Abstract
Interleukin-10 (IL-10) is a mesangial cell growth factor in vivo and in vitro. However, the mechanism by which IL-10 exerts its mitogenic activity is not known. The aim of this study was to determine whether IL-10 induces mesangial cell proliferation in a PDGF-dependent or independent fashion. A well-characterized rat mesangial cell line (1097) was used in a series of cell proliferation experiments in which cells were serum-starved and then incubated with recombinant IL-10 in the presence or absence of STI 571 (a specific inhibitor of signalling via the PDGF-α and β receptors) or a neutralizing anti-PDGF-AB antibody. IL-10 induced significant mesangial cell proliferation at 24 and 48 h after cytokine addition. This response was inhibited totally by the addition of STI-571, demonstrating that IL-10 mitogenic activity has an absolute requirement for signalling through the PDGF receptor. In further studies, it was found that STI-571 could be added 24 h after IL-10 stimulation and still exert a profound inhibition of IL-10 mitogenic activity. The ability of a neutralizing anti-PDGF-AB antibody to inhibit completely IL-10-induced mesangial cell proliferation confirmed that IL-10 acts via induction of an autocrine PDGF response rather than the possibility that IL-10 may transactivate the PDGF receptor in a PDGF-independent fashion. In conclusion, this study has demonstrated that IL-10 induces mesangial cell proliferation via an autocrine PDGF-mediated mechanism. Thus, therapies which antagonize PDGF signalling will also inhibit any contribution of IL-10 to mesangial proliferation.
Keywords: interleukin-10, mesangial, PDGF, proliferation, STI-571
INTRODUCTION
Mesangial proliferation is a characteristic of a number of glomerular diseases, including IgA nephropathy, membranoproliferative glomerulonephritis and lupus nephritis. Mesangial proliferation and expansion is thought to lead to the development of glomerulosclerosis and progressive glomerular dysfunction [1]. Therefore, an understanding of the mechanisms that initiate and sustain mesangial proliferation may provide an insight into the pathogenesis of human disease and might identify potential therapeutic strategies.
Interleukin-10 (IL-10) is a cytokine that is produced predominantly by macrophages and T-cells [2], but also by glomerular mesangial cells [3,4]. Although best known as a regulator of T-cell and macrophage function [2], IL-10 has been shown to induce proliferation of cultured mesangial cells in a dose-dependent manner [4]. Furthermore, administration of IL-10 to normal rats caused glomerular hypercellularity and mesangial proliferation, as demonstrated by the presence of α-smooth muscle actin positive cells within mesangial areas expressing the proliferating cell nuclear antigen (PCNA) [4]. However, the mechanism(s) by which IL-10 exerts its mitogenic effect on mesangial cells is not known.
A variety of growth factors have been shown to be mitogenic for mesangial cells [1]. Of these, platelet-derived growth factor (PDGF) has been shown to be a potent mesangial growth factor in vitro and in vivo [5–10]. Indeed, most mesangial growth factors operate, at least in part, via induction of an autocrine PDGF-mediated mechanism, emphasizing the pivotal importance of PDGF in the regulation of mesangial cell proliferation [1,6].
Having identified IL-10 as a novel mesangial cell growth factor, it is important, therefore, to determine whether it operates in a PDGF-dependent or independent fashion. To this end, we used a well-characterized rat mesangial cell line to examine the effects of STI-571, a specific inhibitor of the Abelson tyrosine kinase and the highly homologous PDGF receptor kinase [11,12], and of a neutralizing anti-PDGF antibody on IL-10-induced mesangial cell proliferation.
MATERIALS AND METHODS
Cell lines
A well-characterized, cloned mesangial cell line (1097) isolated from Sprague–Dawley rats [13], was used between passages 20 and 30. Cells (n = 1097) were cultured in DMEM medium (Sigma-Aldrich, Castle Hill, NSW, Australia) supplemented with 10% fetal calf serum (FCS, Trace Scientific, Melbourne, VIC, Australia), 100 U/ml penicillin and 100 µg/ml streptomycin in humidified 5% CO2 atmosphere at 37°C. NRK52E rat tubular epithelial cells (European Collection of Cell Culture, CAMR, Salisbury, Wiltshire, UK), were cultured under the same conditions.
Reagents
Recombinant cytokines used were murine IL-10 (PeproTech, Rocky Hill, NJ, USA) and human PDGF-AB (Roche Diagnostics, Castle Hill, NSW, Australia). STI 571, a specific inhibitor of the kinase activity of the PDGF-α and β receptors [11,12], was a generous gift from Novartis Pharmaceuticals (Sydney, Australia). STI-571 was dissolved in sterile water to make a stock solution of 10 mmol/l and then diluted in culture medium. A PDGF-AB neutralizing antibody was purchased from Upstate Biotechnology (Lake Placid, NY, USA).
