Abstract
Although T cell involvement in Helicobactor pylori-induced gastritis is known, mechanism about T cell recruitment is not understood. In this study we examined how mucosal addressin cell adhesion molecule-1 (MAdCAM-1) is involved in lymphocyte recruitment in murine chronic gastritis induced by H. pylori. C57 BL/6 mice were infected with Sydney strain (SS1). Six months after infection, the stomach was removed. The expression of adhesion molecules, MAdCAM-1, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), and the cell surface antigens CD4, CD8, CD45R/B220 or β7-integrin were determined by immunohistochemistry. A significant increase in CD4 lymphocytes was observed in the body portion of stomach in SS1-infected mice and most of these CD4 cells express β7-integrin, a known counter ligand for MAdCAM-1 molecule. Strong MAdCAM-1 expression was observed adjacent to these cells in the lamina propria as well as in the submucosa of SS1-infected stomach. Quantitative analysis showed that the area of MAdCAM-1 expression well correlated with the infiltration of β7-integrin positive lymphocytes. On the other hand, expression of ICAM-1 or VCAM-1 in the lamina propria was few even in the SS1-infected stomach. Increased expression of MAdCAM-1 was well correlated to the location of lymphocytes, which express CD4 and β7-integrin. These results suggest the possibility that MAdCAM-1 may be largely involved in the lymphocyte recruitment in the gastritis mucosa with H. pylori.
Keywords: adhesion molecules, β7-integrin, Helicobactor pylori gastritis, lymphocyte migration, mucosal addressin cell adhesion molecule-1 (MAdCAM-1)
INTRODUCTION
Helicobacter pylori plays a causative role in the pathogenesis of gastritis, gastric atrophy and peptic and duodenal ulcer [1]. Infection by this bacterium is also associated with an increased risk of gastric adenocarcinoma, and H. pylori is now classified as a type I human carcinogen [2]; furthermore, a causative relationship between the presence of H. pylori and the occurrence of mucosa-associated lymphoid tissue (MALT) lymphoma was suggested [3]. Despite the presence of high titres of Helicobacter-specific antibodies in the serum and gastric mucosa of H. pylori-infected patients [4], they remain chronically infected and are unable to clear infection.
Infection with Helicobacter species results in the recruitment of CD4+ and CD8+ T cells in gastric tissue [5]. The accumulation of gastric Th1-type H. pylori-specific CD4+ cells has been proposed to account for their failure to generate protective immunity and to contribute to disease pathogenesis [6]. Although many proof of T cell involvement of pathogenesis in H. pylori-induced gastritis, information about recruitment of T cell, including source of lymphocytes or their expression of adhesion molecules have not yet been elucidated.
Lymphocyte homing to both normal tissues and sites of inflammation is, in part, regulated by differential expression of cell surface homing receptors and their selective interactions with tissue selective vascular adhesion molecules at sites of lymphocyte recruitment from the blood [7]. In the mouse, lymphocyte homing to mucosal lymphoid tissue such as Peyer's patches and the intestinal lamina propria involves a single-chain 60-kDa glycoprotein, the mucosal vascular adhesion molecule MAdCAM-1 (mucosal addressin cell adhesion molecule-1) [8] and the heterodimeric alpha4beta7-integrin on leucocytes act as its ligand. Immunohistology demonstrates MAdCAM-1 expression at high levels by HEVs in mucosal-associated lymphoid tissue such as Peyer's patches but not in peripheral lymph nodes. In case of intestinal inflammation, its expression increases and is thought to be a gate of inflammatory cells to the site of inflammation [9,10]; however, it is also observed in the nonmucosal inflamed site in the chronically inflamed pancreas of non-obese diabetic (NOD) mice [11], and weakly on central nervous system venules in chronic relapsing experimental allergic encephalomyelitis [12]. Although MAdCAM-1 involves some infiltration of extra mucosal lesion, involvement to H. pylori-induced gastritis is not elucidated.
In this study, we investigated whether H. pylori infection affect localization of beta7-integrin positive CD4 cells and expression of MAdCAM-1 in the gastric mucosa by using the Sydney strain (SS1) of H. pylori, which has high colonizing ability for the mouse stomach [13].
