Abstract
Periodontitis is an inflammatory bone disease caused by Gram-negative anaerobic bacteria, but the precise mechanism of bone destruction remains unknown. Activated T lymphocytes secrete receptor activator of NF-κB ligand (RANKL) and support the differentiation of monocytes into mature osteoclasts. The purpose of this study was to examine the expression of RANKL and its inhibitor, osteoprotegerin (OPG), in inflamed gingival tissue and to clarify the role of human gingival fibroblasts (HGFs) in osteoclastogenesis regulated by RANKL. HGFs and gingival mononuclear cells (GMCs) were obtained from chronic periodontitis patients during routine periodontal surgery. Expression of OPG and RANKL mRNA in gingival tissue and HGFs was examined with RT-PCR. OPG production was measured using ELISA. Expression of RANKL, CD4, CD8 and CD69 on GMCs was determined by flow-cytometry using RANK-Fc fusion protein and the respective monoclonal antibodies. Osteoclastogenesis by RANKL was assayed by counting the number of tartarate-resistant acid phosphatase (TRAP)-positive cells after culturing human peripheral blood monocytes with recombinant human RANKL and macrophage-colony stimulating factor (M-CSF) for 10 days. OPG and RANKL mRNA were expressed in 80% (16/20) and 25% (5/20) of periodontitis lesions, respectively. OPG, but not RANKL, mRNA was expressed within HGFs. OPG mRNA expression and production by HGFs was augmented by LPS stimulation. All GMC samples expressed CD69, and two of five GMC samples expressed RANKL. The culture supernatant of LPS-stimulated gingival fibroblasts significantly reduced the number of TRAP positive cells generated by culturing monocytes with RANKL and M-CSF. The present study suggests that LPS-stimulated HGFs inhibit monocyte differentiation into osteoclasts through the production of OPG.
Keywords: fibroblasts, osteoprotegerin, periodontitis, RANKL, T-cells
INTRODUCTION
Periodontitis is an inflammatory disease caused by Gram-negative periodontopathic bacteria. Many studies have demonstrated local accumulation of activated lymphocytes, macrophages and neutrophils in inflamed gingival tissue [1]. Infiltrating leucocytes interact with other resident cells in the gingiva to induce inflammatory reactions that degrade connective tissue and enhance alveolar bone resorption. Human gingival fibroblasts (HGFs) are a major constituent of gingival connective tissue and they regulate retention and activation of leucocytes in inflamed gingival tissue through their expression of cell adhesion molecules [2]. The role of gingival fibroblasts in leucocyte-mediated bone destruction, however, is still not understood completely.
Under normal physiological conditions, bone is resorbed periodically by osteoclasts while new bone is formed by osteoblasts [3]. Osteoblasts regulate osteoclastic bone resorption, which involves recruitment of new osteoclasts and activation of mature osteoclasts [3]. The recruitment of new osteoclasts is dependent on the balance between receptor activator of NFκB ligand (RANKL) and its decoy receptor, osteoprotegerin (OPG), in osteoblasts [3,4]. Mice with disrupted RANKL genes exhibited severe osteopetrosis and a complete lack of osteoclast activity as a result of the inability of their osteoblasts to support osteoclastogenesis [5], indicating that osteoclast formation is dependent largely upon osteoblast RANKL production. While T lymphocytes also produce RANKL [6] they may not be involved in bone resorption under normal physiological circumstances, because mice lacking T lymphocytes demonstrated normal bone metabolism [7].
In the presence of inflammatory bone disease, however, activated T lymphocytes might mediate bone destruction through excessive production of soluble RANKL. RANKL mRNA was detected in T lymphocytes isolated from rheumatoid arthritis lesions, and activated T lymphocytes have been observed to support osteoclast formation in vitro [8]. In addition, OPG administration reduced bone destruction by activated RANKL-producing T cells in mice with adjuvant arthritis [7], without affecting inflammatory status.
The purpose of this study was to examine the possible roles of RANKL and OPG in alveolar bone destruction resulting from human chronic periodontitis and to determine the function of gingival fibroblasts in RANKL-mediated osteoclast formation.
MATERIALS AND METHODS
Reagents
Recombinant human RANK/Fc chimera were purchased from Genzyme (MA, USA). Recombinant human soluble RANKL was purchased from Chemicon International (CA, USA). Recombinant human macrophage-colony stimulating factor (M-CSF), polymyxin B and Escherichia coli LPS (serotype 055:B5) were purchased from Sigma (MO, USA). Recombinant human OPG-Fc chimera, monoclonal anti-OPG (no. 438051) and biotinylated anti-OPG (no. 44805) antibodies were purchased from Genzyme.
