment epithelium or the neuroretina could be detected. The expression of marker gene transfer was still detectable in ocular tissues 7 days after injection and dropped thereafter, being undetectable at day 15. The labelling appeared to be more effective with Ad-GFP than Ad-βgal. This could be related to a difference in expression of the marker genes depending on the two differents promoters present in these adenoviruses (CMV for the Ad-GFP, as for Ad-vIL-10, and RSV for the Ad-βgal).
Fig. 5.
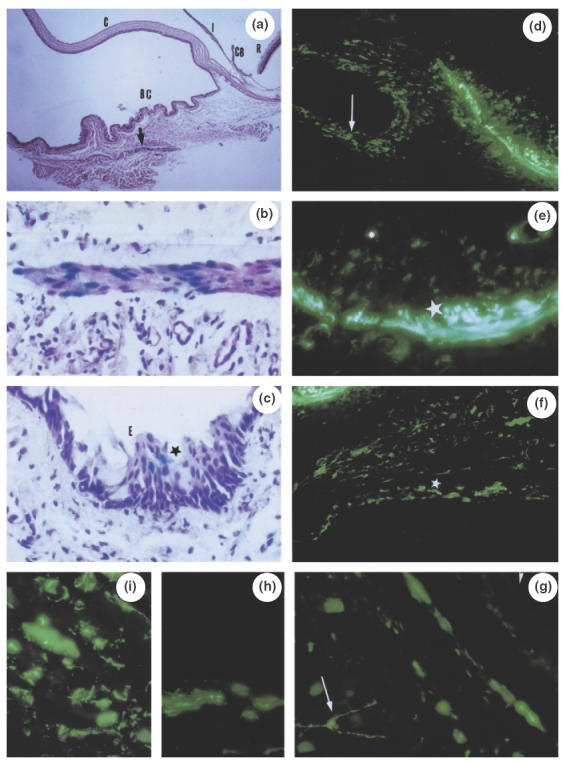
Histochemical examination of rat ocular tissues 3 days after subconjunctival injection of adenovirus expressing βGal (a–c) or GFP (d–i). At low magnification (a), βGal expression is detected in oculomotor muscle cells of the bulbar conjunctiva (BC) (arrow) (C = cornea; I = iris; CB = ciliary body; R = retina). A higher magnification shows βGal blue colour in muscle cells (b), and in some conjunctival epithelial cells (E) (c). In the case of Ad-CMVGFP, numerous conjunctival epithelial cells (arrow) are labelled (d) and, in their vicinity, muscle transduced cells (d, e) show either a weak GFP signal (small asterisk) or a strong GFP signal (large asterisks). Further from the bulbar conjunctiva, oculomotor muscle cells show abundant GFP labelling (asterisks) (f). At the posterior segment level (g, h, i), periocular muscle cells show GFP activities and rare cells with dendritic shape are labelled (arrow); retina and choroid are not transduced nor the retinal pigmented epithelium (arrowhead) (magnification: a: 140; d, f: 400; b, c, e, g: 700; h, i: 870).
Subconjunctival injection of Ad-vIL-10 induces the expression of vIL-10 in aqueous humor and vitreous body
In regard to this local expression of transgene using subconjunctival injection of adenovirus, we then tested adenovirus-mediated vIL-10 expression in the aqueous humor, vitreous body and sera of rats injected intravenously (i.v.) or subconjunctivally. When virus was injected i.v. (3 × 109 pfu), high levels of vIL-10 were detected at day 3 both in aqueous humor and serum (1·5 ng/ml and 35 ng/ml, respectively, n = 1). The kinetics of expression of vIL-10 showed that intravenous administration of 3 × 109 pfu of Ad-vIL-10 resulted in detectable levels of vIL-10 in serum as early as 1 day after administration peaking at day 3–4 (48·4 ± 27·5 ng/ml, n = 8). Then a drop in titre occurred such that by day 9 postinjection, vIL-10 serum level was lower than 1 ng/ml.
When virus was injected by the subconjunctival route in both eyes (109 pfu in 10 µl per eye), an expression of vIL-10 of 0·2–0·4 ng/ml (n = 3) was found in the aqueous humor at day 3. Compared to the intravenous injection, no vIL-10 or very low vIL-10 amount (0–0·075 ng/ml) was detected in the serum of these rats.
