Abstract
Here, CD40L expression and cytokine production have been analysed in peripheral blood cells from orthotopic liver transplantation (OLT) recipients treated with ribavirin for recurrent chronic hepatitis C. The study included 18 OLT recipients treated with ribavirin, eight control OLT recipients and 10 healthy controls. FACS analysis showed that baseline expression of CD40L was not different between ribavirin-treated patients and controls. In contrast, after stimulation with both HCV core antigen and phorbol myristate acetate (PMA) plus ionomycin (IO), the expression of CD40L on CD4 lymphocytes was significantly higher in the ribavirin group compared with controls. In the ribavirin group, the increased expression of CD40L significantly correlated with reduction of HCV RNA levels with respect to pretreatment values. Finally, ribavirin treatment was not associated with modification of PMA-IO-induced cytokine production by T lymphocytes and interleukin (IL)-1β and tumour necrosis-α (TNF)-α production by CD40L-stimulated monocytes. In conclusion, these data indicate that ribavirin upmodulates CD40L expression on CD4 T cells, a property which may account in part for its ability to enhance the antiviral activity of interferon-α in the treatment of chronic HCV infection.
INTRODUCTION
Ribavirin (1-β-D-ribofuranosyl-1,2,4-triazole-3-carboxamide) is a synthetic guanosine nucleoside analogue which possesses in vitro antiviral activity against a range of RNA and DNA viruses [1,2]. Ribavirin has been used as a single antiviral agent to treat patients infected with hepatitis C virus (HCV). Despite the fact that most patients normalized aminotransferase levels transiently during treatment, ribavirin was unable to impact substantially on HCV virus load [3–7]. By contrast, ribavirin proved able to significantly increase the virological response of HCV patients to interferon-α (IFN-α) [8–11]. This suggests that some of the anti-HCV effects of ribavirin may be not directly antiviral. Recent studies in vitro and in animal models have shown that ribavirin may modulate production of immunoregolatory cytokines by T lymphocytes [12–15]. On the basis of these findings, the hypothesis has been raised that ribavirin may act in vivo as an immunomodulant compound.
CD40 ligand (CD40L, also called CD154), is a cell surface molecule present primarily on activated T cells [16,17]. CD40L plays a major role in regulating cellular immune responses. Interactions between CD40L on activated T cells and its receptor, CD40, on antigen presenting cells (APC) result in the priming and expansion of antigen specific CD4+ T cells, induction of co-stimulatory molecules on APCs, and the release of cytokines [18–22]. More important, CD40–CD40L interactions are of pivotal importance in the outcome of viral infections, especially when cytotoxic T lymphocyte (CTL) responses are dependent on CD4 T cell help such as in chronic HCV infection [23–25].
The aim of this study was to extend previous observation on the possible immunomodulatory activity of ribavirin. To this end, we have analysed the CD40L response of T lymphocytes to both mitogens and HCV core protein and the production of immunoregolatory cytokines by activated T cells in patients with recurrent chronic hepatitis C after an orthotopic liver transplant for HCV-related cirrhosis.
PATIENTS AND METHODS
Subjects and treatment
The study included 18 patients treated with ribavirin (600 mg/day (range 400–800) depending on renal function, in two divided doses orally) for a serologically (anti-HCV antibodies and HCV RNA positive) and histologically demonstrated recurrent chronic hepatitis C after liver transplantation for HCV-related cirrhosis. Eight OLT recipients with similar biochemical, virological and histological characteristics, who were not assuming any anti-HCV treatment, were taken as controls. The immunosuppressive regimens were identical for ribavirin-treated patients and controls and consisted in: 1·5 mg/ml cyclosporin A/day. Further characteristics of patients and controls are reported in Table 1. In selected experiments 10 healthy subjects, age- and sex-matched with OLT recipients, were used as further controls. Exclusion criteria included subjects with a previous episode of steroid-resistant acute rejection or chronic rejection, pregnant women, subjects with a leucocyte count <1·5 × 109/l, a platelet count <1·5 × 109/l, or a haemoglobin concentration <10 g/dL at the time of entry. No subject had serological markers of active cytomegalovirus, Epstein–Barr virus or hepatitis B virus infection. Informed consent was obtained from each subject included in the study.
Table 1.