MATERIALS AND METHODS
Inoculation of H. pylori
H. pylori SS1 was grown on tryptic soy agar (TSA) plates (Becton Dickinson, Cockeysville, MD, USA) containing 5% sheep blood (Remel, Leneza, KS, USA) and 100 µg of vancomycin, 3·3 µg of polymixin B, 200 µg of bacitracin, 10·7 µg of nalidixic acid and 50 µg of amphotericin B (Sigma Chemical Co., St Louis, MO, USA) per ml. The plates were incubated for 72–80 h at 37°C in 10% CO2 and 5% O2 in a Trigas incubator (Queue Systems, Ashville, NC, USA). Female 6-week-old specific pathogen-free C57BL/6 mice (Nihon CLEA, Yokohama, Japan) were housed under conventional conditions in our animal facilities. The animals were handled according to the guidelines of Animal Research Committee of National Defense Medical College. The mice had free access to food and water. Mice were inoculated with a bacterial suspension obtained from 2-day liquid cultures of SS1. After overnight fasting, the animals were dosed twice in a 5-day period with 0·5 ml of bacterial suspension (approximately 5 × 108 cfu/ml) using a stomach tube (n = 8). As controls mice were given suspension buffer solution alone (n = 8). After 6 months, the stomach was removed and the excised stomachs were cut along the greater curvature and rinsed with physiological saline.
Blood samples were collected from the left ventricle. Sera were used to determine the titre of anti-H. pylori IgG antibody by enzyme-linked immunosorbent assay (ELISA) (HEL-p Test II, Amrad Operation Pty Ltd, Melbourne, Australia) with the change of the second antibody to antimouse IgG. The antibody titre was expressed by way of an arbitrary index; values>1·5 were accepted as indicating positive detection of H. pylori.
Histological examination and immunohistochemistry for MAdCAM-1
Tissue specimens of gastric mucosa were fixed in 10% buffered formalin and embedded in paraffin after hydration. Consecutive 4 µm sections were stained with haematoxylin and eosin or Giemsa. Degree of mucosal inflammation was assessed in haematoxylin–eosin sections, and the presence of bacteria was confirmed by Giemsa sections. Immunohistochemical study was performed using LSAB (labelled streptavidin–biotin) method. Specimens were fixed in PLP (periodate, lysine-paraformaldehyde) solution, and were vertically embedded carefully in OCT compound (Sakura Fineteck Inc., Tokyo, Japan). Well-orientated 6 µm of cryostat sections were transferred to APS-coated slides and air dried for 1 h at 20°C. After they were washed in phosphate-buffered saline (pH 7·4) containing 1% Triton X for 5 min, sections were incubated in 10% normal goat serum in phosphate buffered saline (PBS). Monoclonal antibodies used in this study and the dilutions were follows. Anti-mouse CD4 antibody (L3T4, PharMingen:10 µg/ml), antimouse CD8 antibody (Ly-2, PharMingen: 10 µg/ml), antimouse CD45R/B220 antibody (RA3–6B2 PharMingen: 10 µg/ml), antimouse β7-integrin (M293, PharMingen: 10 µg/ml), antimouse MAdCAM-1 (MECA-367, PharMingen: 5 µg/ml), antimouse intercellular adhesion molecule-1 (ICAM-1) (3E2, PharMingen: 5 µg/ml), and antimouse and vascular cell adhesion molecule-1 (VCAM-1) (429, PharMingen: 5 µg/ml). Isotype and species matched IgG was used for control study. After overnight incubation at 4°C, sections were treated with subclass- and host-matched biotinylated antibodies for 1 h at room temperature. They were visualized by streptavidin–FITC. Rinsing with PBS containing 1% bovine serum albumin was performed along each step. A cover slip was applied using glycerol jelly. These sections were observed under a fluorescent microscope (BX60, Olympus, Tokyo). In order to stain MPO positive cells, serial sections were incubated for 15–20 min with 0·0125 g/100 ml 3,3′-diaminobenzidine (Sigma) and 0·03% (v/v) hydrogen peroxide in 0·02 mol/l PBS. Methyl-green was used as a counterstain. These sections were observed under a light microscope (BX60, Olympus, Tokyo). In all experiments, pictures were captured in computer, and 5 mm length of muscularis mucosa was analysed using image analyser (NIH Image) as described previously [9,10] (control: n = 8, SS-1 infected: n = 8). The MAdCAM-1 positive vessels in lamina propria were calculated using image analyser and quantified as length of posi-tively stained vessel walls per mm muscularis mucosa. All of the infiltrated cells (CD4, CD8, B cell or MPO positive cells) in the lamina propria and in the submucosa were counted in the section and expressed as the number of cells per mm muscularis mucosa.