In an additional experiment, in which virus was injected in one eye (109pfu in 10 µl), a mean value of 0·25 ng/ml (n = 4) was detected in the vitreous body of the injected eye, with no vIL-10 production in the controlateral non-injected eye. This last point suggests that in vivo vIL-10 inhibitory effect could be restricted to the injected eye, but this needs to be confirmed by further experiments. Again, very low amounts of vIL-10 were found in the serum of these rats (mean: 0·062 ng/ml ± 0·023; n = 4).
Effect of Ad-vIL-10 intravenous and subconjunctival injections on the development of EAU in rats
Consistent with a high level of expression of circulating vIL-10, the intravenous injection of vIL-10 caused significantly inhibition of EAU in treated rats (score: 3·1 ± 0·5, n = 8) (i.e. 43% of protection) compared with controls injected with Ad-vIL-10mut (score: 5·7 ± 0·2, n = 9, p = 0·0001) or saline (score: 5·7 ± 0·1, n = 10, P = 0·0001) (Fig. 6a). Moreover, the protected surface of outer nuclear layer represented 47·7% of the surface of the visual cell layer in Ad-vIL-10-treated rats (n = 3) compared to the complete destruction of this layer in control Ad-vIL-10mut-treated rats (n = 5). However, this protection appeared less important than the one observed in the EAU mouse model, probably as a result of the very strong pathology developed in this rat model.
Fig. 6.
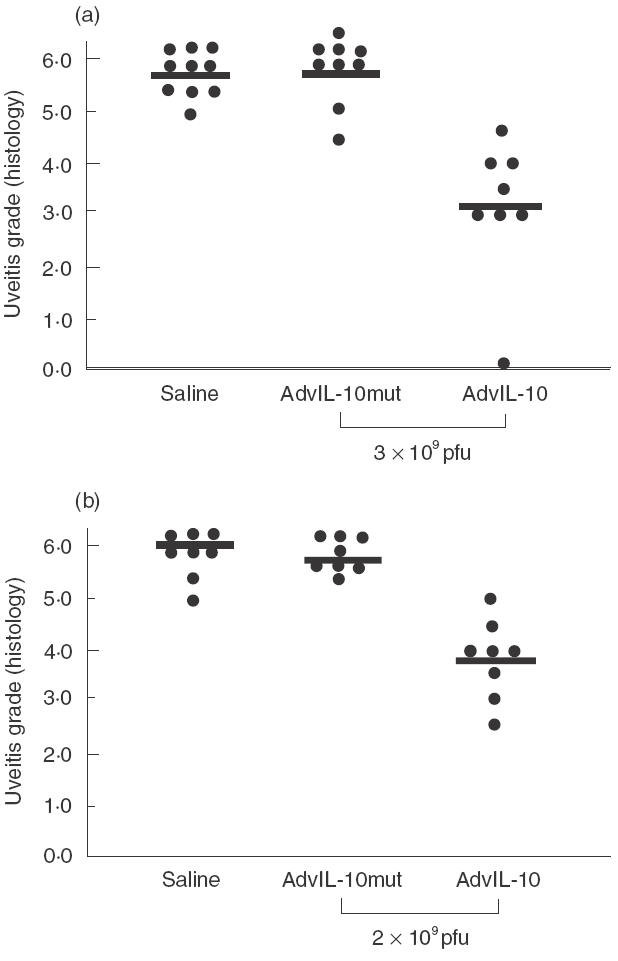
Intravenous injection (a) and subconjunctival injection (b) of Ad-vIL-10 inhibits S-antigen-induced EAU in rats. Lewis rats received 3 × 109 pfu by intravenous injection or 2 × 109 pfu by subconjunctival injection 1 day before immunization with 30 µg of S-Ag in adjuvants in the footpads.The data represents the severity of EAU by histological examination of Ad-vIL-10-injected rats compared to controls injected with the same amount of Ad-vIL-10mut or with saline. Eyes were collected on day 21 after S-Ag-immunization and EAU was scored on a scale of 0–7. Rats were treated on day 1 in four separate experiments as follows: (a), intravenous injection: control groups received one injection of saline (n = 10) or Ad-vIL-10mut (n = 9); rats were treated with one injection of Ad-vIL-10 (n = 8); (b), subconjunctival injection: control groups received one bilateral injection of saline (n = 8) or Ad-vIL-10mut (n = 8); rats were treated with one injection of Ad-vIL-10 (n = 8). Each point is one rat (average of both eyes). The average of each group, is shown by a horizontal bar.