Characteristics of patients at entry into the study
| Baseline data | ||
|---|---|---|
| Variable | Ribavirin | Controls |
| No. of patients | 18 | 8 |
| Mean age (years) | 58 ± 8 | 55 ± 10 |
| Sex (M/F) | 14/2 | 6/2 |
| Time (months) from transplantation | 48 ± 9 | 50 ± 6 |
| Histological grading (mean) | 5·4 | 4·8 |
| Histological staging (mean) | 2·1 | 2 |
| ALT (mU/ml) | 186 ± 92 | 108 ± 54 |
| Pretreatment HCV RNA levels (copies/ml × 103) | 844 ± 579 | 838 ± 230 |
| HCV genotype (no. of patients) | ||
| 1b | 6 | 4 |
| 1a | 2 | 2 |
| 2a | 3 | 1 |
| 4a + 4c not typed | 1 | 0 |
Data are expressed as mean ± s.d. ALT, alanine aminotransferase (normal range <21 mU/ml).
HCV-RNA
The quantification of HCV-RNA in serum samples was performed using a commercially available kit: Amplicor HCV monitorTM test (Roche Diagnostic Systems, Branchburg, NJ, USA). The lowest level of detection of this test is less than 200 HCV-RNA copies/ml of sample.
Preparation of peripheral blood mononuclear cells (PBMC)
Blood from patients and controls was collected by venipuncture 6 months after their entry into the study into sterile EDTA tubes and the PBMC were separated immediately using lymphoprep (Nycomed, Oslo, Norway). PBMC were resuspended at a final concentration of 10 × 106 cells/ml in RPMI-1640 containing 2 mm glutamine, 50 U/ml penicillin, 50 µg/ml streptomycin and 10% heat-inactivated fetal calf serum (complete medium). All cultures were incubated at 37°C in a humidified atmosphere of 5% CO2.
FACS analysis of CD40L expression
PBMC, 2 × 106, were cultured in either 96-well plates in complete medium supplemented with 20 ng/ml phorbol 12-myristate 13-acetate (PMA) plus 500 ng/ml ionomycin (IO) (both from Calbiochem-Novabiochem INTL, La Jolla, CA, USA) or in culture tubes at a 5° slant in complete medium supplemented with 1 µg/ml HCV core antigen (aa 2 to aa 196, Biogenesis Inc., Kingston, NH, USA). At given time-points the cells were washed twice in PBS, collected by centrifugation and stained with the appropiate combination of the following monoclonal antibobies: fluorescein isothiocyanate-conjugated (FITC)-anti-CD40L, Cy-chrome™-conjugated (Cy-chrome)-anti-CD3, Cy-chrome-anti-CD4, phycoerytrin-conjugated (PE)-anti-CD8 (all from PharMingen, San Diego, CA, USA). The cells were then washed and resuspended for 10 min in PBS containing 4% paraformaldehyde. After additional washing the cells were analysed by a FACScan flow cytometer (Becton Dickinson, Mountain View, USA). Lymphocytes and macrophages were differentiated from dead cells on the basis of forward angle and 90° scatter. For each analysis, either 5000 events (mitogen-stimulated cultures) or 50 000 events (HCV core antigen-stimulated cultures) were gated on CD3 or CD4 expression and a light scatter gate designed to include only viable lymphocytes. Isotype-matched negative controls antibodies were used to verify the staining specificity of anti-cytokine antibodies, and as a guide for setting markers to delineate ‘positive’ and ‘negative’ populations. In mitogen-stimulated samples, staining with anti-CD8 antibody enabled T cells to be subdivided into CD8+ and CD8− cells, those cells which did not stain for CD8 were assumed to represent the CD4+ T cell population. These cells could not be stained directly as treatment of lymphocytes with PMA plus IO leads to rapid and complete down-modulation of surface CD4. In HCV core antigen-stimulated samples, no down-modulation of surface CD4 was observed, therefore allowing for direct stain of CD4+ cells with Cy-chrome-anti CD4 antibody.