Proliferation assays
Mesangial cells were plated at 2 × 103 cells per well in 96-well flat-bottomed microtitre plates in DMEM/10% FCS and allowed to adhere overnight. The subconfluent cells were then starved for 3 days in DMEM/0·5% FCS. Recombinant IL-10 or PDGF (in the presence or absence of STI-571 or anti-PDGF antibodies) was added to the cells and proliferation determined 24 or 48 h later by the addition of 0·65 µCi [3H]-thymidine to each well during the last 6 h of culture. After washing twice in PBS, cells were solubilized in 0·2 mol/l NaOH. The lysate then was neutralized with HCl and then UltimaGold scintillation fluid (Packard Bioscience, Groningen, the Netherlands) was added and radioactive emissions determined using a β-counter (Wallace Rack-beta, Wallac Oy, Turku, Finland). Replicates of six wells were used in each experiment. All experiments were performed at least three times.
Additional proliferation assays were performed under serum-free conditions. Mesangial cells were plated in DMEM/10% FCS and allowed to adhere overnight. The subconfluent cells were then starved for 2 days in serum-free DMEM. Recombinant IL-10 or PDGF (in the presence or absence of STI-571) was added to the cells, still under serum-free conditions, and proliferation determined 48 h later.
Statistics
Data were compared by analysis of variance (anova) with the Bonferroni multiple comparison post-test using the GraphPad Prism 3·0 program (GraphPad software, San Diego, CA, USA).
RESULTS
IL-10 induces mesangial cell proliferation via the PDGF receptor
To determine whether the mitogenic activity of IL-10 operates via a PDGF-dependent mechanism, we used STI-571 (previously known as CGP 57148), which is a specific inhibitor of the tyrosine kinase activity of PDGF-α and PDGF-β receptors [11,12]. To this end, we used STI-571 at between 0·5 and 2 µmol/l, a concentration range that we have shown previously to inhibit PDGF-induced mesangial cell proliferation without any toxic effect [10]. As shown in Fig. 1a, the addition of STI-571 to cells 30 min prior to the addition of IL-10 completely inhibited IL-10 mitogenic activity in 24 and 48 h proliferation assays. As a control, STI-571 was also shown to inhibit PDGF-AB induced proliferation (Fig. 1b). In these studies, STI-571 caused no cell detachment, alteration of nuclear morphology or cell death. As an additional specificity control, 2 µmol/l STI-571 was found to have no effect upon proliferation of NRK52E tubular epithelial cells – a cell line which does not proliferate in response to PDGF (data not shown).
Fig. 1.
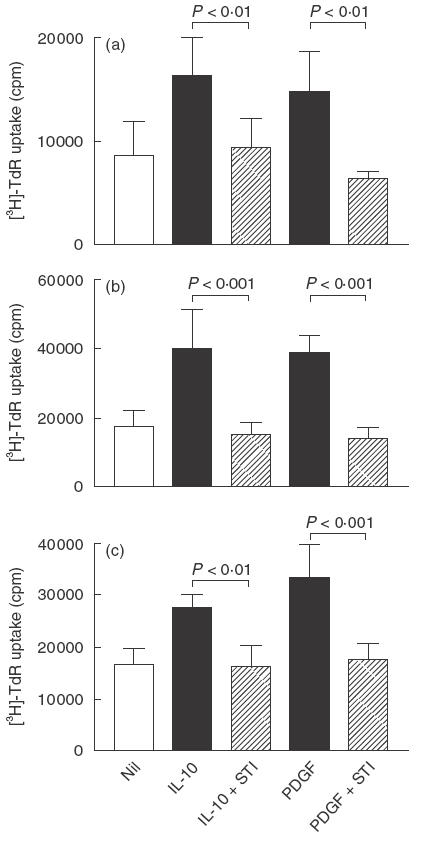
IL-10-induced mesangial cell proliferation is blocked by STI 571, a specific inhibitor of signalling through the PDGF receptor. 1097 rat mesangial cells were starved in 0·5% FCS for 3 days and then incubated with 20 ng/ml IL-10 or 5 ng/ml PDGF-AB (positive control), and proliferation was measured by incorporation of [3H]-thymidine (TdR) during the last 6 h of: (a) 24 h or (b) 48 h assays. Cells were incubated with 2 µm STI-571 for 30 min before the addition of IL-10 or PDGF-AB. (c) 1097 rat mesangial cells were starved in serum-free medium for 2 days and then incubated with 50 ng/ml IL-10 or 5 ng/ml PDGF-AB (positive control), plus or minus 0·5 µm STI-571, and then proliferation assessed 48 h later. Data are shown as mean ± s.d. A comparison between growth factor alone, or with STI-571, was made by anova with Bonferroni post-test analysis. One of five replicate experiments is shown.