Preparation of gingival mononuclear cells (GMCs) and human gingival fibroblasts (HGFs) from gingival tissue
Samples of gingival tissue were obtained from 30 chronic periodontitis patients and two healthy subjects after acquiring informed consent. The patients were diagnosed as chronic periodontitis and had received initial periodontal therapy [9]. Samples were collected from the sites which had responded poorly to the initial therapy and had to be subjected to surgical therapy. Mean and deepest alveolar bone resorption values of the sampled sites were measured according to Schei's method [10], and mean and deepest pocket depths were measured before the surgical procedure.Twenty, five and five samples were used for mRNA extraction, GMC preparation and HGF establishment, respectively.
GMCs were prepared as described previously [11]. Each tissue sample was cut into the smallest possible pieces using scissors, and incubated with 2·4 U/ml of grade II dispase solution (Boehringer-Mannheim Biochemica, Germany) for 30 min at 37°C, with gentle agitation. Cells released into the supernatant were layered onto Lymphoprep (Gallard-Schlesinger Industries, Norway) within a 14-ml conical tube. Following centrifugation at 370 g for 30 min, GMCs were collected from the interface. The GMCs were then suspended in RPMI supplemented with 10% fetal bovine serum at 1·0 × 106 cells/ml.
HGFs were prepared as described previously [2]. Each sample of gingival tissue was cut into small pieces and cultured in α-minimum essential medium (α-MEM) supplemented with 10% fetal bovine serum. Fibroblast cells growing from the explanted tissue were subcultured. HGFs from passage levels 4–6 were used in this study.
RNA extraction and RT-PCR
RNA was extracted from 20 gingival tissue samples and cultured HGFs by the acid guanidium thiocyanate–phenol–chloroform method. Immediately after periodontal surgery, each tissue sample was solubilized in a sterile tube containing RNAzol B solution (Cinna/Biotecx Laboratory, Inc., Houston, TX, USA) with a homogenizer (mini cordless grinder, Funakoshi, Tokyo, Japan). Cultured HGFs were washed three times with α-MEM and collected by use of a sterile scraper after RNAzol B was added to their culture dish. RNA was extracted with phenol and chloroform, precipitated with isopropanol, washed with 80% ethanol and suspended in distilled water. RNA purity was confirmed with 260/280 O.D. spectrophotometry. The 260/280 readings obtained ranged from 1·6 to 1·9. Samples containing 1 µg of RNA were used for reverse transcriptase polymerase chain reaction (RT-PCR). RT-PCR was performed with a one-step RT-PCR kit using rTth DNA polymerase (RT-PCR High-Plus-kit, Toyobo, Osaka, Japan). The cDNA was amplified in the presence of human RANKL, OPG or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers. The RANKL and OPG primers were designed on the basis of sequences described by Horwood [8]. The following primers were used:
RANKL primer R; 5′-TGGATCACAGCACATCAGAGCAG-3′,
RANKL primer F; 5′-TGGGGCTCAATCTATATCTCGAAC-3′,
OPG primer R; 5′-GGGGACCACAATGAACAAGTTG-3′,
OPG primer F; 5′-AGCTTGCACCACTCCAAATCC-3′,
GAPDH primer R; 5′-TCCACCACCCTGTTGCTGTA-3′ and
GAPDH primer F; 5′-ACCACAGTCCATGCCATCAC-3′.
Reactions were carried out at 60°C for 30 min, followed by 94°C for 2 min. Reactions were allowed to continue for 35 cycles (gingival tissue) or 30 cycles (HGFs) at 94°C for 1 min, followed by 60°C for 1·5 min, in a thermal cycler (GeneAmp PCR system 9700, Perkin Elmer, Norwalk, CT, USA). Ten microlitres of PCR product were subjected to electrophoresis on 1% agarose, and visualized using ethidium bromide. Fluorescence intensity was calculated using the SYNGENE Bio Imaging system (SYNGENE, MD, USA).
Flow cytometry
A 100-µl aliquot of diluted GMC or PBMC suspension was reacted with FITC-conjugated anti-CD8, PE-conjugated anti-CD4 and Per-CP conjugated anti-CD69. In order to detect RANKL expression, GMCs or PBMCs were reacted with 10 µg/ml of recombinant human RANK/Fc chimera, followed by FITC-conjugated protein A. Flow cytometric analysis was performed using a FACScan flow cytometer as described previously [12].