Analysis of mitogen- and CD40L-induced cytokine production
PBMC, 2 × 106, were cultured for 18 h in complete medium supplemented with either PMA plus IO as described above or 1 mg/ml recombinant soluble CD40L (provided by Immunex, Seattle, WA, USA), 1 µg/ml brefeldin A (Sigma) was added 1 h after stimulation, to inhibit cytokine export. Dead cells were excluded by trypan blue dye and 2 × 105 PBMC were dispensed in V-bottomed 96-well plates in 20 ml PBS. Cells were then incubated for 15 min with mouse Cy-chrome-anti-CD3 and PE-anti-CD8 or Cy-chrome-anti CD14. The cells were then washed and fixed in 4% paraformaldehyde. The cells were resuspended for 30 min at room temperature in 20 µl PBS containing 0·1% saponin (Sigma), 1% bovine serum albumin (Sigma) and 0·5 µg/million cells of one of the following FITC, mouse antihuman monoclonal antibodies: anti-IL-2, anti-IL-4, anti-IL-10 and anti-IFN-γ (PharMingen). After further washing the cells were analysed by FACS.
Measurement of cytokine production by unfractionated PBMC by enzyme-linked immunosorbent assay (ELISA)
For the determination of cytokine production in supernatants. 4 × 106 PBMC were cutured for 48 h in complete medium supplemented with PMA plus IO as described above, in the absence of brefeldin A. After incubation supernatants were collected and stored at − 80°C. Commercially available sandwich ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to determine the concentration of IL-4, IL-10, IFN-γ and IL-2 in the supernatants. The detection limits of these ELISAs are, respectively, 4·1, 1·5, 15, 7 and 3 pg/ml. According to the manufacturer's specifications, these ELISAs are specific for the relative cytokines. All the samples were tested in duplicate, in a single analytical set. Intra-series variation coefficient was <15%.
Statistics
Data analysis was performed using unpaired, two-tailed Student's t-test after logarithm transformation of all values. P-values <0·05 were considered statistically significant. After analysis the results were converted back to the original scale for reporting.
RESULTS
Response to treatment
After 12 months of treatment, serum aminotransferase levels normalized in four of 17 patients receiving ribavirin (23·5%) and in one of eight patients receiving no therapy (12·5%). Mean HCV RNA levels were 6 × 105 ± 5·6 × 105 and 7·44 × 105 ± 5·1 × 105 copies/ml in the ribavirin group and in controls, respectively (see Table 1 for pretreatment HCV RNA levels).
Effect of ribavirin treatment on the expression of CD40L by peripheral CD4+ lymphocytes
In the absence of activation, no CD40L expression was documented in patients and controls. Stimulation with HCV core protein resulted in a CD40L response in 12 of 18 (66%) of ribavirin-treated patients and in one of eight (12·5%) of the control group (mean percentage of CD40L-positive cells: 0·015 ± 0·012 versus 0·0032 ± 0·009, P = 0·009) (Fig. 1). Following PMA-IO stimulation, the expression of CD40L peaked at 24 h in all subjects and then decreased to near baseline at 48 h. However, 24 h after stimulation, both the percentage of CD40L-positive cells and the intensity of CD40L expression were significantly higher in ribavirin-treated patients compared with OLT controls and healthy subjects (Fig. 2). The expression of CD40L was significantly higher in the ribavirin patients that reduced their HCV RNA levels of at least 0·5 log with respect to pretreatment values, compared with those that did not (Table 2). In contrast, no correlation was found between CD40L expression and ALT levels, different genotypes or liver histology (data not shown). Shown in Fig. 3 are representative FACS plots from data presented in Figs 1 and 2.
Fig. 1.