In a separate series of experiments using a 48-h assay, it was shown that IL-10-induced mesangial cell proliferation was inhibited completely by STI-571, even when drug addition was delayed 1 or 24 h after IL-10 stimulation (Fig. 2). In this context, it is important to remember that proliferation was measured by the addition of [3H]-thymidine to cells during the last 6 h of culture.
Fig. 2.
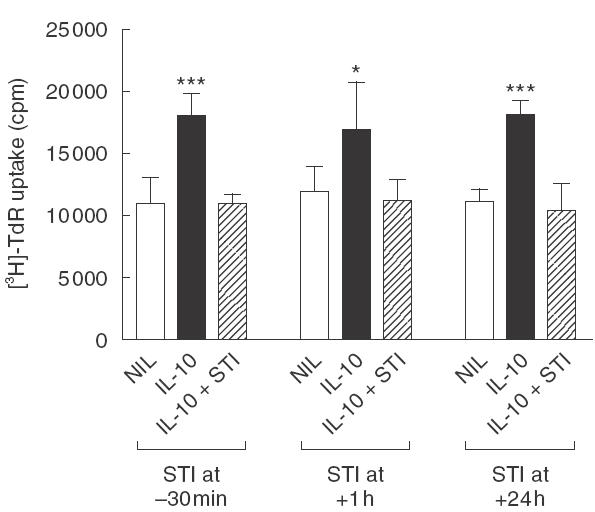
Delayed addition of STI-571, an inhibitor of PDGF receptor signalling, blocks IL-10-induced mesangial cell proliferation. STI-571 (2 µmol/l) was added to 1097 rat mesangial cells starved in 0·5% FCS at 30 min prior to, at 1 h after, or at 24 h after the addition of 100 ng/ml IL-10. Cell proliferation was determined 48 h after growth factor addition by incorporation of [3H]-thymidine (TdR) during the last 6 h of culture. Data are shown as mean ± s.d. *P < 0·05, **P < 0·01, ***P < 0·001 versus Nil and versus growth factor plus STI-571 by anova with Bonferroni post-test analysis. One of three replicate experiments is shown.
The mitogenic effect of IL-10 was not dependent upon the presence of small amounts of serum in the culture as demonstrated by the ability of IL-10 to stimulate mesangial cell proliferation under serum-free conditions. IL-10 induced mesangial cell proliferation under serum-free conditions was completely inhibited by the addition of STI-571 (Fig. 1c).
IL-10 operates via an autocrine PDGF mechanism
The finding that STI-571 inhibits IL-10 induced mesangial cell proliferation demonstrates that signalling via the PDGF receptor is required for IL-10 mitogenic activity. However, recent studies have shown that growth factors, such as angiotensin II, can transactivate the PDGF receptor kinase and thus induce PDGF receptor signalling — without the need for PDGF binding to its receptor [14,15]. Therefore, we determined the role of PDGF in IL-10 mitogenic activity using a neutralizing rabbit polyclonal antibody which blocks the mitogenic effects of the different forms of PDGF (AA, BB, AB). Using a concentration of antibody known to inhibit PDGF-AB induced mesangial cell proliferation, it was found that the anti-PDGF-AB antibody completely inhibited IL-10-induced mesangial cell proliferation (Fig. 3).
Fig. 3.
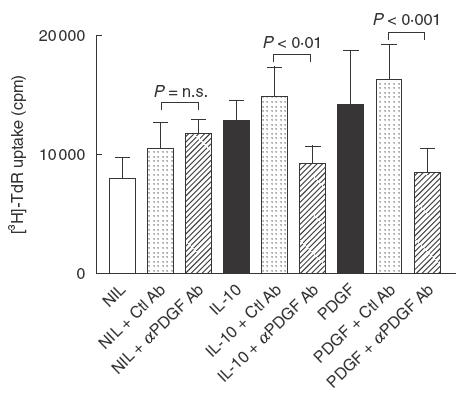
Anti-PDGF antibody inhibits IL-10-induced mesangial cell proliferation. 1097 rat mesangial cells were starved in 0·5% FCS and then stimulated with 50 ng/ml IL-10 or 2 ng/ml PDGF-AB (positive control), in the presence or absence of 25 µg/ml of a neutralizing anti-PDGF-AB antibody (Ab) or normal rabbit IgG (Ctl Ab), and proliferation measured 48 h later by incorporation of [3H]-thymidine (TdR) during the last 6 h of culture. Data are expressed as mean ± s.d. A comparison between growth factor with control antibody or anti-PDGF antibody was made by anova with Bonferroni post-test analysis. One of three replicate experiments is shown.