In the case of the subconjunctival route of administration, a significant reduction in the pathological manifestations of EAU was also observed in the group of Ad-vIL-10-treated animals at day 21 after immunization (EAU score: 3·8 ± 1·3, n = 8) (i.e. 26% of protection) compared to controls injected with saline (EAU score: 5·8 ± 1, n = 8; P = 0·0002) or with Ad-vIL-10mut (EAU score 5·1 ± 0·7, n =8; P = 0·01). The protected surface of outer nuclear layer represented 14% of the visual cell layer in Ad-vIL10-treated rats (n = 3) compared to 5% in Ad-vIL10mut-treated rats (n = 3).
We also investigated the effect of the vIL-10 treatment on the immune response to Ag-S either by examination of the rat footpads, site of the immunization or by DTH analysis. Rats that received an intravenous injection of Ad-vIL-10 showed a marked decrease in the footpad granulomatous infiltration (experiment 1: 0·5 ± 0·16, n = 4, experiment 2: 0·4 ± 0·14) compared to controls that received Ad-vIL-10mut (experiment 1: 1·75 ± 0·48, n = 4, experiment 2: 0·9 ± 0·29) or saline (experiment 1: 2 ± 0·31, n = 5). In contrast, rats treated by local subconjunctival injection of Ad-vIL-10 presented an increased footpad reactivity (3 ± 0·003, n = 3) compared to controls injected with Ad-vIL-10mut (1·67 ± 0·33, n = 3) or saline (1·5 ± 0·76, n = 3), indicating that the protective effect was not related to a diminished induction of the immune response at the site of immunization. In addition, a decrease in DTH response was noted in animals injected intravenously with Ad-vIL-10 (0·71 ± 0·05) compared to animals injected with Ad-vIL-10mut (0·91 ± 0·26), confirming a systemic effect of vIL-10. In contrast, no difference was observed between the animals injected subconjunctivally with Ad-vIL-10 compared to those injected with Ad-vIL-10mut (0·87 ± 0·05 versus 0·91 ± 0·26).
DISCUSSION
Numerous works emphasize the role of IL-10 in the down-regulation of ocular inflammation and in the immune privilege of the eye [13]. During EAU, IL-10mRNA is detected in the eyes both at the onset of EAU [49] and at the time of the resolution of the disease [49]. IL-10 seems not to be implicated in the development of the disease since IL-10-deficient mice develop EAU [7]. Moreover, mice with a targeted disruption of the NOS-2 gene develop EAU with a delayed onset and reduced severity, together with high levels of IL-10 mRNA in the spleen [42]. Finally, experiments showing decreased EAU after repeated administration of IL-10 and increased EAU severity after neutralization of IL-10 with anti-IL-10 antibodies led to the conclusion that IL-10 is critical for the down-regulation of uveitis [7]. Interestingly, decreased IL-10 was detected in the anterior chamber of patients with idiopathic uveitis [50]. Moreover, IL-10 does not induce inflammation when injected into the eye [26], allowing its use to be considered in local immunotherapy.
The present study demonstrated that a single vIL-10 expressing adenovirus injection decreased the severity of autoantigen-induced uveitis in mice and rats significantly, and that this protection could be achieved by systemic and subconjunctival treatment. The viral IL-10 was preferred over cellular IL-10 as it exhibits only the immunosuppressive properties of this molecule.