Elisa
HGFs were seeded in 96-well flat-bottomed culture plates at 1 × 105 cells per well, and were grown to confluence (mean; 2 × 105 cells per well). Once confluent, the fibroblasts were cultured with or without 1 µg/ml of E. coli LPS. OPG in the culture supernatants was measured using ELISA, and expressed as ng/1 × 106 cells. Human OPG ELISA was performed according to the manufacturer's protocol (Genzyme). In brief, 2 µg/ml of monoclonal anti-OPG antibody (Genzyme) was used to coat 96-well ELISA plates, which were then incubated overnight at room temperature. The plates were blocked with PBS containing 1% bovine serum albumin for 1 h at room temperature. After washing, samples were added to each of the wells and incubated for 2 h. The recombinant human OPG-Fc chimera was used as standard OPG protein. The plates were washed and 100 ng/ml of biotinylated anti-OPG antibody (Genzyme) was added to each well and allowed to incubate for 2 h. After washing, streptavidin alkaline phosphatase (Genzyme) was added to each well and the wells were incubated for another 30 min. Following washing, substrate solution (Genzyme) was added and absorbance recorded using an ELISA reader.
Effect of supernatant from HGF culture on the differentiation of human PBMCs into preosteoclasts
Human peripheral blood was collected from healthy normal donors. PBMCs were isolated by centrifugation, washed and resuspended at 2·0 × 106 cells/ml in α-MEM supplemented with 10% fetal bovine serum and polymyxin B. PBMCs were then cultured for 10 days in 96-well plates (1 × 106 cells/well) in the presence or absence of human RANKL, human M-CSF, anti-human OPG and supernatant from cultures of gingival fibroblasts. After culture for 10 days, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP). Cells were fixed with 10% formalin for 10 min, and stained with acid phosphatase in the presence of 0·05 m sodium tartrate (Sigma). The substrate used was naphthol AS-BI phosphate (Sigma).
Statistical analysis
The Mann–Whitney U-test was used for statistical analysis.
RESULTS
RANKL and OPG mRNA were expressed in inflamed gingival tissue
We obtained PCR products of RANKL and OPG from gingival tissue. The PCR products obtained were of the same size as estimated from the DNA sequences of RANKL and OPG. OPG and RANKL mRNA were expressed in 80% (16/20) and 25% (5/20) of the inflamed gingival tissue samples, respectively (Table 1). OPG was also expressed in healthy tissue (2/2), whereas RANKL was not detected in these tissues (0/2). Sites expressing RANKL had deeper pocket depth compared with RANKL-negative sites (Table 1, P < 0·05).
Table 1.
RNA was extracted from gingival tissue, and RT-PCR analysis for OPG and RANKL was carried out as described in the Materials and methods. Expression intensity of OPG and GAPDH mRNA was calculated with a SYNGENE Bio Imaging system. Clinical parameters of the sites expressing OPG and RANKL mRNA are shown
| Expression (n) | Mean pocket depth (mm) | Deepest pocket depth (mm) | Mean bone loss (%) |
|---|---|---|---|
| RANKL | |||
| +(n = 5) | 3·83 ± 0·55* | 8·00 ± 1·78 | 52·5 ± 12·8 |
| −(n = 15) | 27·5 ± 0·20* | 56·7 ± 0·77 | 37·5 ± 12·8 |
| OPG | |||
| +(n = 16) | 2·96 ± 0·25 | 5·64 ± 0·78 | 39·5 ± 4·27 |
| −(n = 4) | 2·45 ± 0·23 | 5·50 ±0·50 | 43·5 ± 8·50 |
P < 0·05; mean ± s.e.
Time-course of OPG mRNA expression in LPS-stimulated HGFs
OPG and RANKL mRNA expression in LPS-stimulated HGFs was analysed by RT-PCR. HGFs expressed OPG (Fig. 1a), but not RANKL (data not shown). The fluorescence intensity of OPG and GAPDH mRNA was calculated, and the OPG/GAPDH ratio was determined (Fig. 1b). After stimulation with LPS, OPG/GAPDH ratio increased until 24 h, and then returned to normal after 48 h.
Fig. 1.
