Abstract
We studied the in vitro effects of butyric acid on differentiation, maturation and function of dendritic cells (DC) and macrophages (MΦ) generated from human monocytes. A non-toxic dose of butyrate was shown to alter the phenotypic differentiation process of DC as assessed by a persistence of CD14, and a decreased CD54, CD86 and HLA class II expression. The more immature differentiation stage of treated cells was confirmed further by their increased phagocytic capability, their altered capacity to produce IL-10 and IL-12, and their weak allostimulatory abilities. Butyrate also altered DC terminal maturation, regardless of the maturation inducer, as demonstrated by a strong down-regulation of CD83, a decreased expression of CD40, CD86 and HLA class II. Similarly, butyrate altered MΦ differentiation, down-regulating the expression of the restricted membrane antigens and reducing the phagocytic capacity of treated cells. To investigate further the mechanism by which butyrate hampers the monocyte dual differentiation pathway, we studied the effects of 1,25(OH)2D3 alone or in combination with butyrate on the phenotypic features of DC. Unlike 1,25(OH)2D3, butyrate inhibited DC differentiation without redirecting it towards MΦ. Combined treatment gave rise to a new cell subset (CD14high, CD86 and HLA-DRlow) phenotypically distinct from monocytes. These results reveal an alternative mechanism of inhibition of DC and MΦ differentiation. Altogether, our data demonstrate a novel immune suppression property of butyrate that may modulate both inflammatory and immune responses and support further the interest for butyrate and its derivatives as new immunotherapeutic agents.
Keywords: butyrate, dendritic cells, immunosuppression, macrophages, monocytes
INTRODUCTION
A novel form of immune intervention mediated by the G1 blocker butyric acid has been proposed recently for the tolerance induction to allografts [1,2] and the treatment of autoimmune diseases [3]. Butyrate and other short-chain fatty acids are end-products of fibre polysaccharide metabolism in the intestine and affect key functions of the colonic epithelium in vivo [4]. Butyrate has been studied primarily as an antineoplastic agent able to inhibit proliferation and to induce differentiation in a variety of transformed cell types in vitro (for a review, see [5]). In addition to its effects on tumour cells, butyrate has also been shown to possess anti-inflammatory properties [6–8] and to inhibit T cell activation in response to mitogens [9,10], alloantigens [11] and soluble antigens [12]. Modulation of proliferative T cell responses was reported to result from both direct interference with cell cycle progression [13] and alteration of the stimulatory function of antigen presenting cells (APC) [14]. Based on the observation that pretreatment of monocytes with butyrate suppresses subsequent T cell proliferation responses and down-regulates expression of B7-1 (CD80), ICAM-1 (CD54) and LFA-3 (CD58) on monocytes, it has been speculated that altered APC function by butyrate was a consequence of their inability to deliver critical costimulatory signals [14]. However, these effects were observed only with high doses of butyrate which resulted in alteration of membrane integrity owing to apoptotic cell death [14] and, consequently, cell toxicity may represent the critical mechanism underlying altered APC function in the monocyte model.
Important clues to understand the anti-APC action of butyrate might come from the analysis of the cytodifferentiation capabilities of this agent on monocyte-derived cells. Indeed, peripheral blood monocytes can differentiate in vivo into both macrophages (MΦ) and dendritic cells (DC), depending upon their cytokine environment [15]. This indicates a dichotomy of monocyte differentiation either into antigen-presenting DC leading to specific immunity or into phagocytosing MΦ, thus connecting non-specific and specific immune responses [16]. Over the past years, methods have been described to differentiate MΦ [17,18] and DC [19,20] from blood monocytes by in vitro culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) or GM-CSF plus IL-4, respectively. In addition cultured DC, which show functional and phenotypic characteristics typical of the immature stage of differentiation, can be differentiated further in vitro into mature DC in the presence of tumour necrosis factor-alpha (TNF-α) and prostaglandin E2 (PGE2) [21]. PGE2 is a mediator found in inflamed tissues and secreted by tumours (reviewed in [22]). Therefore, using such an in vitro cultured cell model, the objective of the present study was to investigate whether butyrate affects differentiation, maturation and function of monocyte-derived MΦ and DC, thus contributing to the anti-inflammatory and immunosuppressive properties of this molecule.
MATERIALS AND METHODS
Cytokines and reagents
All culture media, chemicals and reagents were purchased from Gibco-BRL (Cergy-Pontoise, France) unless specified otherwise. Recombinant human GM-CSF was obtained from Schering Plough (Levallois Perret, France), recombinant human IL-1 beta (IL-1β) and IL-4, TNF-α, γ-interferon (IFN-γ) from R&D Systems (Abingdon, UK). PGE2, indomethacin, LPS, butyrate and 1,25(OH)2D3 were obtained from Sigma (L’Isle d’Abeau Chesnes, France), Staphylococcus aureus cowan 1 strain (SAC) from Calbiochem (Meudon, France) and human pooled AB serum from Valbiotech (Paris, France).
Cell separation
After giving informed consent, healthy blood donors underwent mononuclear cell leukapheresis using a continuous-flow cell separator (Spectra, COBE laboratory, Lakewood, CO, USA) and ACD-A anticoagulant (Maco Pharma, Tourcoing, France). Purification of both monocytes and lymphocytes was performed by countercurrent centrifugal elutriation using the J6 MC centrifuge equipped with the JE 5·0 rotor (Beckman, Roissy, France). Briefly, the rotor was sterilized by autoclaving (20 min at 120°C) and primed by a solution supplemented with 1% human albumin (Bruneau, Boulogne-Billancourt, France). The initial cell loading flow rate was set to 40 ml/min (initial rotor speed, 2000 r.p.m. at 4°C). Elutriation was performed by stepwise increments of the flow rate. The size and concentration of elutriated cells were closely monitored by flow cytometry using an EPICS XL flow-cytometer (Coulter, Villepinte, France). At a flow rate of 45–55 ml/min, lymphocyte-rich fraction was collected. The flow was slowed progressively to 30 ml/min to reduce the dilution of the elutriated sample. Monocyte-rich fraction was elutriated by progressively decreasing the rotor speed to 1400 r.p.m. and collected on ice. Cell viability assessed by the trypan blue exclusion test was always >98% for both lymphocytes and monocytes. T lymphocyte and monocyte purity as determined by CD2 and CD14 expression, respectively, was always >90%.