In the uveitis mouse model, a single injection in the retro-orbital sinus venosus of vIL-10-expressing adenovirus, made the day before immunization, allowed to obtain a clear-cut reduction of the degree of ocular inflammation compared to controls with a trend to a dose-dependence of this inhibitory effect. Both Ad-vIL-10 and Ad-vIL-10mut induced an antibody production when injected into mice (data not shown), but this antivirus immune response appeared not to interfere with the development of the disease. Indeed, no difference in disease intensity was observed between the group of Ad-vIL-10mut and the saline-injected mice indicating that the immune response directed against the virus did not interfere with the autoimmune response. Using this type of administration, high systemic levels of vIL-10 were obtained in the sera of animals. These levels peaked at 3–4 days after injection and then dropped to be undetectable at 10 days after viral infection. In the present experiment, the inhibitory effect of Ad-vIL-10 injection was evidenced in the eye that received the Ad-vIL-10 injection and also to a similar extent in the controlateral non-injected eye. The retro-orbital sinus venosus injection in mice is equivalent to an intravenous injection and led to high levels of vIL-10 in the serum. It also led to some expression in the periocular tissues (such as the limbus of the cornea, and periocular muscles but not the retina, the choroid and the retinal pigmented epithelium as demonstrating using GFP reporter gene. Compared to the injected eye, only a low GFP expression in the fellow non-injected eye was detected in the iris vessels and the limbus of the cornea. Interestingly, it has been reported that retrobulbar injection of IFN alpha-2a led to cytokine diffusion in the choroid from the retrobulbar depot with a peak concentration at 2 h, whereas the amount in the serum was <1% of the choroidal concentration [51]. Therefore, we cannot exclude completely a possible diffusion of vIL-10 from the retrobulbar depot. However, the effect of local compared to systemic vIL-10 appeared limited because in all the experiments we never observed a difference in the protection observed between the two eyes. The involvement of vIL-10 in the protection observed was confirmed by the complete neutralization of the inhibitory effect by injection of anti-vIL-10 MoAb. Indeed, mice that received vIL-10 treatment plus anti-vIL-10 antibody showed an equivalent disease to control mice injected with Adv-IL-10mut or saline. This inhibitory effect of administration of vIL-10 on EAU was associated with a depression of the Th1 response of splenic cells (IL-2 and IFN-γ) without affecting the Th2 response (IL-4 and IL-10), and with a decrease of both IgG1 and IgG2a antibody response to IRBP. This is consistent with previous results observed in IL-10-treated mice, indicating that IL-10 can suppress the Th-1 type response without promoting a Th-2 shift in the system [7]. The above data showed that IL-10 reduced uveitis, probably by inhibiting the induction of auto-immunity. However, in an immunotherapy approach, the ideal interven-tion would consist of acting on a yet to be established ocular inflammation. We thus investigated the effect of vIL-10 treatment on ongoing EAU, by injecting the adenovirus 10 days after immunization. Preliminary results indicated a trend to a protection against uveitis, although less than the early treatment (mean EAU score: 0·5 ± 0·6 for Ad-vIL-10 versus 1·4 ± 0·9 for Ad-vIL-10mut, although not significant: P = 0·15, n = 5), associated with a clear reduction of Th1 cytokine production in the cellular supernatant of IRBP-stimulated spleen cells (9·5 ± 0·6 ng/ml versus 58 + 1·2 ng/ml for IFNγ; 168·5 ± 6·7 pg/ml versus 385·7 ± 2·6 pg/ml for IL-2).
A problem arising from our gene therapy approach is the possible induction of a general immunosuppression by IL-10 that could be deleterious in the case of an arising infection. Uveitis is induced in a systemic way but the expression of the pathology is restricted to the eye. To determine whether vIL-10 expressed locally by transfected ocular tissues would be effective in inhibiting EAU, we then developped the more convenient EAU Lewis rat model to perform a subconjunctival injection of the Ad-vIL-10, the day of rat immunization. This approach allowed us to investigate whether a treatment at the site of pathology expression is efficient and could be considered as a protocol of immunointervention in uveitis, which is a systemic disease.
The efficacy of periocular, subconjunctival injection to deliver drugs into the eye has been reported in experimental ocular inflammation [52–54] and in patients [55]. Indeed, subconjunctival injection of vancomycin was found to be more effective than intravenous injection to deliver high amounts of vancomycin in human aqueous humor compared to the systemic route [56]. Subconjunctival administration of triamcinolone was highly efficacious in treating non-necrotizing anterior scleritis [55]. Anterior subconjunctival injection of kerolac tromethamine led to short-lived levels of drug in aqueous and vitreous and inhibited ocular inflammation of short duration induced by intravitreal injection of TNF [52].