DISCUSSION
This study has demonstrated that IL-10 stimulates mesangial cell proliferation via an autocrine PDGF-dependent mechanism. This is based upon two different experimental approaches: inhibition of PDGF receptor signalling via STI-571 and antibody-mediated neutralization of PDGF.
This study confirms the predominant role of PDGF in regulating mesangial cell proliferation in vitro and in vivo [1,5–10]. IL-10 can now be added to the list of growth factors which stimulate mesangial cell proliferation in a PDGF-dependent fashion, including: epidermal growth factor, thrombin, basic fibroblast growth factor, endothelin-1, thrombospondin and angiotensin II [6,16–20]. There are two major mechanisms by which growth factors can trigger PDGF-dependent signalling.
First, growth factors such as epidermal growth factor (EGF) induce mesangial cells to secrete PDGF [6,17]. The secreted PDGF then binds to its cell-surface receptor, triggering autophosphorylation of the receptor cytoplasmic domain, which then becomes an active tyrosine kinase. The receptor kinase then phosphorylates cytoplasmic kinases which, via a cascade of phosphorylation reactions, activate the extracellular-regulated kinase (ERK), thereby inducing entry into the cell cycle [1,21].
Secondly, growth factors whose receptors have a tyrosine kinase activity, such as angiotensin II, have been shown to transactivate the PDGF receptor independently of PDGF interact-ing with its receptor. For example, angiotensin II binding to the angiotensin II receptor induces phosphorylation of a 66-kDa Shc kinase which, in turn, phosphorylates and so activates the PDGF receptor [14,15].
We found that IL-10 induces mesangial cell proliferation via the first mechanism. This is based upon the ability of STI-571 to inhibit mesangial cell proliferation even when added 24 h after IL-10 stimulation and the ability of a neutralizing anti-PDGF antibody to inhibit IL-10-induced mesangial cell proliferation. The fine detail of the mechanism by which IL-10 stimulates autocrine PDGF-mediated proliferation has not been examined. While it is clear that IL-10 induces PDGF release from mesangial cells in order to mediate autocrine proliferation, it is not known whether this involves increased PDGF gene transcription, PDGF mRNA stabilization, increased PDGF protein synthesis, or changes in expression of the PDGF receptor.
An intriguing aspect of IL-10 is that it has opposite effects on the proliferative response depending upon the cell type examined. We have shown that IL-10 stimulates autocrine PDGF mediated mesangial cell proliferation – a mechanism that operates via the ERK pathway [21]. In contrast, M-CSF dependent macrophage proliferation – that also operates via the ERK pathway – is inhibited by IL-10 [22,23]. This emphasizes that the action of individual growth factors can be highly cell-type-specific.
In summary, this study has demonstrated that IL-10 induces mesangial cell proliferation via an autocrine PDGF-mediated mechanism. Thus, therapies which antagonize PDGF signal-ling will also inhibit any contribution of IL-10 to mesangial proliferation.