Human DC and MΦ cultures
DC and MΦ were generated from purified monocytes in Teflon bags under adherence-free conditions as described previously [23,24]. Briefly, MΦ were differentiated in IMDM supplemented with 2 mm l-glutamine, 1% non-essential amino acids, 100 mm sodium pyruvate, 3 × 10−5 m 2-mercaptoethanol, 5% human pooled AB serum, 5 × 10−6m indomethacin and 250 U/ml GM-CSF, in the presence or absence of butyrate. Similarly, immature DC were differentiated from monocytes cultured in RPMI-1640 supplemented with 2 mm l-glutamine, 5% human pooled AB serum, 400 U/ml GM-CSF and 500 U/ml IL-4, in the presence or absence of butyrate. In some experiments various concentrations of 1,25(OH)2D3 ranging from 10−10 m to 10−7 m were added concomitantly to butyrate.
DC maturation was induced by adding 1000 U/ml TNF-α and 3 µg/ml PGE2, on 5-day cultured DC, in the presence or absence of butyrate. Cells were recovered after 48 h of culture. Some experiments were performed using 1000 U/ml TNF-α plus 15 µg/ml IL-1β, or 1 µg/ml LPS as maturation inducers.
Cell immunophenotyping
Cell staining was performed using the following mouse monoclonal antibodies, all from Immunotech (Marseille, France) except when indicated: anti-CD14 PE (Dako, Trappes, France), anti-CD16 FITC, anti-CD40 PE, anti-CD54 PE, anti-CD80 FITC, anti-CD83 PE, anti-CD86 PE (Becton Dickinson, Pont-de-Claix, France), anti-HLA-DR FITC (Becton Dickinson, Pont-de-Claix, France) and anti-HLA-DQ FITC.
IL-10 and IL-12 production
Activated DC were assessed for their cytokine production according to the method described by Hilkens et al. [25]. Briefly, 1 × 106 cells were stimulated in 12-well flat-bottom culture plates in serum-free AIM-V medium containing 1% pyruvate in a final volume of 1 ml. SAC strain (75 µg/ml) or SAC strain (75 µg/ml) plus 1000 UI/ml IFN-γ were used to induce either IL-10 or IL-12 secretion. After 24 h, supernatants were collected and cytokine production was determined by enzyme-linked immunosorbent assay (ELISA) using commercial kits purchased from R&D Systems (Abingdon, UK) according to the manufacturer's instructions. The detection limit of this ELISA was <0·5 pg/ml for IL-10 and IL-12.
Antigen capture
Single cell phagocytosis quantification was assessed according to the method adapted from Dunn and Tyrer [26]. A suspension of 2 µm FITC-dextran microbeads (Polysciences, Warrington, PA, USA) was added in Teflon bags. After a 4-h incubation period at 37°C, cells were recovered and washed extensively with cold Dulbecco's PBS to stop phagocytosis. Then, cells were resuspended in PBS at a final concentration of 5 × 105 cells/ml and analysed by flow cytometry. In this assay, cell fluorescence intensity depends on the number of beads that have been phagocytized. Therefore, when the number of cells is analysed as a function of the fluorescence intensity, each peak depicted clusters of cells that have phagocytized 1, 2, 3 or more microbeads.
Allogeneic mixed leucocyte reaction (MLR)
The stimulation of purified T lymphocytes by immature or mature DC cultured in the presence or absence of butyrate was evaluated in the MLR assay as described elsewhere [27]. Briefly, stimulator cells were added in graded doses to 2 × 105 allogeneic responder T cells in 96-well flat-bottomed tissue culture plates (0·2 ml of medium per well). Cell proliferation during the last 18 h was quantified by incubation of cells with 1 µCi (37 kBq) of [3H]-thymidine (Amersham, Les Ulis, France). The cells were harvested onto filters and radioactivity was measured in a scintillation counter.
Statistical analysis
Statistical analysis was performed using StatView IV software (Abacus Concepts INC, Berkeley, CA, USA). Results are expressed as mean ± s.e.m. Comparisons were performed using analysis of variance followed by Fisher's exact test when appropriate. A P-value ≤0·05 was considered statistically significant.
RESULTS
Phenotypic characterization of monocyte-derived cells used in this study
Flow cytometric analyses were performed to confirm phenotypic differences between monocytes, DC and MΦ in our culture system. DC and MΦ were generated from monocytes cultured for 7 days in the presence of either GM-CSF plus IL-4 or GM-CSF alone, respectively, according to the method described previously [24]. As shown in Fig. 1a, the DC population expressed the typical phenotype of immature DC [19,20,24], with low CD80, CD86 and no CD83, whereas MΦ grown in parallel showed a different pattern of surface marker expression, as assessed by increased CD14 and CD16 expression. We next investigated the phenotypic changes in immature DC exposed to the cocktail TNF-α plus PGE2 during the last 2 days of their culture. Cultured cells exhibited a phenotype commonly referred to as mature DC [21], characterized by increased CD83, CD86 and HLA class II molecule expression (Fig. 1b).
Fig. 1.
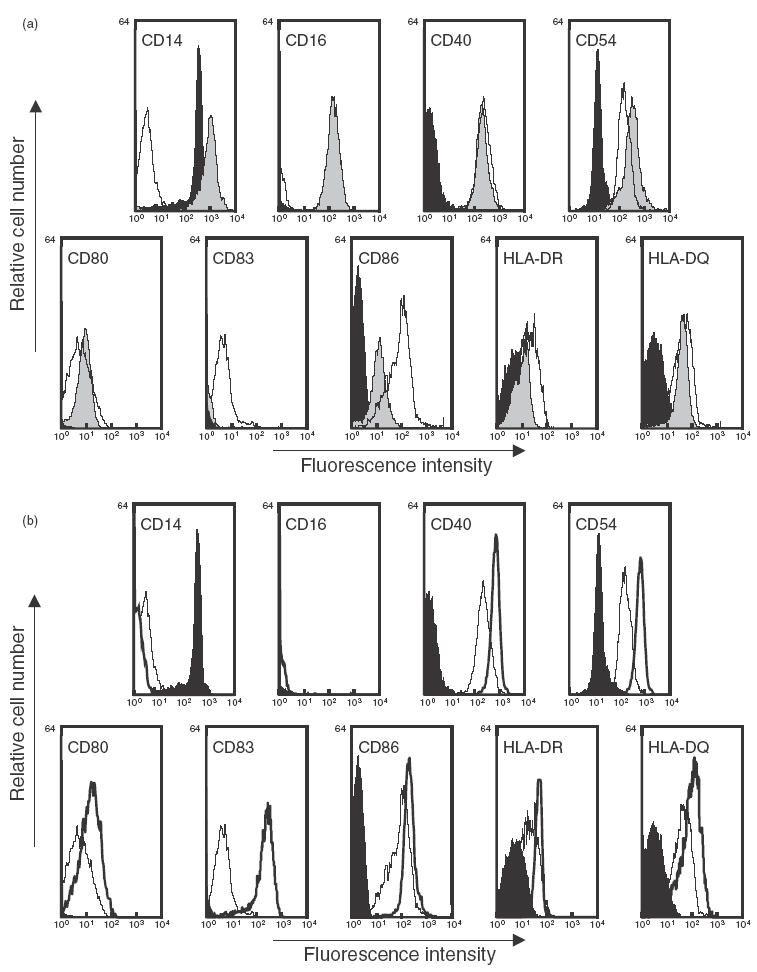
Phenotypic characterization of the monocyte-derived cells generated in the absence of butyrate. Immature DC and MΦ were generated from monocytes cultured for 7 days in the presence of GM-CSF plus IL-4 or GM-CSF alone, respectively. Mature DC were derived from 5-day immature DC cultured for additional 2 days with TNF-α plus PGE2. On day 7, cells were harvested and analysed using flow cytometry. (a) Immature DC (white profile) and MΦ (grey profile) were compared to freshly isolated monocytes (black profile) from the same individual. (b) Immature (white profile, thin line) and mature DC (white profile, bold line) were compared to monocytes (black profile). Data shown are representative of three separate experiments performed with three different donors.