Using this route of administration of Ad-vIL-10, we were able to reduce the target photoreceptor cell alterations without affecting the systemic immune response, as shown by the identical inflammatory reaction observed in the footpads, site of the immunization or by the absence of difference in the DTH test to S-Ag compared to controls. The infection by the adenovirus was restricted mainly to periocular muscle cells without any infection of the choroid, the retina or the retinal pigment epithelium of the retina, thus avoiding a possible neuroretina destruction by the immune response developped against adenovirus-infected cells. Moreover, this route of injection led to very low levels of vIL-10 in the serum but induced vIL-10 to diffuse into the eye, as shown by RT-PCR and specific ELISA, suggesting that even if not produced directly by RPE and neuroretinal cells, IL-10 is present at the site of autoimmune tissue destruction and can protect the ocular structures. This is consistent with results showing that rats resistant to develop EAU had higher levels of IL-10 mRNA in the eyes than susceptible rat strains [57]. All these results found in the case of subconjunctival administration of Ad-vIL-10 compared to Ad-vIL-10mut: low amount of serum vIL-10, no modification of the inflammation at the immunization site, no effect on the specific DTH are arguments in favour of a local inhibitory effect on the ocular inflammation not affecting the systemic immune response. Nevertheless, this periocular administration appeared less effective than the systemic intravenous injection of virus. This could be related to the lower levels of vIL-10 found in aqueous humor and vitreous body at day 3 after subconjunctival injection compared to those obtained after intravenous injection. Experiments are now being undertaken to provide a more targeted delivery of this cytokine at the site of the visual cells, i.e. in the vitreous body, in the anterior chamber cavity or in the subretinal space. Visual cells were shown to be permissive to adenoviral infection [58] and reintroduction of these ex vivo-infected cells could also be an alternative to deliver the cytokine directly into the eye without affecting resident cells.
In this work, we have demonstrated that an adenoviral vector strategy is convenient to deliver this cytokine, either systemically or locally, during a period sufficient to protect against uveitis. Moreover, the absence of integration of this virus allows only a transient expression of transgene, thus avoiding a chronic interference of the expressed cytokine with the immune system. Experimental autoimmune uveoretinitis is an experimental model for immune-mediated diseases affecting the posterior segment of the eye in man. Some uveitis are limited to the eye (such as sympathetic ophthalmia and birdshot retinopathy) and others are part of a generalized disease such as sarcoidosis, Behçet's disease and Vogt-Koyonagy Harada [59,60]. Uveitis is a chronic disease with relapses suggesting that a chronic recruitment of antigen-specific cells is operating. The local administration of Ad-vIL-10 with viral expression located exclusively in the eye could be useful as a clinical approach to treatment of autoimmune manifestations occurring into the eye. Nevertheless, for future clinical development, more optimized targetting and more inert vector will be required. The use of anti-inflammatory cytokines and especially IL-10 holds promise as a therapeutic approach in the treatment of autoimmune pathological disorders.
Acknowledgments
This work was supported by grants from INSERM, CNRS and Association Française Retinitis Pigmentosa (AFRP). We wish to express our gratitude to Dr Florence Apparailly for invaluable help during the initiation of this study. We also thank Anne Delanoye for performing lymphokine production assays.
REFERENCES
- 1.Faure JP. Autoimmune disease of the retina. In: Bona CA, Siminovitch KA, Zanetti M, Theofilopoulos AN, editors. The Molecular Pathology of Autoimmune Diseases. Langhorne, PA: Harwood Academic Publications; 1993. pp. 651–72. [Google Scholar]