Double immunolabelling and laser scanning confocal microscopy
For double staining of CD4 and β7, essentially the same immunohistochemistry procedure was used as for normal fluorescent microscopy. Briefly, sections were incubated with both primary antibodies against biotinylated antiβ7-integrin and FITC-conjugated anti-CD4 antibody overnight. In a second step, after rinsing with PBS, sections were incubated with rhodamine-conjugated streptavidin (streptavidin–rhodamine) (Amersham International plc, Buckinghamshire, UK) for 30 min at room temperature. Fluorescent preparations were examined using a Carl Zeiss laser scan microscope equipped with an argon laser (488 nm excitation for FITC), and rhodamine fluorescence was examined with the 543 nm laser. An appropriate emission filter system was used, and scanning with the 543 and 488 nm laser was performed sequentially (Carl Zeiss, Jena, Germany).
Statistics
Results are expressed as median and range. Data were statistically analysed by Kruskal–Wallis and Scheffé's F-test (among subsets of infiltrating cells) or Mann–Whitney test (between control group and SS1 infection group). P-values of 0·05 or less were considered to be statistically significant. Association among parameters was assessed by use of the Spearman rank-correlation technique.
RESULTS
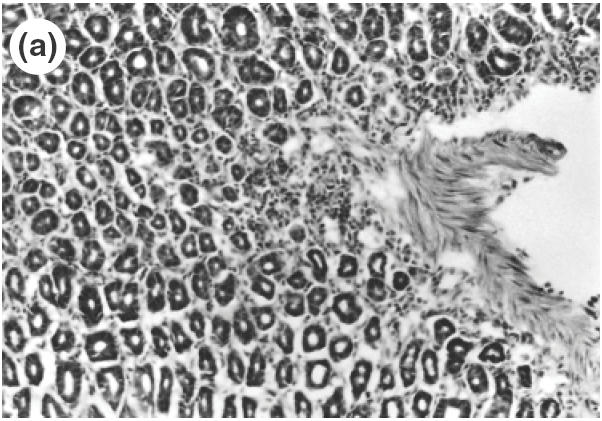
The serum antibody (IgG) against H. pylori was positive for all mice and was negative for all animals of control groups. As the gastric histological specimens revealed the presence of the bacterial body of H. pylori bacteria in all stomachs in the SS1-inoculated group, persistent infection was confirmed in the SS1 group during the observation period. A significant cell infiltration was observed not only in the submucosa but extended to the upper part of the mucosa of SS1-infected mice compared with non-infected control mice by H&E staining (Fig. 1a,b).
Fig. 1.
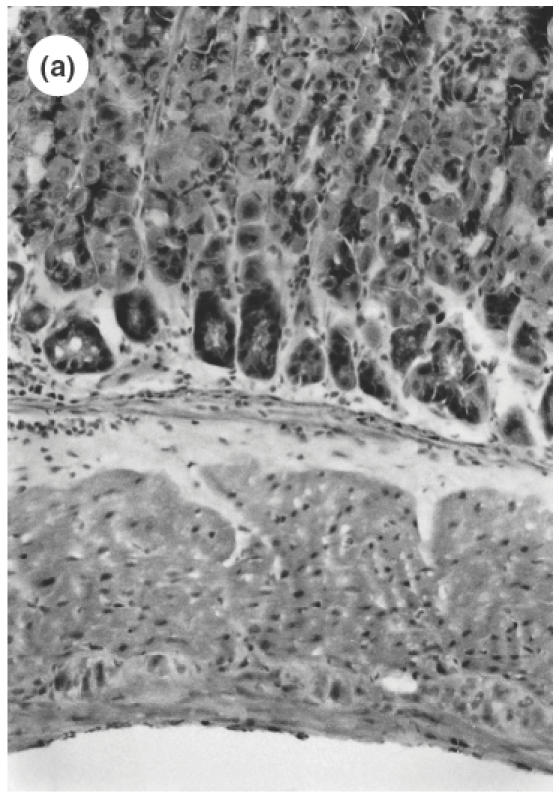
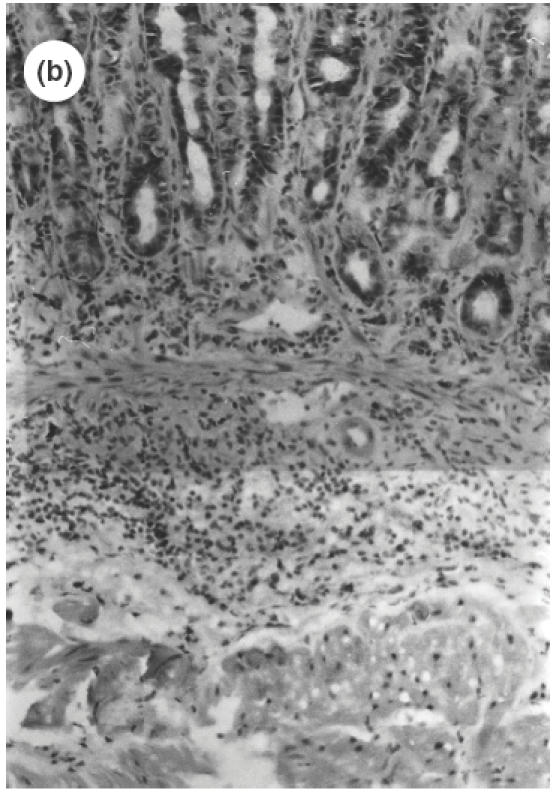

Microscopic pictures of the gastric mucosa of SS1-infected mice compared with noninfected control mice. (a) Control mice (H&E staining, ×100). (b) SS1-infected mice (H&E staining, ×100). (c) Immunohistochemical study of CD4 positive cells in the stomach of control mice. (d) Immunohistochemical study of CD4-positive cells in the stomach of SS1-infected mice. The same specimens as (a) and (b) were observed.