Butyrate alters the phenotypic differentiation of monocyte-derived immature DC and MΦ
To examine the toxicity of butyrate in our culture system, dose-response curves, ranging from 0·1 to 10 mm, were carried out based on cell recovery and viability. As calculated from five independent experiments, induction of cell death was dose-dependent with a decrease in DC yields from 94 ± 4% (mean percentage ± s.e.m.) in the presence of 0·5 mm butyrate to 52 ± 2% in the presence of 10 mm butyrate. In the same way, MΦ yields decreased from 91 ± 5% to 47 ± 6% in the presence of 0·5 mm and 10 mm butyrate, respectively. Therefore, a standard butyrate concentration of 0·5 mm was chosen for subsequent experiments.
Phenotypic characteristics of immature DC and MΦ cultured in the presence or absence of butyrate were analysed using flow cytometry and compared to freshly isolated monocytes from the same individual (Fig. 2). Untreated DC down-regulated CD14, neo-expressed CD40, CD54, CD80, CD86 and up-regulated the expression of MHC class II products HLA-DR and HLA-DQ. As shown in Fig. 2a, treatment by butyrate partially inhibited this phenotypic differentiation process and resulted in a maintained CD14 expression and in a limited increase in CD40, CD54, CD86 and MHC II molecule expression. Similarly, the main phenotypic features acquired by MΦ during culture (i.e. up-regulated CD14, CD16, CD54, CD86 and MHC II molecules) were restrained by butyrate (Fig. 2b).
Fig. 2.
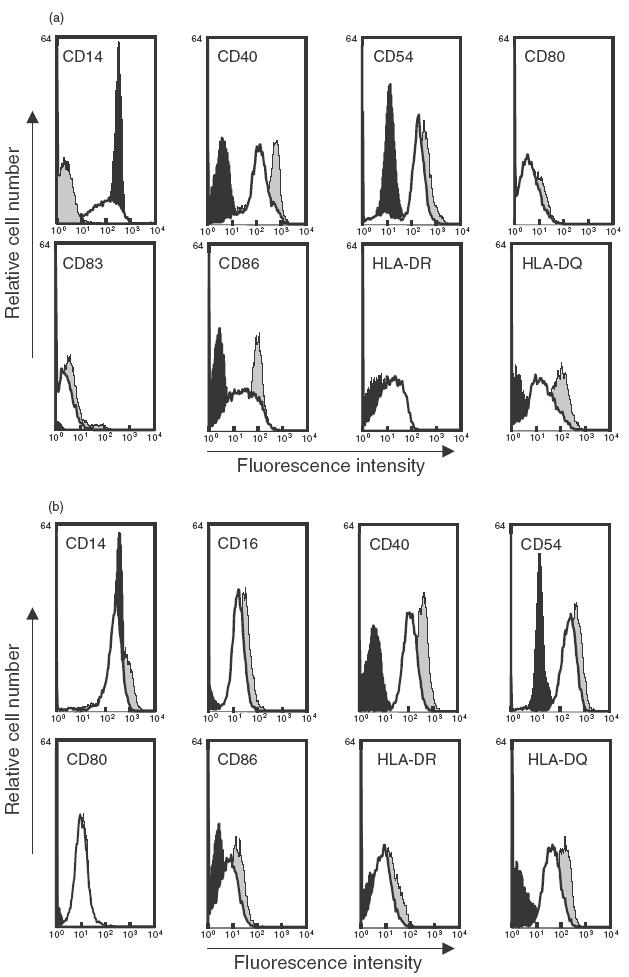
Effect of butyrate on the phenotypic features of 7-day cultured DC and MΦ differentiation. Immature DC and MΦ were generated from monocytes cultured in the presence of GM-CSF plus IL-4 or GM-CSF alone, in the absence or in the presence of 0·5 mm butyrate. On day 7, cultured cells were harvested and analysed using flow cytometry. Immature DC (a) and MΦ (b) differentiated in the absence (grey profile) or in the presence (white profile) of 0·5 mm butyrate were compared to freshly isolated monocytes from the same individual (black profile). Data shown are representative of three separate experiments performed with three different donors.
Butyrate alters the terminal maturation of DC
Upon activation by various stimuli, DC undergo terminal maturation associated with the modulation of relevant surface antigens [28]. To study the effect of butyrate on this maturation process, immature DC obtained after 5 days of standard culture were incubated for 2 additional days with TNF-α plus PGE2 in the presence or absence of butyrate, and membrane phenotypes of mature cells were compared. As depicted in Fig. 3, butyrate strongly interfered with the DC maturation process by inhibiting the expression of the CD83 maturation marker and by reducing the cytokine-driven up-regulation of CD40, CD54, CD86 and MHC II molecules. Similar inhibition was observed when DC were matured using LPS or TNF-α plus IL-1β (Table 1).
Fig. 3.