- 2.Nussenblatt RB, Gery I. Experimental autoimmune uveitis and its relationship to clinical ocular inflammatory diseases. J Autoimmunity. 1996;9:575–85. doi: 10.1006/jaut.1996.0077. [DOI] [PubMed] [Google Scholar]
- 3.Caspi RR, Roberge FG, Chan CC, et al. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–5. [PubMed] [Google Scholar]
- 4.de Kozak Y, Mirshahi M, Boucheix C, Faure JP. Inhibition of experimental autoimmune uveoretinitis in rats by S-antigen specific antibodies. Eur J Immunol. 1985;15:1107–11. doi: 10.1002/eji.1830151108. [DOI] [PubMed] [Google Scholar]
- 5.Caspi RR, Roberge FG, McAllister CC, et al. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in rat. J Immunol. 1986;136:928–33. [PubMed] [Google Scholar]
- 6.Caspi RR, Silver PB, Chan CC, et al. Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J Immunol. 1996;157:2668–75. [PubMed] [Google Scholar]
- 7.Rizzo LV, Xu H, Chan CC, Wiggert B, Caspi RR. IL-10 has a protective role in experimental autoimmune uveoretinitis. Int Immunol. 1998;10:807–14. doi: 10.1093/intimm/10.6.807. [DOI] [PubMed] [Google Scholar]
- 8.Saoudi A, Hurez V, de Kozak Y, et al. Human immunoglobulin preparations for intravenous use prevent experimental autoimmune uveoretinitis. Int Immunol. 1993;5:1559–67. doi: 10.1093/intimm/5.12.1559. [DOI] [PubMed] [Google Scholar]
- 9.Rizzo LV, Morawetz RA, Miller-Rivero NE, et al. IL-4 and IL-10 are both required for the induction of oral tolerance. J Immunol. 1999;162:2613–22. [PubMed] [Google Scholar]
- 10.Laliotou B, Duncan L, Dick AD. Intranasal administration of retinal antigens induces transient T cell activation within drainage lymph nodes but not spleen. J Autoimmunity. 1999;12:145–55. doi: 10.1006/jaut.1998.0269. [DOI] [PubMed] [Google Scholar]
- 11.Hara Y, Caspi RR, Wiggert B, Chan CC, Wilbanks GA, Streilein JW. Suppression of experimental autoimmune uveitis in mice by induction of anterior chamber-associated immune deviation with interphotoreceptor retinoid-binding protein. J Immunol. 1992;15:1685–92. [PubMed] [Google Scholar]
- 12.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA. Fas Ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–92. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 13.Ferguson TA. The molecular basis of anterior associated immune deviation (ACAID) Ocul Immunol Inflamm. 1997;5:213–5. doi: 10.3109/09273949709116897. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentino DF, Bond MW, Mossman TM. Two types of mouse helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein–Barr virus gene BCRF1. Science. 1990;248:1230–4. doi: 10.1126/science.2161559. [DOI] [PubMed] [Google Scholar]
- 16.O'Garra A, Stapleton G, Dhar V, et al. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2:821–32. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- 17.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes. an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):120–9. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vieira P, de Waal-malefyt R, Dang MN, et al. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein–Barr virus open reading frame BCRF1. Proc Natl Acad Sci USA. 1991;88:1172–6. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Go NF, Castle BE, Barrett R, et al. Interleukin-10, a novel B cell stimulatory factor. unresponsiveness of X chromosome-linked im-munodeficiency B cells. J Exp Med. 1990;172:1625–31. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen WF, Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991;14:528–34. [PubMed] [Google Scholar]
- 21.Hsu DH, de Waal Malefyt R, Fiorentino DF, et al. Expression of interleukin-10 activity by Epstein–Barr virus protein BCRF1. Science. 1990;250:830–2. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 22.Thompson-Snipes L, Dhar V, Bond MW, Mossman TR, Moore KW, Rennick DM. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173:507–10. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jinquan T, Larsen CG, Gesser B, Matsushima K, Thestrup-Pedersen K. Human IL-10 is a chemoattractant for CD8+ T lymphocytes and an inhibitor of IL-8-induced CD4+ T lymphocyte migration. J Immunol. 1993;151:4545–51. [PubMed] [Google Scholar]