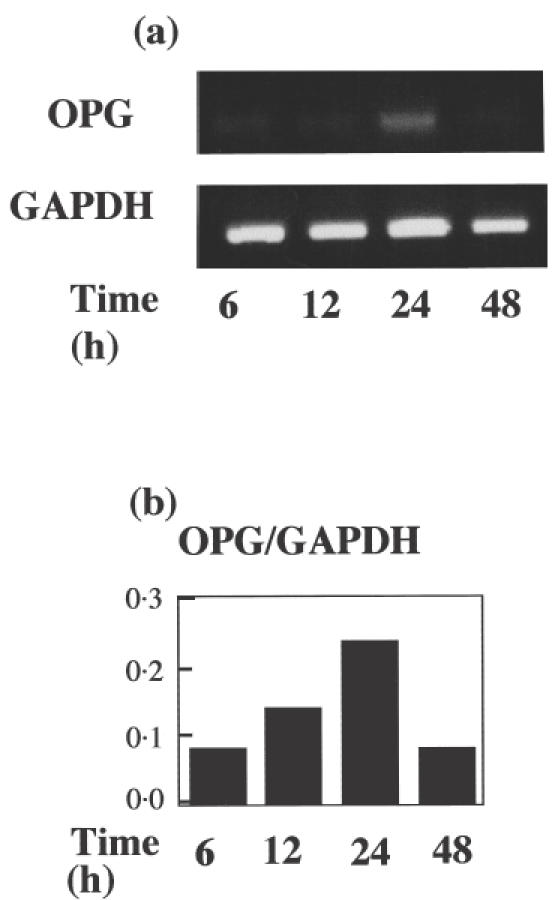
(a) Time-course of OPG and GAPDH mRNA expression in HGFs stimulated with E. coli LPS. HGFs were stimulated with E. coli LPS and collected 6, 12, 24 and 48 h after stimulation. RT-PCR analysis for OPG and GAPDH was carried out as described in the Materials and methods. The results were representative of five timed experiments. (b) OPG/GAPDH mRNA ratio in HGFs stimulated with E. coli LPS. Expression intensity of OPG and GAPDH mRNA was calculated with a SYNGENE Bio Imaging system and the OPG/GAPDH mRNA ratio was calculated. The results were representative of five experiments.
Production of OPG by HGFs stimulated with LPS
OPG and RANKL expression was examined in different HGF lines by RT-PCR. Representative results from three HGF lines are shown in Fig. 2a. All HGF lines constitutively expressed OPG mRNA and expression was augmented by culturing fibroblasts with LPS for 24 h. RANKL mRNA expression was not observed in any HGF line, either before or after stimulation with LPS (data not shown).
Fig. 2.
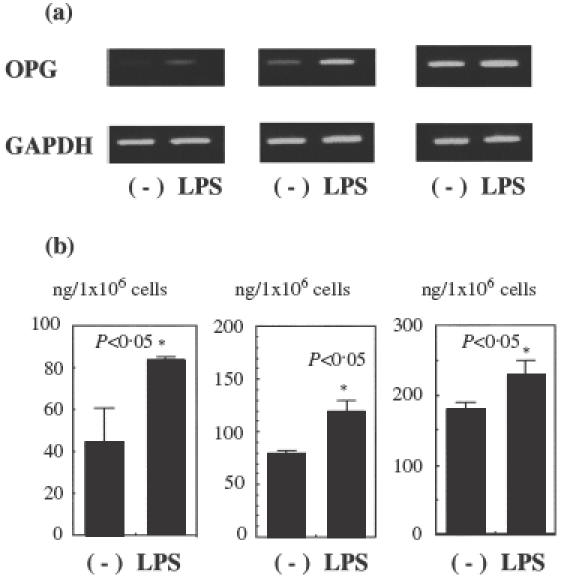
(a) Expression of OPG and GAPDH mRNA in different HGF lines following stimulation with E. coli LPS. HGFs were stimulated with E. coli LPS and collected 24 h after stimulation. RT-PCR analysis for OPG and GAPDH was carried out as described in the Materials and methods. The results were representative of five experiments. (b) Production of OPG in different HGF lines stimulated with E. coli LPS. HGFs were stimulated with E. coli LPS and their culture supernatant collected 24 h after stimulation. The relative amount of OPG within the supernatant of each culture was measured using ELISA as described in the Materials and methods. The results were representative of five experiments.
Secretion of OPG by HGFs was examined using ELISA, and representative results of three fibroblast lines are shown in Fig. 2b. All HGF lines produced OPG and production was augmented by LPS stimulation. The relative amount of OPG produced varied among the three HGF lines examined, ranging from 40 to 300 ng/ml.