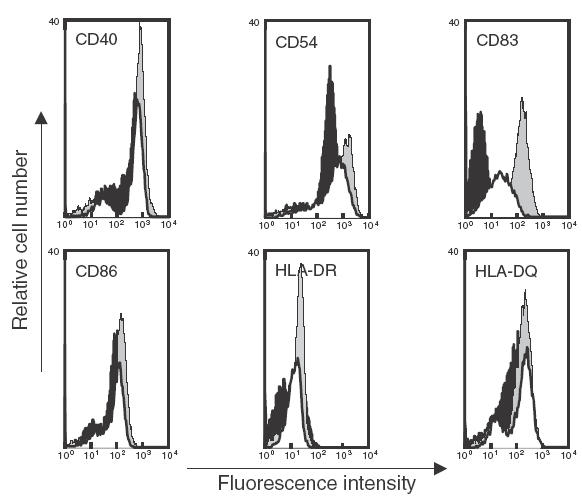
Effect of butyrate on the phenotypic changes induced during DC maturation. Day 5 immature DC were cultured for 2 additional days with TNF-α plus PGE2 in the absence (grey profile) or in the presence (white profile) of 0·5 mm butyrate. On day 7, cells were analysed by flow cytometry and compared to day 7 immature DC from the same individual (black profile). Data shown are representative of three separate experiments performed with three different donors.
Table 1.
Effect of butyrate on the terminal maturation of DC. Day 5 DC were matured with TNF-α plus PGE2 or TNF-α plus IL-1β or LPS in the absence or in the presence of 0·5 mm butyrate. On day 7, cells were harvested and analysed by flow cytometry. Data represent the inhibitory effect of butyrate on CD83, CD86 and HLA-DR and are mean ± s.e.m. inhibition percentage of three separate experiments performed with three different donors
| Maturation inducers | |||
|---|---|---|---|
| Membrane markers | TNF-α plus PGE2 | TNF-α plus IL-1β | LPS |
| CD83 | 49·4 ± 1·2% | 32·6 ± 0·9% | 54·4 ± 2·4% |
| CD86 | 26·3 ± 1·9% | 35·4 ± 1·8% | 32·3 ± 1·5% |
| HLA-DR | 50·2 ± 2·3% | 45·9 ± 3·1% | 48·6 ± 2·1% |
Butyrate modulates cytokine production by DC
We further investigated the capacity of butyrate to interfere with DC cytokine production. Compared to monocytes, control DC differentiated in the absence of butyrate exhibited a 20-fold decreased IL-10 secretion. In contrast, the presence of butyrate prevented this decrease partially and led to DC that exhibited an intermediate capacity to produce IL-10 (Fig. 4a). Kinetics of IL-12 secretion by DC cultured in the absence of butyrate (Fig. 4b) was characterized by a 10-fold increase on day 2 when compared to monocytes, followed by a drastic decrease. DC differentiated for 7 days in the presence of butyrate exhibited a preserved IL-12 secretion capacity similar to that observed on day 3 with untreated cultured cells (Fig. 4b). Both IL-10 and IL-12 data are consistent with the phenotypic and functional changes described above and confirm that butyrate hampers monocyte differentiation into DC.
Fig. 4.
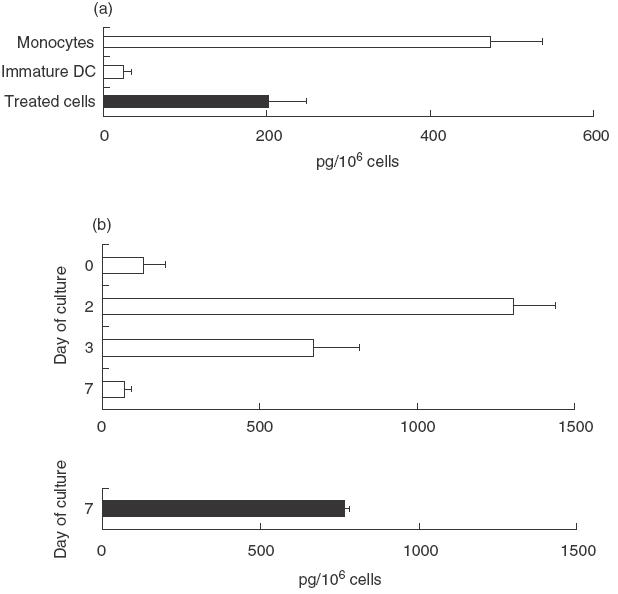
Effect of butyrate on DC cytokine release. Immature DC were generated from monocytes in the absence or in the presence of 0·5 mm butyrate and assessed for their cytokine production. (a) IL-10 production by cells activated for 24 h with SAC strain. Results are expressed as mean pg/106 cells ± s.e.m. of six separate experiments performed with six different donors. (b) IL-12 production by cultured DC harvested at days 2, 3 or 7 and activated subsequently for 24 h with SAC strain plus IFN-γ. Results are expressed as mean pg/106 cells ± s.e.m. of six separate experiments performed with six different donors.
Butyrate modulates antigen capture ability of immature DC and MΦ
Phagocytic capacity of DC and MΦ grown in the presence or absence of butyrate were analysed and compared to fresh monocytes. In a typical experiment shown in Fig. 5, 51% of immature DC are phagocytic (i.e. engulfed at least one microsphere) compared to 72% of monocytes. When focusing on cells expressing a high phagocytic capacity (more than 3 microspheres engulfed), the proportion decreased to 32% of DC versus 59% of monocytes. Altogether, these data suggest that monocyte differentiation into DC resulted in down-regulated phagocytic activity. The presence of butyrate inhibited this process totally, because 74% and 60% of treated cells engulfed at least 1 and more than 3 microspheres, respectively (Fig. 5b). Conversely, phagocytosis activity of monocytes was up-regulated upon differentiation into MΦ, with 80% and 67% of MΦ which engulfed at least 1 and more than 3 microspheres, respectively. Butyrate also inhibited monocyte differentiation into MΦ, because 64% of treated cells engulfed at least 1 and 37% more than 3 microspheres, respectively (Fig. 5c).
Fig. 5.