- 24.MacNeil IA, Suda T, Moore KW, Mossman TR, Zlotnik A. IL-10, a novel growth cofactor for mature and immature T cells. J Immunol. 1990;145:4167–73. [PubMed] [Google Scholar]
- 25.Hayashi S, Guez-Crosier Y, Delavaux T, Velu T, Roberge FR. Interleukin 10 inhibits inflammatory cells infiltration in endotoxin-induced uveitis. Graefe's Arch Clin Exp Ophthalmol. 1996;234:633–6. doi: 10.1007/BF00185297. [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum JT, Angell E. Paradoxical effects of IL-10 in endotoxin-induced uveitis. J Immunol. 1995;155:4090–4. [PubMed] [Google Scholar]
- 27.Qin L, Chavin KD, Ding Y, et al. Retrovirus-mediated transfer of viral IL-10 gene prolongs murine cardiac allograph survival. J Immunol. 1996;156:2316–23. [PubMed] [Google Scholar]
- 28.David A, Chetritt J, Guillot C, et al. Interleukin-10 produced by recombinant adenovirus prolongs survival of cardiac allografts in rats. Gene Ther. 2000;7:505–10. doi: 10.1038/sj.gt.3301114. [DOI] [PubMed] [Google Scholar]
- 29.Mathisen P, Yu M, Johnson J, Drazba J, Tuohy V. Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells. J Exp Med. 1997;186:159–64. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apparailly F, Verwaerde C, Jacquet C, Auriault C, Sany J, Jorgensen C. Adenovirus-mediated transfer of viral IL-10 gene inhibits murine collagen-induced arthritis. J Immunol. 1998;160:5213–20. [PubMed] [Google Scholar]
- 31.Ma Y, Thornton S, Duwel L, et al. Inhibition of collagen-induced arthritis in mice by viral IL-10 gene transfer. J Immunol. 1998;161:1516–24. [PubMed] [Google Scholar]
- 32.Pennline KJ, Roque-Gaffney E, Monahan M. Recombinant human IL-10 prevents the onset of diabetes in the nonobese diabetic mouse. Clin Immunol Immunopathol. 1994;71:169–75. doi: 10.1006/clin.1994.1068. [DOI] [PubMed] [Google Scholar]
- 33.Lechman E, Jaffurs D, Ghivizzani S, et al. Direct adenoviral gene transfer of viral IL-10 to rabbit knee with experimental arthritis ameliorates disease in both injected and controlateral knees. J Immunol. 1999;163:2202–8. [PubMed] [Google Scholar]
- 34.Whalen JD, Lechman EL, Carlos CA, et al. Adenoviral transfer of the viral IL-10 gene periarticularly to mouse paws suppresses development of collagen-induced arthritis in both injected and uninjected paws. J Immunol. 1999;162:3625–32. [PubMed] [Google Scholar]
- 35.Quattrocchi E, Dallman MJ, Dhillon AP, Quaglia A, Bagnato G, Feldmann M. Murine IL-10 gene transfer inhibits established collagen-induced arthritis and reduces adenovirus-mediated inflammatory responses in mouse liver. J Immunol. 2001;166:5970–8. doi: 10.4049/jimmunol.166.10.5970. [DOI] [PubMed] [Google Scholar]
- 36.Mashhour B, Couton D, Perricaudet M, Briand P. In vivo adenovirus-mediated gene transfer into ocular tissues. Gene Ther. 1994;1:122–6. [PubMed] [Google Scholar]
- 37.Hoffman LM, Maguire AM, Bennett J. Cell-mediated immune response and stability of intraocular transgene expression after adenovirus-mediated delivery. Invest Ophthalmol Vis Sci. 1997;38:2224–33. [PubMed] [Google Scholar]
- 38.Di Polo A, Aigner LJ, Dunn RJ, Bray GM, Aguayo AJ. Prolonged delivery of brain-derived neurotrophic factor by adenovirus-infected Muller cells temporarily rescues injured retinal ganglion cells. Proc Natl Acad Sci U S A. 1998;95:3978–83. doi: 10.1073/pnas.95.7.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akimoto M, Miyatake S, Kogishi J, et al. Adenovirally expressed basic fibroblast growth factor rescues photoreceptor cells in RCS rats. Invest Ophthalmol Vis Sci. 1999;40:273–9. [PubMed] [Google Scholar]
- 40.Weise J, Isenmann S, Klocker N, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–23. doi: 10.1006/nbdi.2000.0285. [DOI] [PubMed] [Google Scholar]
- 41.Pepperberg D, Okajima T, Ripps H, Chader GJ, Wiggert B. Functional properties ofinterphotoreceptor retinoid-binding protein. Photochem Photobiol. 1991;54:1057–60. doi: 10.1111/j.1751-1097.1991.tb02129.x. [DOI] [PubMed] [Google Scholar]
- 42.Thillaye-Goldenberg B, Goureau O, Naud MC, de Kozak Y. Delayed onset and decreased severity of experimental autoimmune uveoretinitis in mice lacking nitric oxide synthase 2. J Neuroimmunol. 2000;110:31–44. doi: 10.1016/s0165-5728(00)00313-1. [DOI] [PubMed] [Google Scholar]