Serial stomach sections of control and infected mice were investigated for the distribution of MPO-positive cells, CD4 T cells, CD8 T cells, B cells and for the expression of cell adhesion molecules such as β7-integrin and vascular endothelial cell adhesion molecules. Figure 2 shows accumulation of leucocytes after SS-1 infection and compared the number of infiltrated cells among different subsets in the lamina propria (Fig. 2a) and in the submucosa (Fig. 2b). In control mice, there were a few CD4 T cells in the gastric mucosa. On the other hand, in SS1-infected mice, there was a marked infiltration of CD4 lymphocytes not only in the lamina propria (control, 1·14/mm versus SS1, 288·2/mm; P < 0·01), but also in the submucosa (control, 1·31/mm versus SS1145·2/mm; P < 0·01) extending to the upper part of the gastric mucosa (Fig. 1c,d). A significant increase in MPO positive cells (mainly consisting of neutrophils) was also demonstrated in the lamina propria (control, 1·12/mm versus SS1, 75·1/mm SS1; P < 0·01) and in the submucosa (control, 1·52/mm versus SS1, 94·5/mm; P < 0·01) of infected mice. In control mice some B cells were observed, mainly in the lamina propria (3·12/mm), but B cells were observed mainly in the submucosa around the large vessels of SS1-infected mice and a significant increase in B cell accumulation was observed in the submucosa (control, 3·1/mm versus SS1, 21·5/mm SS1; P < 0·01). There were a few CD8 (1·06/mm) T cells in the gastric mucosa of control mucosa, but this subset did not increase significantly in the infected mice. Therefore, the dominantly infiltrated cells were CD4 positive cells in the SS1-infected gastric mucosa, and their number was markedly greater than those of CD8 T cells or B cells (P < 0·01) and MPO-positive cells (P < 0·05).
Fig. 2.
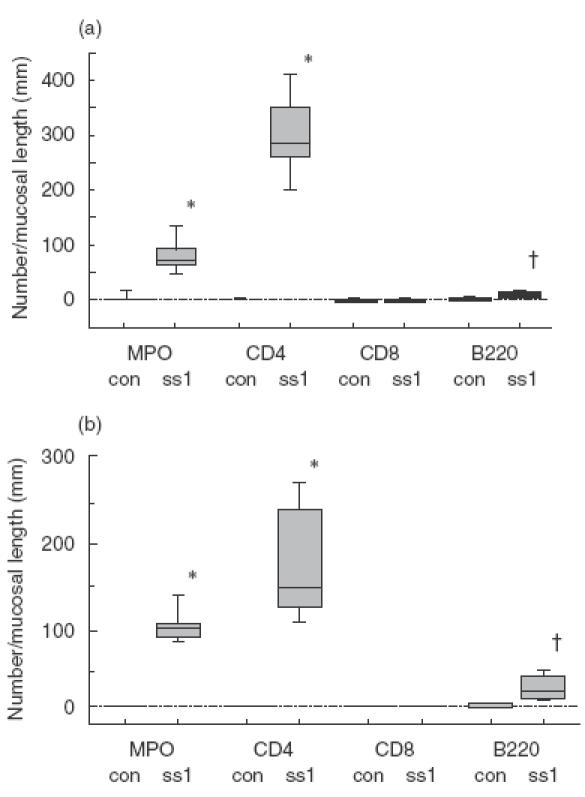
The number of infiltrated cells of different subsets in the lamina propria (a) and submucosa (b) in control mice (con) and SS1-infected mice (SS1). The number of MPO positive cells, CD4, CD8 and B220 positive cells were determined as cells per mm of mucosal length. Values are expressed median and range (box) and minimal and vertical values (vertical line) from eight animals. *P < 0·01 compared with control groups. †P < 0·05 compared with control groups.