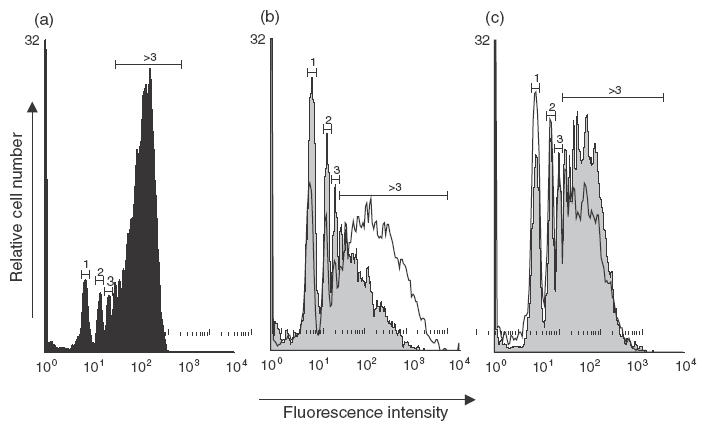
Effect of butyrate on the phagocytic activity of immature DC and MΦ. Immature DC and MΦ were generated from monocytes cultured with GM-CSF plus IL-4 or GM-CSF alone, in the absence or in the presence of 0·5 mm butyrate. On day 7, the antigen capture capacity of immature DC (b) and MΦ (c) was evaluated using flow cytometry to assess single cell fluorescent microsphere engulfment and compared to data obtained with freshly isolated monocytes (a). The histograms of FITC-latex bead capture show a series of fluorescence peaks corresponding to clusters of cells that phagocytized 1, 2, 3 … beads. Numbers above peaks indicate the number of ingested particles per cell. (a) Fresh monocytes (black profile) showed a high capture capacity assessed by a majority of cells that phagocytized more than three beads. (b) This phagocytosis activity is down-regulated upon differentiation into immature DC (grey profile) as demonstrated by the decreased number of cells that engulfed more than three beads. The presence of butyrate inhibits this phenomenon and leads to DC (white profile) with intermediate capture capacity. Conversely, phagocytosis activity of monocytes is up-regulated upon differentiation into MΦ (grey profile) as shown by the shift of the peak of cells engulfing a high number of beads. Butyrate partially inhibited this phenomenon (white profile). Data shown are representative of three separate experiments performed with three different donors.
Butyrate down-regulates T cell stimulatory activity of DC
The ability to prime T cells constitutes a unique and critical DC function that is related to the persistent expression of high levels of membrane MHC products and co-stimulatory molecules [28]. Therefore, as butyrate was found to down-modulate the expression of these antigens, we examined its effect on the ability of immature and mature DC to induce the MLR, a model for graft rejection in which DC are the most potent cells for priming naive T cells to alloantigens [27]. The study was performed using various APC/T cell ratios ranging from 1/10 to 1/1000 (Fig. 6). As expected, mature DC and to a lesser extent immature DC showed a strong capacity to activate T lymphocyte proliferation. In both cases, DC grown in the presence of butyrate exhibited reduced allostimulatory capability, as the allogeneic proliferation induced by treated cells was threefold and 1·5-fold less important than that induced by control immature and mature DC, respectively. This inhibitory effect of butyrate was more marked, for immature DC whose activity was nearly abrogated at the lower APC/T cell ratios (P < 0·01). These results indicate that the phenotypic changes induced by butyrate at key stages of DC differentiation correlate with the decreased allostimulatory properties of treated cells.
Fig. 6.
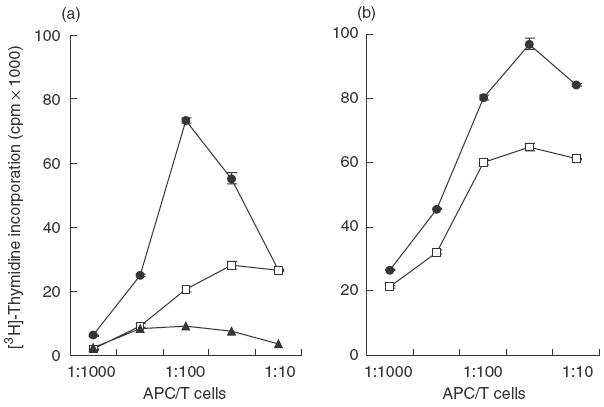
Effect of butyrate on the T-cell allostimulatory capacity of DC. Immature DC were generated from monocytes cultured with GM-CSF plus IL-4 in the absence or in the presence of 0·5 mm butyrate. In a second set of experiments, 5-day immature DC were further matured for 48 h with TNF-α plus PGE2. On day 7, cells were harvested and used as stimulators of allogeneic T cell proliferation. Thymidine incorporation was measured on day 5 by an 18-h pulse with [3H]-thymidine. (a) Immature DC were compared to freshly isolated monocytes from the same individual. (b) Mature DC. Data from quadruplicate assays are representative of three separate experiments performed with three different donors. (a) •, Immature DC; □, treated cells; ▴, monocytes. (b) •, Mature DC; □, treated cells.
Butyrate synergistically acts with 1,25(OH)2D3 on DC phenotypic differentiation
As 1,25(OH)2D3 was shown to interfere with monocyte differentiation [29,30], we therefore studied the individual and combined effect of butyrate and 1,25(OH)2D3 on the phenotypic differentiation of monocytes into DC. As shown in Fig. 7a, treatment with either butyrate or 1,25(OH)2D3 resulted in a preserved CD14 expression (mean fluorescence intensity: 13 ± 2 and 41 ± 9, re-spectively) when compared to untreated cells (0·7 ± 0·1). Furthermore, combination of both treatments led to a syner-gistic effect on CD14 expression resulting in differentiated cells which exhibited a twofold increased CD14 membrane density when compared to fresh monocytes. In addition, Fig 7b shows that combined treatment further reduced CD86 and HLA-DR expression, when compared to cells treated with each drug alone.
Fig. 7.
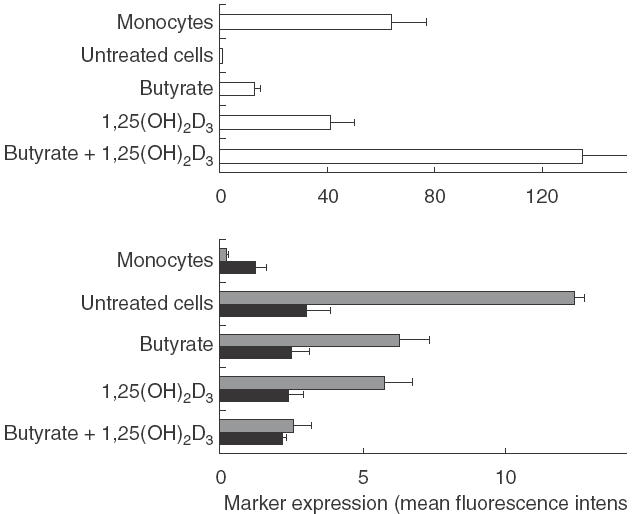
Effect of treatment with butyrate and 1,25(OH)2D3 on the phenotypic differentiation of monocytes into DC. DC were generated from monocytes cultured in the presence of 0·5 mm butyrate, 10−7m 1,25(OH)2D3 or a combination of both drugs, respectively. On day 7, cells were analysed for CD14, CD86 and HLA-DR expression using flow cytometry and compared to untreated cells and fresh monocytes from the same individual. Data shown are the mean ± s.e.m. of three separate experiments performed with three different donors. □, CD14;  , CD86; ▪, HLA-DR.