Acknowledgments
This work was funded by the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Rupprecht HD, Schocklmann HO, Sterzel RB. Glomerular mesangial cells. In: Neilson EG, Couser WG, editors. Immunologic renal diseases. Philadelphia: Lippincott, Williams & Wilkins; 2001. pp. 657–91. [Google Scholar]
- 2.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 3.Fouqueray B, Boutard V, Phillipe C, et al. Mesangial cell derived interleukin-10 modulates mesangial cell response to lipopolysaccharide. Am J Pathol. 1995;147:176–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Chadban SJ, Tesch GH, Foti R, Atkins RC, Nikolic-Paterson DJ. Interleukin-10 is a mesangial cell growth factor in vitro and in vivo. Lab Invest. 1997;5:619–27. [PubMed] [Google Scholar]
- 5.Shultz PJ, DiCorleto PE, Silver BJ, Abboud HE. Mesangial cells express PDGF mRNAs and proliferate in response to PDGF. Am J Physiol. 1988;255:F674–84. doi: 10.1152/ajprenal.1988.255.4.F674. [DOI] [PubMed] [Google Scholar]
- 6.Sliver BJ, Jaffer FE, Abboud HE. Platelet-derived growth factor synthesis in mesangial cells: induction by multiple peptide mitogens. Proc Natl Acad Sci USA. 1989;86:1056–60. doi: 10.1073/pnas.86.3.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson RJ, Raines EW, Floege J, et al. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992;175:1413–6. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Floege J, Eng E, Young BA, et al. Infusion of platelet-derived growth factor or basic fibroblast growth factor induces selective glomerular mesangial cell proliferation and matrix accumulation in rats. J Clin Invest. 1993;92:2952–62. doi: 10.1172/JCI116918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Floege J, Ostendorf T, Janssen U, et al. Novel approach to specific growth factor inhibition in vivo: antagonism of platelet-derived growth factor in glomerulonephritis by aptamers. Am J Pathol. 1999;154:169–79. doi: 10.1016/S0002-9440(10)65263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert RE, Kelly DJ, McKay T, et al. PDGF signal transduction inhibition ameliorates experimental mesangial proliferative glomerulonephritis. Kidney Int. 2001;59:1324–32. doi: 10.1046/j.1523-1755.2001.0590041324.x. [DOI] [PubMed] [Google Scholar]
- 11.Druker BJ, Tamura S, Buchdunger E, et al. Effects of selective inhibitor of the Abl tyrosine kinase in the growth of Bcr-abl positive cells. Nat Med. 1996;2:561–6. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 12.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–42. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 13.Kakizaki Y, Kraft N, Atkins RC. Differential control of mesangial cell proliferation by interferon-gamma. Clin Exp Immunol. 1991;85:157–63. doi: 10.1111/j.1365-2249.1991.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linseman DA, Benjamin CW, Jones DA. Convergence of angiotensin II and platelet-derived growth factor receptor signaling cascades in vascular smooth muscle cells. J Biol Chem. 1995;270:12563–8. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- 15.Heeneman S, Haendeler J, Saito Y, Ishida M, Berk BC. Angiotensin II induces transactivation of two different populations of the platelet-derived growth factor beta receptor. Key role for the p66 adaptor protein Shc. J Biol Chem. 2000;275:15926–32. doi: 10.1074/jbc.M909616199. [DOI] [PubMed] [Google Scholar]
- 16.Shultz PJ, Knauss TC, Mene P, Abboud HE. Mitogenic signals for thrombin in mesangial cells: regulation of phospholipase C and PDGF genes. Am J Physiol. 1989;257:F366–74. doi: 10.1152/ajprenal.1989.257.3.F366. [DOI] [PubMed] [Google Scholar]
- 17.Gesualdo L, Di Paolo S, Ranieri E, Schena FP. Trapidil inhibits human mesangial cell proliferation: effect on PDGF beta-receptor binding and expression. Kidney Int. 1994;46:1002–9. doi: 10.1038/ki.1994.360. [DOI] [PubMed] [Google Scholar]
- 18.Jaffer FE, Knauss TC, Poptic E, Abboud HE. Endothelin stimulates PDGF secretion in cultured human mesangial cells. Kidney Int. 1990;38:1193–8. doi: 10.1038/ki.1990.333. [DOI] [PubMed] [Google Scholar]
- 19.Marinides GN, Suchard SJ, Mookerjee BK. Role of thrombospondin in mesangial cell growth: possible existence of an autocrine feedback growth circuit. Kidney Int. 1994;46:350–7. doi: 10.1038/ki.1994.281. [DOI] [PubMed] [Google Scholar]
- 20.Higueruelo S, Romero R. Angiotensin II requires PDGF-BB to induce DNA synthesis in rat mesangial cells cultured in an exogenous insulin-free medium. Nephrol Dial Transplant. 1997;12:694–700. doi: 10.1093/ndt/12.4.694. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury GG, Karamitsos C, Hernandez J, Gentilini A, Bardgette J, Abboud HE. PI-3-kinase and MAPK regulate mesangial cell proliferation and migration in response to PDGF. Am J Physiol. 1997;273:F931–8. doi: 10.1152/ajprenal.1997.273.6.F931. [DOI] [PubMed] [Google Scholar]
- 22.O'Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaworowski A, Wilson NJ, Christy E, Byrne R, Hamilton JA. Roles of the mitogen-activated protein kinase family in macrophage responses to colony stimulating factor-1 addition and withdrawal. J Biol Chem. 1999;274:15127–33. doi: 10.1074/jbc.274.21.15127. [DOI] [PubMed] [Google Scholar]