Expression of CD69 and RANKL on GMCs
Activated T cells are known to express CD69 on their cell surface. CD69 was detected on 65 ± 11% of CD4+ GMCs and 65 ± 2·2% of CD8+ GMCs, whereas less than 10% of CD4+ and CD8+ PBMCs expressed CD69 (Fig. 3a).
Fig. 3.
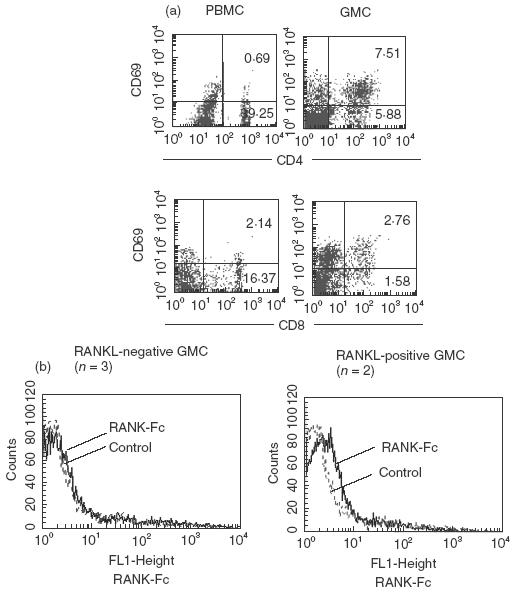
(a) Expression of CD69 in CD4+ and CD8+ GMCs. GMCs were prepared from inflamed gingival tissue and stained with anti-CD4, CD8 and CD69 antibodies. Representative results from five experiments are shown. (b) Expression of RANKL on GMCs. GMCs were prepared from inflamed gingival tissue and stained with RANK-Fc fusion protein, followed by FITC-conjugated protein A. Representative results from five experiments are shown.
Expression of RANKL on GMCs was examined with the use of RANK-Fc fusion protein. Figure 3b shows the representative results of RANKL expression on GMCs. Of five GMC samples, two samples stained positively with RANK-Fc fusion protein.
Culture supernatant of LPS-stimulated gingival fibroblasts inhibited differentiation of monocytes into mature osteoclasts
Human peripheral blood monocytes were cultured with RANKL and M-CSF for 10 days and observed for differentiation into TRAP-positive cells. The number of TRAP-positive cells increased according to the concentration of RANKL in culture (Fig. 4a). Addition of supernatant from HGF culture partially inhibited the generation of TRAP-positive cells; moreover, supernatant from LPS-stimulated HGFs significantly inhibited the generation of TRAP-positive cells (Fig. 4a).
Fig. 4.
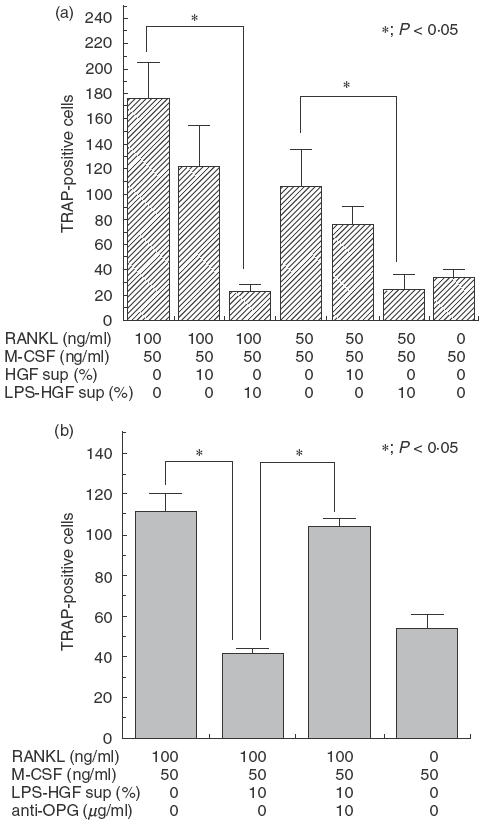
(a) Effect of HGF culture supernatant on the differentiation of monocytes into TRAP-positive cells. Peripheral blood monocytes were cultured with RANKL and M-CSF in the presence or absence of supernatant from HGF culture (HGF sup) or supernatant from an E. coli LPS-stimulated HGF culture (LPS-HGF sup). The number of TRAP-positive cells in each sample was calculated 10 days after cultivation. The results were representative of five experiments. (b) Effect of anti-OPG neutralizing antibody on the inhibitory effect of HGF culture supernatant. Peripheral blood monocytes were cultured with RANKL, M-CSF and supernatant from E. coli LPS-stimulated HGF culture (LPS-HGF sup) in the presence or absence of anti-OPG neutralizing antibody. The number of TRAP-positive cells in each sample was calculated 10 days after cultivation. The results were representative of five experiments. *P < 0·05.