, CD86; ▪, HLA-DR.
DISCUSSION
To our knowledge, this study represents the first to examine the effect of short-chain fatty acid butyrate on the differentiation and maturation process of DC and MΦ from monocytes. We demonstrate that butyrate partially inhibited the cytokine-driven phenotypic differentiation process of both cells, thus modulating their antigen capture efficiency. Moreover, butyrate altered the terminal maturation of DC and, consequently, profoundly decreased their ability to prime alloreactive naive T cells. Because DC have the unique property to induce primary immune response, and because MΦ are a major component of the innate and adaptive immune responses [31], suppression of DC and MΦ APC function by butyrate may represent a new, promising approach in immunotherapy.
Upon stimulation with GM-CSF and IL-4, monocytes differentiated into DC displaying phenotypic and functional characteristics. Compared to precursor monocytes, DC down-regulated CD14, neoexpressed adhesion and co-activation molecules and up-regulated antigen-presentation molecules. In the presence of butyrate this phenotypic differentiation was inhibited partially, as we observed a persistence of CD14 and a decreased CD54, CD86 and HLA class II expression. This more immature differentiation stage was assessed further by the increased phagocytic capacity of the treated cells whose bead-engulfing capability was found intermediary between those of monocytes and untreated DC. Similarly, DC differentiated in the presence of butyrate exhibited an altered capacity to produce both IL-10 and IL-12 and developed only weak allostimulatory capacities.
Moreover, DC triggered to maturation through TNF-α plus PGE2 in the presence of butyrate were unable (1) to express CD83, a marker highly restricted to mature DC, (2) to acquire highly level of co-stimulatory and antigen-presentation molecules such as CD40, CD86 and HLA class II and (3) to optimally stimulate allogeneic naive T cells. This effect of butyrate was independent of the type of maturation inducers used, because similar changes were observed with LPS or TNF-α plus IL-1β.
Similarly, butyrate was found to alter the monocyte-derived MΦ differentiation, reducing the expression of restricted membrane antigens and the phagocytic capacity of treated cells. Overall, our data demonstrate clearly that butyrate modulates the differentiation potential of monocytes and leads to the generation of DC and MΦ with altered function. Whether butyrate simply blocks the dual monocyte differentiation or rather redirects this process towards distinct functional programmes is still a matter of speculation.
Previous studies have shown that pretreatment of fresh monocytes with butyrate down-regulates their ability to stimulate T cell responses [14,32]. Altered APC function was considered to result from a down-regulation of distinct adhesion and/or co-stimulatory receptors on monocytes. However, these effects were observed only at high T cell/APC ratios and, more importantly, at high butyrate concentrations (1–2 mm), and such concentrations were shown by us and others [14] to result in a high level of monocyte death and apoptosis. At lower concentrations, butyrate did not significantly alter cell viability but virtually no APC suppression effect was observed in the fresh monocyte model [14]. Our results, obtained with a cultured cell model at a non-toxic butyrate concentration (0·5 mm), demonstrate a novel immune suppression property of butyrate that results in monocyte inability to differentiate into potent professional APC.
The active metabolite of the immunomodulatory hormone vitamin D3, 1,25(OH)2D3, has also been shown to inhibit cytokine-driven differentiation, maturation and function of DC [30,33]. In addition, it has been demonstrated that butyrate in combination with 1,25(OH)2D3 acts synergistically on the differentiation of several neoplastic cell lines [34–36]. We therefore decided to study the effects of these treatments, alone or in combination, on the phenotypic changes induced by monocyte differentiation into DC in our physiologically more relevant model. Both butyrate and 1,25(OH)2D3 show an inhibitory effect on monocyte differentiation into DC, as assessed by the evolution of the phenotypic profile observed (Fig. 7). Moreover, when combined these treatments resulted in an additive effect on the inhibition of CD86 and HLA-DR expression, and a synergistic effect on CD14 expression which membrane density even exceeded that observed on fresh monocytes. Gaschott et al. [37] have suggested that the synergistic effect of butyrate and 1,25(OH)2D3, observed on tumoral cell lines could be due to an up-regulation of vitamin D receptors by butyrate. However, despite a similar synergistic effect on DC differentiation when both treatments were combined, 1,25(OH)2D3 and butyrate have been shown to alter monocyte differentiation in a different way, when used alone. 1,25(OH)2D3 has been shown to skew monocyte differentiation away from antigen-presenting DC development and towards the direction of mature phagocytosing MΦ [29], when butyrate inhibits monocyte differentiation into DC without redirecting towards MΦ (Fig. 2). Moreover, 1,25(OH)2D3 has been shown to promote the differentiation of monocytes towards MΦ [30], while in our study butyrate prevents both DC and MΦ differentiation (Fig. 2). Therefore, our results suggest that, at least in part, another mechanism might explain the effect of butyrate on the dual differentiation of monocytes.
Among other mechanisms by which butyrate could modulate differentiation and maturation, one can speculate a possible role for the NF-κB/Rel transcription factor family. NF-κB, identified initially as a constitutive immunoglobulin κ light-chain enhancer-binding protein present in the nuclei of B lymphocytes [38], has been found to play a central role in regulating the expression of various cytokines and cell adhesion molecules involved in immune and inflammatory responses [39]. Recently, gradual differences in the DNA binding activities of different NF-κB homo- and heterodimers have been shown to correlate with differentiation stages of monocytes, MΦ and DC [40]. In the same way, butyrate has been shown to inhibit the activation of NF-κB on cultured cells [41]. This inhibitory effect could result from direct alteration of the expression of one or more genes that function subsequently to regulate NF-κB activity, but also from inhibition of NF-κB activation by TNF-α [41]. Therefore, inhibition of NF-κB activity by butyrate is likely to have a role in impaired DC and MΦ differentiation and DC maturation.