Next we investigated the expression of adhesion molecule β7-integrin in infiltrating lymphocytes. In control mice, there were few β7-integrin-positive cells in the gastric mucosa. On the other hand, we observed that the markedly infiltrating cells both in the gastric mucosa (control, 1·41/mm versus SS1, 270·6/mm; P < 0·01) and in the submucosa (control, 1·64/mm versus SS1, 167·2/mm; P < 0·01) expressed β7-integrin, a known marker for mucosal homing lymphocytes. In order to examine whether the infiltrating lymphocytes, which consisted of mainly CD4 cells, co-express β7-integrin molecules, dual labelling for CD4 and β7-integrin was performed in the gastric mucosa of SS1-infected mice. A confocal microscopic image demonstrated that there were many FITC positive CD4-positive cells in the lamina propria (Fig. 3a) and that the rhodamine-positive–β7-integrin-positive cells were also seen in the same area (Fig. 3b). The cells shown in yellow in Fig. 3c indicated that the almost all of CD4 lymphocytes co-expressed β7-integrin.
Fig. 3.
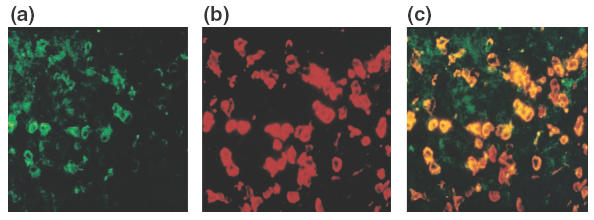
Expression of CD4 and β7 in the lamina propria of gastric mucosa of SS1-infected mice observed by double immunolabelling (×400). A confocal microscopic image demonstrates the FITC-positive CD4 positive cells (a) and the rhodamine positive–β7-integrin-positive cells in the same area (b). The CD4 lymphocytes co-express β7-integrin were indicated in yellow (c).
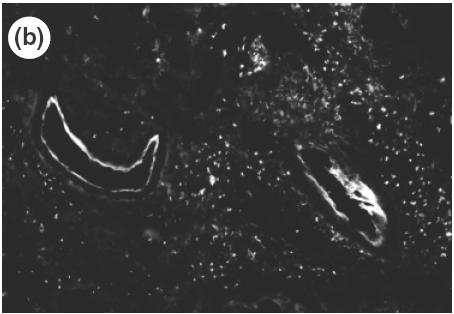
To investigate which adhesion molecules are involved when cells are infiltrated from the microvascular beds to the gastric tissue, the expression of vascular adhesion molecules, ICAM-1, VCAM-1 and MAdCAM-1 in the gastric mucosa was determined by immunohistochemistry. The specificity of antibodies was verified by the absence of non-specific staining in negative controls. In control mice, there was no detectable expression of ICAM-1 or VCAM-1 in the gastric tissue. In SS1-infected mice, expression of ICAM-1 was observed mainly in the vascular endothelium in the submucosa, and a weak expression of ICAM-1 was also shown in the vessels of lamina propria as well as on the surface of infiltrating cells in the inflamed mucosa (Fig. 4a). On the other hand, a strong expression of VCAM-1 was observed on venular endothelium in the submucosa (Fig. 4b), although no expression was observed in the mucosal area. In addition to venular endothelium, VCAM-1 was also observed on the cells of lamina propria in the infected mucosa. MAdCAM-1, a known counter ligand for β7-integrin, was not observed in control non-infected mice, either in the submucosa or lamina propria (Fig. 5a). A strong expression of MAdCAM-1 was observed clearly both in the lamina propria and in the submucosa of infected stomach (Fig. 5b), and the length of positively stained vessels reached 4·2 ± 1·2 × 102 µm/mm muscularis mucosae (control 0·0 × 102µm/mm; P < 0·01). These significant expressions of MAdCAM-1 in the lamina propria were usually seen around the infiltrating cells expressing β7-integrin (Fig. 6). The correlation between the number of infiltrating cells and expression of MAdCAM-1 were determined from serial sections using NIH image and plotted. A statistically significant correlation could be found between the length of MAdCAM-1 positive vessels and the number of β7-positive infiltrating lymphocytes in the infected gastric mucosa (r = 0·915, P < 0·01).
Fig. 4.