The contribution of OPG to the inhibitory effect of LPS-stimulated HGFs was examined using anti-OPG neutralizing antibody. Culture-supernatant from LPS stimulated HGFs significantly inhibited the generation of TRAP-positive cells, and this inhibitory effect was significantly reduced upon addition of anti-OPG neutralizing antibody (Fig. 4b).
DISCUSSION
In the present study, RANKL and OPG mRNA expression was detected in inflamed gingival tissue. Hence, OPG and RANKL might interact in periodontitis. Osteoclast formation through RANKL is mediated by osteoblasts or activated T-cells [6]. Activated T-cells were observed to cause severe bone destruction, following migration into periodontal tissue in a rat experimental model of periodontitis [13]. Adoptive transfer of T cells from periodontitis patients into SCID mice also caused severe bone destruction and the resorption was suppressed by OPG, suggesting that this destruction was mediated by soluble RANKL produced from T cells [14]. The expression of CD69 on CD4+ and CD8+ GMCs in this study indicates that gingival T cells were activated, and some GMCs expressed RANKL, suggesting that T cells might take part in the RANKL expression in inflamed gingival tissue. Further study is necessary to determine the relevance of RANKL expression by T cells for the osteoclast formation in periodontitis.
LPS-stimulated HGFs produced OPG, and their culture supernatant inhibited the ability of soluble RANKL to stimulate monocyte differentiation into osteoclasts. HGFs expressed toll-like receptor-2 (TLR-2) and TLR-4 [15,16], which is a competent receptor for LPS. It is noteworthy that HGFs appear to recognize LPS through TLR-4 expression [15,16], but fibroblasts from other tissues do not express TLR-4 [16]. As HGFs are challenged by a large variety of bacteria, they might have receptors for bacterial products. In this context, production of OPG by LPS-stimulated HGFs might be a protective mechanism against bacterial challenge.
All the known periodontopathogens, such as Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis and Bacteroides forthysus, possess LPS. The structure of P. gingivalis LPS is different from E. coli LPS, and lacks hepatose and 2-keto-3-deoxyoctonate [17]. The structure of A. actinomycetemcomitans LPS is known to resemble that of E. coli LPS [18]. Although HGFs could recognize P. gingivalis LPS through TLR-4 [15,16], it remains to be determined whether P. gingivalis LPS augments OPG production by HGFs.
Sakata and co-workers have reported that OPG is expressed by HGFs, periodontal ligament cells and dental pulp cells, but not epithelial cells [19]. In light of the fact that periodontal ligament cells secrete OPG which inhibits osteoclast production [20] and that TNF-α and IL-1β increase OPG mRNA expression in human periodontal ligament cells [19], it appears that dental mesenchymal cells play a protective role against osteoclastic bone resorption during periodontal inflammation.
Numerous reports have focused on the pathological role of HGFs in periodontitis, as they have been observed to produce inflammatory mediators in response to bacterial products [21–24]. These mediators include IL-1, IL-6 and PGE2, which augment RANKL expression on the surface of osteoblasts [4]. It remains to be determined whether OPG produced by HGFs is sufficient to suppress RANKL expression in osteoblasts activated by IL-1, IL-6 and PGE2 in periodontitis tissue, but OPG might slow down bone resorption. It is also possible that HGF lines used in this study were different from pathological HGFs and they were derived from periodontal lesions in a healing stage, as the patients had received initial therapy before surgery.