Butyrate or related compounds have recently been suggested to have potential clinical relevance for the treatment of autoimmune diseases as well as for the inhibition of alloresponses [2,3,42]. Butyrate enemas or a high-fibre diet that increase colonic butyrate concentrations have been shown to be effective treatments for mucosal inflammation in both humans and animal models of colitis [43–47]. In addition, supplementation of drinking water with butyrate resulted in a modest, but significant prolongation of skin allograft survival in mice [1]. The therapeutic potential of butyrate, which is limited by its short half-life in vivo [48], has been improved by the synthesis of prodrugs of n-butyric acid extending the residence time of potentially releasable n-butyric acid in vivo [3,49–53]. These compounds have been shown to retain the majority of the biological properties in vitro as well as in vivo [32,54–56]. Evidence for an anti-inflammatory effect of these drugs on human monocytes [42], as well as prolongation of heart allograft survival in animals [2], have been obtained. Our results, demonstrating impairment of DC and MΦ differentiation by butyrate, represent important clues to understand the mechanism of action of butyrate on both inflammatory and alloresponses, and support further the interest for butyrate and its derivatives as new immunotherapeutic reagents.
Acknowledgments
This work was supported by grants from the Ligue Nationale Contre le Cancer, comité de la Marne. A.L.M. is a recipient of a fellowship from Région Champagne-Ardenne.
REFERENCES
- 1.Suthanthiran M, Rubin AL, Novogrodsky A, Stenzel KH. Immunosuppressive properties of polar organic compounds that induce cellular differentiation in Friend erythroleukemia cells. Transplantation. 1982;33:534–40. doi: 10.1097/00007890-198205000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Bohmig GA, Krieger PM, Saemann MD, et al. Stable prodrugs of n-butyric acid: suppression of T cell alloresponses in vitro and prolongation of heart allograft survival in a fully allogeneic rat strain combination. Transpl Immunol. 1999;7:221–7. doi: 10.1016/s0966-3274(99)80006-9. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert KM, Wahid R, Fecher NP, Freeman JP, Fifer EK. Potential clinical use of butyric acid derivatives to induce antigen-specific T cell inactivation. J Pharmacol Exp Ther. 2000;294:1146–53. [PubMed] [Google Scholar]
- 4.Velazquez OC, Lederer HM, Rombeau JL. Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol. 1997;427:123–34. [PubMed] [Google Scholar]
- 5.Scheppach W, Bartram HP, Richter F. Role of short-chain fatty acids in the prevention of colorectal cancer. Eur J Cancer. 1995;31A:1077–80. doi: 10.1016/0959-8049(95)00165-f. [DOI] [PubMed] [Google Scholar]
- 6.Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23–8. doi: 10.1056/NEJM198901053200105. [DOI] [PubMed] [Google Scholar]
- 7.Chapman MA, Grahn MF, Boyle MA, Hutton M, Rogers J, Williams NS. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut. 1994;35:73–6. doi: 10.1136/gut.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad MS, Krishnan S, Ramakrishna BS, Mathan M, Pulimood AB, Murthy SN. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut. 2000;46:493–9. doi: 10.1136/gut.46.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenzel KH, Schwartz R, Rubin AL, Novogrodsky A. Chemical inducers of differentiation in Friend leukaemia cells inhibit lymphocyte mitogenesis. Nature. 1980;285:106–8. doi: 10.1038/285106a0. [DOI] [PubMed] [Google Scholar]
- 10.Novogrodsky A, Rubin AL, Stenzel KH. A new class of inhibitors of lymphocyte mitogenesis: agents that induce erythroid differentiation in Friend leukemia cells. J Immunol. 1980;124:1892–7. [PubMed] [Google Scholar]
- 11.Bohmig GA, Csmarits B, Cerwenka A, Alaei P, Kovarik J, Zlabinger GJ. Induction of alloantigen-specific hyporesponsiveness in vitro by the short-chain fatty acid N-butyrate. Transplantation. 1995;59:1500–3. doi: 10.1097/00007890-199505270-00029. [DOI] [PubMed] [Google Scholar]
- 12.Kyner D, Zabos P, Christman J, Acs G. Effect of sodium butyrate on lymphocyte activation. J Exp Med. 1976;144:1674–8. doi: 10.1084/jem.144.6.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert KM, Weigle WO. Th1 cell anergy and blockade in G1a phase of the cell cycle. J Immunol. 1993;151:1245–54. [PubMed] [Google Scholar]
- 14.Bohmig GA, Krieger PM, Saemann MD, Wenhardt C, Pohanka E, Zlabinger GJ. n-Butyrate downregulates the stimulatory function of peripheral blood-derived antigen-presenting cells: a potential mechanism for modulating T-cell responses by short-chain fatty acids. Immunology. 1997;92:234–43. doi: 10.1046/j.1365-2567.1997.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4 (+) T cells. J Exp Med. 1997;185:2133–41. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rappolee DA, Mark D, Banda MJ, Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988;241:708–12. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- 17.Andreesen R, Scheibenbogen C, Brugger W, et al. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: a new approach to cancer immunotherapy. Cancer Res. 1990;50:7450–6. [PubMed] [Google Scholar]
- 18.Lopez M, Martinache C, Canepa S, Chokri M, Scotto F, Bartholeyns J. Autologous lymphocytes prevent the death of monocytes in culture and promote, as do GM-CSF, IL-3 and M-CSF, their differentiation into macrophages. J Immunol Meth. 1993;159:29–38. doi: 10.1016/0022-1759(93)90138-w. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–8. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 23.Eymard JC, Lopez M, Cattan A, Bouche O, Adjizian JC, Bernard J. Phase I/II trial of autologous activated macrophages in advanced colorectal cancer. Eur J Cancer. 1996;32A:1905–11. doi: 10.1016/0959-8049(96)00233-x. [DOI] [PubMed] [Google Scholar]
- 24.Bernard J, Ittelet D, Christoph A, et al. Adherent-free generation of functional dendritic cells from purified blood monocytes in view of potential clinical use. Hematol Cell Ther. 1998;40:17–26. [PubMed] [Google Scholar]
- 25.Hilkens CM, Kalinski P, de Boer M, Kapsenberg ML. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–6. [PubMed] [Google Scholar]
- 26.Dunn PA, Tyrer HW. Quantitation of neutrophil phagocytosis, using fluorescent latex beads. Correlation of microscopy and flow cytometry. J Laboratory Clin Med. 1981;98:374–81. [PubMed] [Google Scholar]