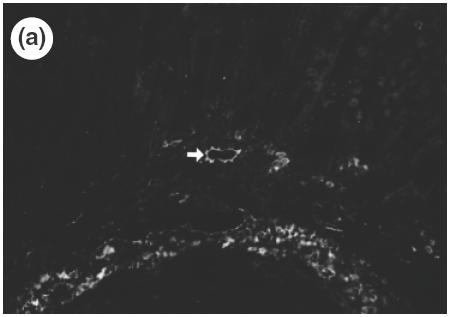
Expression of vascular adhesion molecules, ICAM-1 (a) (×100, arrow) and VCAM-1 (b) (×200) in the gastric mucosa of SS1-infected mice determined by immunohistochemistry.
Fig. 5.
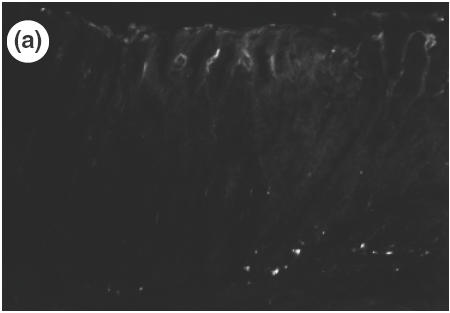
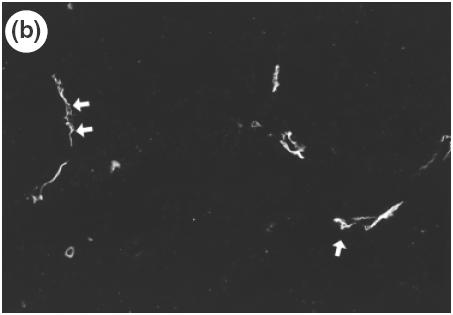
Expression of vascular adhesion molecule, MAdCAM-1 in the gastric mucosa of control mice (a) and SS1-infected mice (b) determined by immunohistochemistry (×200). MAdCAM-1 expression is observed in the lamina propria (left arrows) and in the submucosa (right arrow) of infected mucosa (b).
Fig. 6.
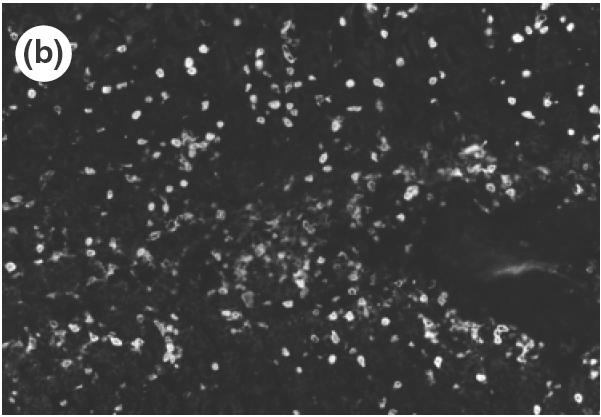
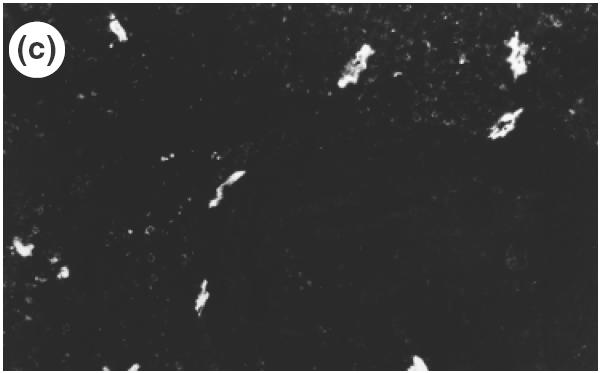
Localization of MAdCAM-1 expression and β7-integrin-positive cells in the lamina propria of SS1-infected mice using serial section of gastric mucosa (×200). (a) H&E staining. (b) β7-integrin-positive cells determined by immunohistochemistry of the serial section of the H&E picture. (c) MAdCAM-1 expression in the lamina propria determined by immunohistochemistry using the serial section.
DISCUSSION
Although there are many reports of CD4 lymphocytes participating in cell impairment and in the formation of H. pylori-induced gastritis [5,14,15], they do not fully explain the origin of lymphocytes or from which vascular addressin these cells were recruited to the gastric mucosa. Recently, Michetti et al. reported that gastric infection with H. felis induced a recruitment of CD4 T cells into gastric lamina propria and that a high proportion of them expressed alpha4beta7 integrin [16], although those authors did not analyse these cells quantitatively in histological sections. In this study, using well-orientated specimens, we studied the location and number of infiltrating cells in situ by immunohistochemistry. The present finding of a significant increase in CD4 T cell infiltration after infection into both the lamina propria and submucosa is consistent with their study, but the significant increase in B cell infiltration into the submucosa, which was not observed in the previous study, might result from the difference in the infection period (6 months versus 8 weeks) or a difference in Helicobacter species.