In addition, several reports showed the differentiation and activation of osteoclasts besides RANKL [25,27]. IL-1 has been found to induce multi-nucleation and bone-resorption activity in preosteoclasts when osteoblast/stromal cells are not present [25]. While OPG has been observed to inhibit bone resorption induced by IL-1 in mice [26], complete inhibition of calcium release by mouse calvaria stimulated with IL-1 has not been observed [27]. HGFs produce IL-1, but the amount of IL-1 reportedly produced by HGFs varied among researchers [28–30]. Dongari reported that basal IL-1 production by HGF was limited, ranging from 0 to 75 pg/0·1 ml, and stimulation of HGF with A. actinomycetemcomitans cells did not enhance IL-1 levels [28]. Imatani and co-workers stimulated HGFs with various doses (0·1–10 mg/ml) of bacterial LPS, including E. coli LPS, A. actinomycetemcomitans LPS or P. gingivalis LPS, and none of them enhanced production of IL-1 significantly [29]. On the other hand, Sismey-Durrant reported that LPS from P. gingivalis stimulated dose-related increases in IL-1 release at the concentrations of LPS tested (0·1–10 µg/ml) [30]. While HGFs suppress differentiation of monocytes into TRAP-positive cells, they might augment survival and fusion of prefusion osteoclasts through the production of IL-1. Different HGF lines produced different amounts of OPG in our study, suggesting that HGFs are heterogeneous. Thus HGFs might function differently, depending on the balance between OPG and IL-1 production within tissue.
The present study demonstrates that gingival fibroblasts produce OPG in response to LPS stimulation. Induction of OPG in gingival fibroblasts might be a self-defence mechanism to inhibit alveolar bone destruction during periodontal inflammation.
Acknowledgments
We thank Dr Geena Koshy for her help in preparation of the manuscript. This work was supported by the Ministry of Education, Culture, Sports, Science and Technology (numbers 12771321, 13470459, 14571977 and 12672028).
REFERENCES
- 1.Okada H, Kida T, Yamagami H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect Immun. 1983;41:365–74. doi: 10.1128/iai.41.1.365-374.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayashi J, Saito I, Ishikawa I, et al. Effects of cytokines and periodontopathic bacteria on the leukocyte function-associated antigen 1/intercellular adhesion molecule 1 pathway in gingival fibroblasts in adult periodontitis. Infect Immun. 1994;62:5205–12. doi: 10.1128/iai.62.12.5205-5212.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suda T, Udagawa N, Takahashi N. The molecular mechanism of osteoclast differentiation and activation. Dentistry Japan. 2000;36:42–6. [Google Scholar]
- 4.Kong Y-Y, Boyle WJ, Penninger JM. Osteoprotegerin ligand. a regulator of immune responses and bone physiology. Immunol Today. 2000;21:495–502. doi: 10.1016/s0167-5699(00)01718-7. [DOI] [PubMed] [Google Scholar]
- 5.Kong Y-Y, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 6.Joisen R, Wong BR, Li HL, et al. TRANCE, a TNF family member, is differentially expressed on T cell subsets and induces cytokine production in dendritic cells. J Immunol. 1999;162:2562–8. [PubMed] [Google Scholar]
- 7.Kong YY, Feige U, Sarosi I, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9. doi: 10.1038/46303. [DOI] [PubMed] [Google Scholar]
- 8.Horwood NJ, Kartsogannis V, Quinn JMW, Romas E, Martin TJ, Gillespie MT. Activated T lymphocytes support osteoclast formation in vitro. Biochem Biophys Res Commun. 1999;265:144–50. doi: 10.1006/bbrc.1999.1623. [DOI] [PubMed] [Google Scholar]
- 9.Armitage CC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Schei O, Waerhaug J, Lovdal A, Arno A. Alveolar bone loss as related to oral hygiene and age. J Clin Periodontol. 1959;30:7–16. [Google Scholar]
- 11.Aramaki M, Nagasawa T, Koseki T, Ishikawa I. Presence of activated B-1 cells in chronic inflamed gingival tissue. J Clin Immunol. 1998;18:421–9. doi: 10.1023/a:1023234823783. [DOI] [PubMed] [Google Scholar]
- 12.Nagasawa T, Nitta H, Watanabe H, Ishikawa I. Reduced CD8+ peripheral blood T lymphocytes in rapidly progressive periodontitis. Archs Oral Biol. 1995;40:605–8. doi: 10.1016/0003-9969(95)00025-k. [DOI] [PubMed] [Google Scholar]