- 27.Crow MK, Kunkel HG. Human dendritic cells: major stimulators of the autologous and allogeneic mixed leucocyte reactions. Clin Exp Immunol. 1982;49:338–46. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreutz M, Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. 1990;76:2457–61. [PubMed] [Google Scholar]
- 30.Canning MO, Grotenhuis K, de Wit H, Ruwhof C, Drexhage HA. 1-alpha,25-Dihydroxyvitamin D3 (1,25 (OH) (2) D (3) hampers the maturation of fully active immature dendritic cells from monocytes. Eur J Endocrinol. 2001;145:351–7. doi: 10.1530/eje.0.1450351. [DOI] [PubMed] [Google Scholar]
- 31.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 32.Bohmig GA, Kovarik J, Holter W, Pohanka E, Zlabinger GJ. Specific down-regulation of proliferative T cell alloresponsiveness by interference with CD2/LFA-3 and LFA-1/ICAM-1 in vitro. J Immunol. 1994;152:3720–8. [PubMed] [Google Scholar]
- 33.Piemonti L, Monti P, Sironi M, et al. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–51. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida M, Tanaka Y, Eguchi T, Ikekawa N, Saijo N. Effect of hexafluoro-1,25-dihydroxyvitamin D3 and sodium butyrate combination on differentiation and proliferation of HL-60 leukemia cells. Anticancer Res. 1992;12:1947–52. [PubMed] [Google Scholar]
- 35.Yoneda T, Aya S, Sakuda M. Sodium butyrate (SB) augments the effects of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) on neoplastic and osteoblastic phenotype in clonal rat osteosarcoma cells. Biochem Biophys Res Commun. 1984;121:796–801. doi: 10.1016/0006-291x(84)90748-4. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka Y, Bush KK, Klauck TM, Higgins PJ. Enhancement of butyrate-induced differentiation of HT-29 human colon carcinoma cells by 1,25-dihydroxyvitamin D3. Biochem Pharmacol. 1989;38:3859–65. doi: 10.1016/0006-2952(89)90596-0. [DOI] [PubMed] [Google Scholar]
- 37.Gaschott T, Werz O, Steinmeyer A, Steinhilber D, Stein J. Butyrate-induced differentiation of Caco-2 cells is mediated by vitamin D receptor. Biochem Biophys Res Commun. 2001;288:690–6. doi: 10.1006/bbrc.2001.5832. [DOI] [PubMed] [Google Scholar]
- 38.Singh H, Sen R, Baltimore D, Sharp PA. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986;319:154–8. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- 39.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–79. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 40.Ammon C, Mondal K, Andreesen R, Krause SW. Differential expression of the transcription factor NF-kappaB during human mononuclear phagocyte differentiation to macrophages and dendritic cells. Biochem Biophys Res Commun. 2000;268:99–105. doi: 10.1006/bbrc.1999.2083. [DOI] [PubMed] [Google Scholar]
- 41.Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-kappaB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724–34. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- 42.Saemann MD, Bohmig GA, Osterreicher CH, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. Faseb J. 2000;14:2380–2. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 43.Scheppach W, Sommer H, Kirchner T, et al. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51–6. doi: 10.1016/0016-5085(92)91094-k. [DOI] [PubMed] [Google Scholar]
- 44.Butzner JD, Parmar R, Bell CJ, Dalal V. Butyrate enema therapy stimulates mucosal repair in experimental colitis in the rat. Gut. 1996;38:568–73. doi: 10.1136/gut.38.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kanauchi O, Andoh A, Iwanaga T, et al. Germinated barley foodstuffs attenuate colonic mucosal damage and mucosal nuclear factor kappa B activity in a spontaneous colitis model. J Gastroenterol Hepatol. 1999;14:1173–9. doi: 10.1046/j.1440-1746.1999.02025.x. [DOI] [PubMed] [Google Scholar]
- 46.Andoh A, Bamba T, Sasaki M. Physiological and anti-inflammatory roles of dietary fiber and butyrate in intestinal functions. JPEN J Parenter Enteral Nutr. 1999;23:S70–3. doi: 10.1177/014860719902300518. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-Banares F, Hinojosa J, Sanchez-Lombrana JL, et al. Randomized clinical trial of Plantago ovata seeds (dietary fiber) as compared with mesalamine in maintaining remission in ulcerative colitis. Spanish Group for the Study of Crohn's Disease and Ulcerative Colitis (GETECCU) Am J Gastroenterol. 1999;94:427–33. doi: 10.1111/j.1572-0241.1999.872_a.x. [DOI] [PubMed] [Google Scholar]
- 48.Daniel P, Brazier M, Cerutti I, et al. Pharmacokinetic study of butyric acid administered in vivo as sodium and arginine butyrate salts. Clin Chim Acta. 1989;181:255–63. doi: 10.1016/0009-8981(89)90231-3. [DOI] [PubMed] [Google Scholar]
- 49.Pouillart P, Cerutti I, Ronco G, Villa P, Chany C. Butyric monosaccharide ester-induced cell differentiation and anti-tumor activity in mice. Importance of their prolonged biological effect for clinical applications in cancer therapy. Int J Cancer. 1991;49:89–95. doi: 10.1002/ijc.2910490117. [DOI] [PubMed] [Google Scholar]
- 50.Planchon P, Raux H, Magnien V, et al. New stable butyrate derivatives alter proliferation and differentiation in human mammary cells. Int J Cancer. 1991;48:443–9. doi: 10.1002/ijc.2910480323. [DOI] [PubMed] [Google Scholar]
- 51.Pouillart P, Cerutti I, Ronco G, Villa P, Chany C. Enhancement by stable butyrate derivatives of antitumor and antiviral actions of interferon. Int J Cancer. 1992;51:596–601. doi: 10.1002/ijc.2910510416. [DOI] [PubMed] [Google Scholar]
- 52.Chen ZX, Breitman TR. Tributyrin: a prodrug of butyric acid for potential clinical application in differentiation therapy. Cancer Res. 1994;54:3494–9. [PubMed] [Google Scholar]
- 53.Planchon P, Magnien V, Starzec A, Prevost G. Selection of a highly tumorigenic breast cancer cell line sensitive to estradiol to evidence in vivo the tumor-inhibitory effect of butyrate derivative Monobut-3. Life Sci. 1994;55:951–9. doi: 10.1016/0024-3205(94)00541-9. [DOI] [PubMed] [Google Scholar]
- 54.Jenkins MK, Chen CA, Jung G, Mueller DL, Schwartz RH. Inhibition of antigen-specific proliferation of type 1 murine T cell clones after stimulation with immobilized anti-CD3 monoclonal antibody. J Immunol. 1990;144:16–22. [PubMed] [Google Scholar]
- 55.Gilbert KM, Hoang KD, Weigle WO. Th1 and Th2 clones differ in their response to a tolerogenic signal. J Immunol. 1990;144:2063–71. [PubMed] [Google Scholar]
- 56.Liu Y, Jones B, Aruffo A, Sullivan KM, Linsley PS, Janeway CA., Jr Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J Exp Med. 1992;175:437–45. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan P, Anasetti C, Hansen JA, et al. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–73. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]