In our study most of the CD4 lymphocytes in the gastric mucosa of SS1-infected mice expressed β7-integrin, a lymphocyte marker originating from gut-associated mucosa. The critical role of β7-integrin in lymphocyte recruitment for the formation of the gut-associated lymphoid tissue has been proposed [17]. In the inflamed tissue, a significant number of mucosal lymphocytes could migrate aberrantly to non-originating organs, where these cells are seldom seen under non-inflamed conditions [18]. Dogan et al. examined the expression of α4β7 or B cells in the lymphoid follicle of the stomach induced by H. pylori infection [19]. It might be considered that H. pylori infection induces a host environment favourable for the accumulation of these lymphocytes as an autoimmune-like condition.
MAdCAM-1 is an immunogloblin superfamily adhesion molecule expressed on mucosal endothelium and a counter ligand for integrin α4β7 [8]. It should be noted that in this study increased expression of MAdCAM-1 was seen not only in the submucosa, but also in the lamina propria of the infected mucosa where β7 positive cells were infiltrated densely, whereas almost no MAdCAM-1 expression was seen in control stomach. So, it is considered that in H. pylori-gastritis inflammatory cells preferentially migrated into the gastric mucosa through venular endothelium which expresses MAdCAM-1. In this study up-regulation of VCAM-1 expression was limited only on venular endothelium in the submucosa, not in the mucosal area. Since VCAM-1 binds lymphocytes which express α4β1, but not α4β7 [20], we speculate that VCAM-1 plays little role in lymphocyte infiltration to the mucosal site. The differences in lymphocyte subsets binding to either MAdCAM-1 or VCAM-1 may be correlated to the delineation between mucosal and non-mucosal trafficking compartments, allowing for separation of specialized immune responses. In this study there were also VCAM-1-positive cells in the lamina propria of inflamed mucosa. Although we could not identify these cells, they may possibly include dendritic cells [21], fibroblast-like cells [22] or T cells [23]. In the H. pylori-infected stomach, ICAM-1 was observed in the submucosa and only a weak expression of ICAM-1 was observed in the lamina propria. However, because it is considered that ICAM-1 has a role in neutrophil infiltration in gastric epithelial cells [24], this adhesion molecule may not be involved actively in the attraction of CD4-β7 positive lymphocytes to the mucosal site.
In H. pylori-induced gastritis it is uncertain how MAdCAM-1 expressed aberrantly. Sikorski et al. investigated inducer of MAdCAM-1 in mouse venular endothelium [25]. They proved that LPS strongly induce MAdCAM-1 expression as well as TNF-α or IL-1β. LPS exists also in H. pylori, although it is considered to have less toxicity than that of Esherichia coli [26]. There is a possibility that H. pylori LPS itself can induce MAdCAM-1 expression in gastric mucosal venules, causing gastritis by aberrantly migrated β7-positive cells.
Acknowledgments
This study was supported in part by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture of Japan, and by grants from National Defense Medical College and Keio University, School of Medicine.
REFERENCES
- 1.Kuipers EJ, Thijs JC, Festen HP. Helicobacter and peptic ulcer desease. Aliment Pharmacol Ther. 1995;9(Suppl. 2):59–69. [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Scistosomes, liver flukes and Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–241. [PMC free article] [PubMed] [Google Scholar]
- 3.Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. N Engl J Med. 1994;330:1267–71. doi: 10.1056/NEJM199405053301803. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt JI, Rathbone BJ, Heatley RV. Local immune response to gastric Campylobacter in non-ulcer dyspepsia. J Clin Pathol. 1986;39:863–70. doi: 10.1136/jcp.39.8.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamford KB, Fan X, Crowe SE, et al. Lymphocytes in the human gastric mucosa during Helicobacter pylori have a T helper cell 1 phenotype. Gastroenterology. 1998;114:482–92. doi: 10.1016/s0016-5085(98)70531-1. [DOI] [PubMed] [Google Scholar]
- 6.D’Elios MM, Magnhetti M, De Carli M, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7. [PubMed] [Google Scholar]
- 7.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–6. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 8.Mackay CR, Andrew DP, Briskin M, Ringler DJ, Butcher EC. Phenotype, and migration properties of three major subsets of tissue homing T cells in sheep. Eur J Immunol. 1996;26:2433–9. doi: 10.1002/eji.1830261025. [DOI] [PubMed] [Google Scholar]