- 43.Verwaerde C, Thiam K, Delanoye A, Fernandez-Gomez R, D’Halluin JC, Auriault C. Systemic delivery of an adenovirus expressing EBV-derived IL-10 in mice infected with Schistosoma mansoni or Leishmania amazonensis: controversial effects on the development of pathological parameters. Eur Cytokine Netw. 1999;10:161–6. [PubMed] [Google Scholar]
- 44.Graham FL, Prevec L. Adenovirus-based expression vectors and recombinant vaccines. Biotechnology. 1992;20:363–90. doi: 10.1016/b978-0-7506-9265-6.50022-1. Review. [DOI] [PubMed] [Google Scholar]
- 45.Rolling F, Shen WY, Tabarias H, Constable I, Kanagasingam C, Rakoczy PE. Evaluation of adeno-associated virus-mediated gene transfer into the rat retina by clinical fluorescence photography. Human Gene Ther. 1999;10:641–8. doi: 10.1089/10430349950018715. [DOI] [PubMed] [Google Scholar]
- 46.Pouvreau I, Zech JC, Thillaye-Goldenberg B, Naud MC, Van Rooijen N, de Kozak Y. Effect of macrophage depletion by liposomes containing dichloromethylene diphosphonate on endotoxin-induced uveitis. J Neuroimm. 1998;86:171–81. doi: 10.1016/s0165-5728(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 47.Caspi RR, Chan CC, Leake W, Higushi M, Wiggert B, Chader GJ. Experimental autoimmune uveoretinitis in mice. Induction by simple elicitating event and dependence on quantitative parameters of immunization. J Autoimm. 1990;3:237–46. doi: 10.1016/0896-8411(90)90143-g. [DOI] [PubMed] [Google Scholar]
- 48.Ramanathan S, de Kozak Y, Saoudi A, et al. Recombinant IL-4 aggravates experimental autoimmune uveoretinitis in rats. J Immunol. 1996;157:2209–15. [PubMed] [Google Scholar]
- 49.Okada A, Sakai J, Usui M, Mizukichi J. Intraocular cytokine quantification of experimental autoimmune uveoretinitis in rats. Ocul Immunol Inflamm. 1998;6:111–20. doi: 10.1076/ocii.6.2.111.4046. [DOI] [PubMed] [Google Scholar]
- 50.Calder VL, Shaer B, Muhaya M, et al. Increased CD4+ expression and decreased IL-10 in the anterior chamber in idiopathic uveitis. Invest Ophthalmol Vis Sci. 1999;40:2019–24. [PubMed] [Google Scholar]
- 51.Lincoff H, Stanga P, Movshovich A, et al. Choroidal concentration of interferon after retrobulbar injection. Invest Ophthalmol Vis Sci. 1996;37:2768–71. [PubMed] [Google Scholar]
- 52.Rabiah PK, Fiscella RG, Tessler HH. Intraocular penetration of periocular ketorolac and efficacy in experimental uveitis. Invest Ophthalmol Vis Sci. 1996;37:613–8. [PubMed] [Google Scholar]
- 53.Rootman J, Bussanich N, Gudauskas G, Kumi C. Effects of subconjunctivally injected antineoplastic agents on three models of corneal inflammation. Can J Ophthalmol. 1985;20:142–6. [PubMed] [Google Scholar]
- 54.de Kozak Y, Verwaerde C. Cytokines in immunotherapy of experimental uveitis. Intern Rev Immunol. 2002;21:1–23. doi: 10.1080/08830180212060. [DOI] [PubMed] [Google Scholar]
- 55.Tu EY, Culbertson WW, Pflugfelder SC, Huang A, Chodosh JC. Therapy of non-necrotizing anterior scleritis with subconjunctival corticosteroid injection. Ophthalmology. 1995;102:718–24. doi: 10.1016/s0161-6420(95)30963-3. [DOI] [PubMed] [Google Scholar]
- 56.Souli M, Kopsinis G, Kavouklis E, Gabriel L, Giamarellou H. Vancomycin levels in human aqueous humour after intravenous and subconjunctival administration. Int J Antimicrob Agents. 2001;18:239–43. doi: 10.1016/s0924-8579(01)00375-2. [DOI] [PubMed] [Google Scholar]
- 57.Sun B, Sun SH, Chan CC, Caspi RR. Evaluation of in vivo cytokine expression in EAU-susceptible and resistant rats: a role for IL-10 in resistance. Exp Eye Res. 2000;70:493–502. doi: 10.1006/exer.1999.0808. [DOI] [PubMed] [Google Scholar]
- 58.de Kozak Y, Thillaye-Goldenberg B, Naud MC, Auriault C, Verwaerde C. In vivo transfer of vIL-10 by injection into rat vitreous of rat retinal Müller glial cells infected with recombinant adenovirus. Invest Ophthalmol Vis Sci. 2001;42:S914. [Google Scholar]
- 59.Nussenblatt RB, Palestine AG. Uveitis: fundamentals and clinical practice. Chicago: Yearbook Medical Publishers; 1989. [Google Scholar]
- 60.Dick AD. Immune mechanisms of uveitis: insights into disease pathogenesis and. Treatment Int Ophthalmol Clin. 2000;40:1–18. doi: 10.1097/00004397-200004000-00003. [DOI] [PubMed] [Google Scholar]