- 13.Kawai T, Eisen-Lev R, Seki M, et al. Requirement of B7 costimulation for Th1-mediated inflammatory bone resorption in experimental periodontal disease. J Immunol. 2000;164:2102–9. doi: 10.4049/jimmunol.164.4.2102. [DOI] [PubMed] [Google Scholar]
- 14.Teng Y-TA, Nguyen H, Gao X, et al. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabeta K, Yamazaki K, Akashi S, et al. Toll-like receptors confer responsiveness to lipopolysaccharide from Porphyromonas gingivalis in human gingival fibroblasts. Infect Immun. 2000;68:3731–5. doi: 10.1128/iai.68.6.3731-3735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang PL, Azuma Y, Shinohara M, Ohura K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochem Biophys Res Commun. 2000;273:1161–7. doi: 10.1006/bbrc.2000.3060. [DOI] [PubMed] [Google Scholar]
- 17.Mayrand D, Holt SC. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988;52:134–52. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masoud H, Weintraub ST, Wang R, Cotter R, Holt SC. Investigation of the structure of lipid A from Actinobacillus actinomycetemcomitans strain Y4 and human clinical isolate PO 1021–7. Eur J Biochem. 1991;200:775–81. doi: 10.1111/j.1432-1033.1991.tb16244.x. [DOI] [PubMed] [Google Scholar]
- 19.Sakata M, Shiba H, Komatsuzawa H, et al. Expression of osteoprotegerin (osteoclastogenesis inhibitory factor) in cultures of human dental mesenchymal cells and epithelial cells. J Bone Miner Res. 1999;14:1486–92. doi: 10.1359/jbmr.1999.14.9.1486. [DOI] [PubMed] [Google Scholar]
- 20.Wada N, Maeda H, Tanabe K, et al. Periodontal ligament cells secrete the factor that inhibits osteoclastic differentiation and function: the factor is osteoprotegerin/osteoclastogenesis inhibitory factor. J Periodont Res. 2001;36:56–63. doi: 10.1034/j.1600-0765.2001.00604.x. [DOI] [PubMed] [Google Scholar]
- 21.Hanazawa H, Hirase K, Ohmori Y, Amano S, Kitano S. Bacteroides gingivalis fimbriae stimulate production of thymocyte activating factor by human gingival fibroblasts. Infect Immun. 1988;56:272–4. doi: 10.1128/iai.56.1.272-274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sismey-Durrant HJ, Hopps RM. Effects of LPS from P. gingivalis on prostaglandin E2 and interleukin-1β release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1991;6:378–80. doi: 10.1111/j.1399-302x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]
- 23.Bartold PM, Haynes DR. Interleukin-6 production by human gingival fibroblasts. J Periodont Res. 1991;26:339–45. doi: 10.1111/j.1600-0765.1991.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 24.Dongari-Bagtzoglou AI, Ebersole JL. Production of inflammatory mediators and cytokines by human gingival fibroblasts following bacterial challenge. J Periodont Res. 1996;31:90–8. doi: 10.1111/j.1600-0765.1996.tb00469.x. [DOI] [PubMed] [Google Scholar]
- 25.Jimi E, Nakamura I, Takahashi N, Hirata N, Suda T. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res. 1999;247:84–93. doi: 10.1006/excr.1998.4320. [DOI] [PubMed] [Google Scholar]
- 26.Morony S, Capparelli C, Lee R, et al. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1β, TNF-α, PTH, PTHrP, and 1,25 (OH) 2D3. J Bone Miner Res. 1999;14:1478–85. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- 27.Tsukii K, Shima N, Mochizuki S, et al. Osteoclast differentiation factor mediates an essential signal for bone resorption induced by 1α,25-dihydroxyvitamin D3, prostaglandin E2, or parathyroid hormone in the microenvironment of bone. Biochem Biophys Res Commun. 1998;246:337–41. doi: 10.1006/bbrc.1998.8610. [DOI] [PubMed] [Google Scholar]
- 28.Dongari-Bagtzoglou AI, Ebersole JL. Gingival fibroblast cytokine profiles in Actinobacillus actinomycetemcomitans-associated periodontitis. J Periodontol. 1996;67:871–8. doi: 10.1902/jop.1996.67.9.871. [DOI] [PubMed] [Google Scholar]
- 29.Imatani T, Kato T, Okuda K. Production of inflammatory cytokines by human gingival fibroblasts stimulated by cell-surface preparations of Porphyromonas gingivalis. Oral Microbiol Immunol. 2001;16:65–72. doi: 10.1034/j.1399-302x.2001.016002065.x. [DOI] [PubMed] [Google Scholar]
- 30.Sismey-Durrant HJ, Hopps RM. Effect of lipopolysaccharide from Porphyromonas gingivalis on prostaglandin E2 and interleukin-1β release from rat periosteal and human gingival fibroblasts in vitro. Oral Microbiol Immunol. 1991;6:378–80. doi: 10.1111/j.1399-302x.1991.tb00510.x. [DOI] [PubMed] [Google Scholar]