- 9.Kato S, Hokari R, Matsuzaki K, et al. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther. 2000;295:183–9. [PubMed] [Google Scholar]
- 10.Hokari R, Kato S, Matsuzaki K, et al. Involvement of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the pathogenesis of granulomatous colitis in rats. Clin Exp Immunol. 2001;126:259–65. doi: 10.1046/j.1365-2249.2001.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanninen A, Taylor C, Streeter PR, et al. Vascular addressins are induced on islet vessels during insulitis in nonobese diabetic mice and are involved in lymphoid cell binding to islet endothelium. J Clin Invest. 1993;92:2509–15. doi: 10.1172/JCI116859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill JK, Butter C, Baker D, et al. Expression of vascular addressins and ICAM-1 by endothelial cells in the spinal cord during chronic relapsing experimental allergic encephalomyelitis in the Biozzi AB/H mouse. Immunology. 1991;72:520–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Lee A, O'Rourke J, Corazon de Ungria M, et al. A standerdized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–97. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 14.Cacciarelli AG, Marano BJ, Jr, Gualtieri NM, et al. Lower Helicobacter pylori infection and peptic ulcer disease prevalence in patients with AIDS and suppressed CD4 counts. Am J Gastroenterol. 1996;91:1783–4. [PubMed] [Google Scholar]
- 15.Mohammadi M, Czinn S, Redline R, et al. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–38. [PubMed] [Google Scholar]
- 16.Michetti M, Kelly CP, Kraehenbuhl JP, Bouzourene H, Michetti P. Gastric mucosal α4β7-integrin-positive CD4 T lymphocytes and immune protection against Helicobacter infection in mice. Gastroenterology. 2000;119:109–18. doi: 10.1053/gast.2000.8548. [DOI] [PubMed] [Google Scholar]
- 17.Wagner N, Löhler J, Kunkel EJ, et al. Critical role for beta 7-integrin in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–70. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- 18.Hanninen A, Salmi M, Simell O, et al. Recirculation and homing of lymphocyte subsets: dual homing specificity of beta 7-integrin (high)-lymphocytes in nonobese diabetic mice. Blood. 1996;88:934–44. [PubMed] [Google Scholar]
- 19.Dogan AM, Du M, Koulis A, et al. Expression of lymphocyte homing receptors and vascular addressins in low-grade gastric B-cell lymphomas of mucosa-associated lymphoid tissue. Am J Pathol. 1997;151:1361–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Bradley LM, Watson SR. Lymphocyte migration into tissue: the paradigm derived from CD4 subsets. Curr Opin Immunol. 1996;8:312–20. doi: 10.1016/s0952-7915(96)80118-x. [DOI] [PubMed] [Google Scholar]
- 21.Koopman G, Parmentier HK, Schuurman HJ, Newman W, Meijer CJ, Pals ST. Adhesion of human B cells to follicular dendritic cells involves both the lymphocyte function-associated antigen 1/intercellular adhesion molecule 1 and very late antigen 4/vascular cell adhesion molecule 1 pathways. J Exp Med. 1991;173:1297–304. doi: 10.1084/jem.173.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marlor CW, Webb DL, Bombara MP, Greve JM, Blue ML. Expression of vascular cell adhesion molecule-1 in fibroblastlike synoviocytes after stimulation with tumor necrosis factor. Am J Pathol. 1992;140:1055–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Kitani A, Nakashima N, Matsuda T, et al. T cells bound by vascular cell adhesion molecule-1/CD106 in synovial fluid in rheumatoid arthritis: inhibitory role of soluble vascular cell adhesion molecule-1 in T cell activation. J Immunol. 1996;156:2300–8. [PubMed] [Google Scholar]
- 24.Mori N, Wada A, Hirayama T, Parks YP, et al. Activation of intercellular adhesion molecule 1 expression by Helicobacter pylori is regulated by NF-kappa B in gastric epithelial cancer cells. Infect Immun. 2000;68:1806–14. doi: 10.1128/iai.68.4.1806-1814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Sikorski EE, Hallmann R, Berg EL, et al. The Peyer's patch high endothelial receptor for lymphocytes, the mucosal vascular addressin, is induced on murine endothelial cell line by tumor necrosis factor-alpha and IL-1. J Immunol. 1993;151:5239–50. [PubMed] [Google Scholar]
- 26.Birkholz S, Knipp U, Nietzki C, et al. Immunological activity of lipopolysaccharide of Helicobacter pylori on human peripheral mononuclear blood cells in comparison to lipopolysaccharides of other intestinal bacteria. FEMS Immunol Med Microbiol. 1993;4:317–24. doi: 10.1111/j.1574-695X.1993.tb00344.x. [DOI] [PubMed] [Google Scholar]