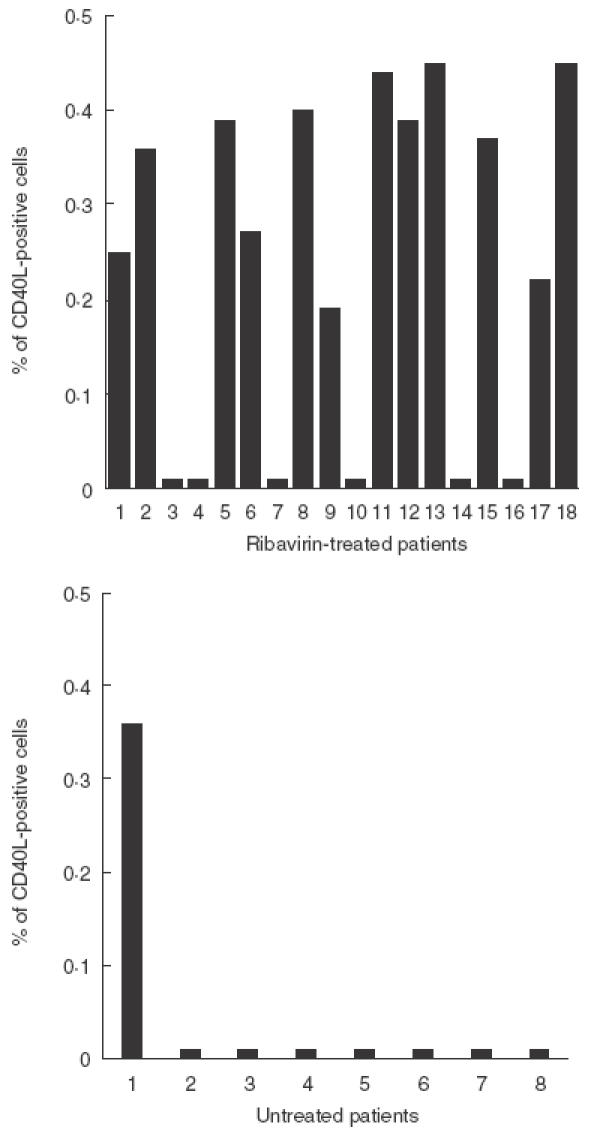
HCV core protein-induced expression of CD40L on CD4 lymphocytes from control and ribavirin-treated patients: expression of CD40L was determined by FACS after 6 h incubation of PBMC with 1 µg/ml HCV core antigen. 50 000 events gated on viable CD4-positive lymphocytes were analysed in each sample. The percentage of CD40L-positive cells in samples incubated without core protein were consistently less than 0·02.
Fig. 2.
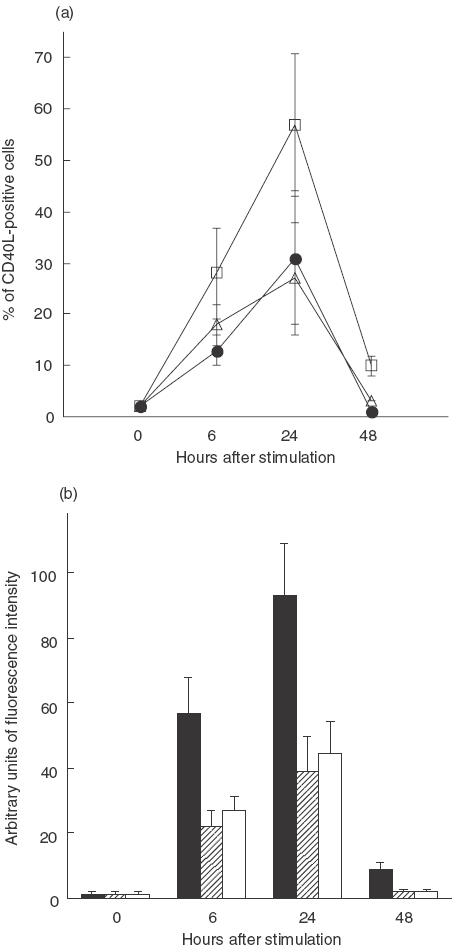
Spontaneous and mitogen-induced expression of CD40L on CD4 lymphocytes from control and ribavirin-treated patients. (a) Percentage of CD40L positive cells as assessed by FACS just before (time 0) and at different time-points after stimulation. (b) Intensity of expression of CD40L just before and at different time-points after stimulation, calculated on 1000 positive events as the value of intensity of fluorescence (arbitrary units) of the sample stained with anti-CD40L MoAb minus the value of intensity of fluorescence of the same sample stained with the control antibody. The error bars represent s.d. (a) □, Ribavirin; • controls; ▵, healthy subjects. (b) ▪, Ribavirin;  , controls; □, healthy subjects.
, controls; □, healthy subjects.
Table 2.
Correlation between percentages of CD40L-positive cells and HCV-RNA reduction
| % of CD40L-positive cells | ||
|---|---|---|
| Stimulation | >0·5 log | <0·5 log |
| PMA-IO | 76·4 | 39·8 |
| HCV core antigen | 0·13 | 0·033 |
CD40L expression was determined by FACS 24 h after mitogen stimulation or 6 h after HCV-core antigen stimulation.
Fig. 3.
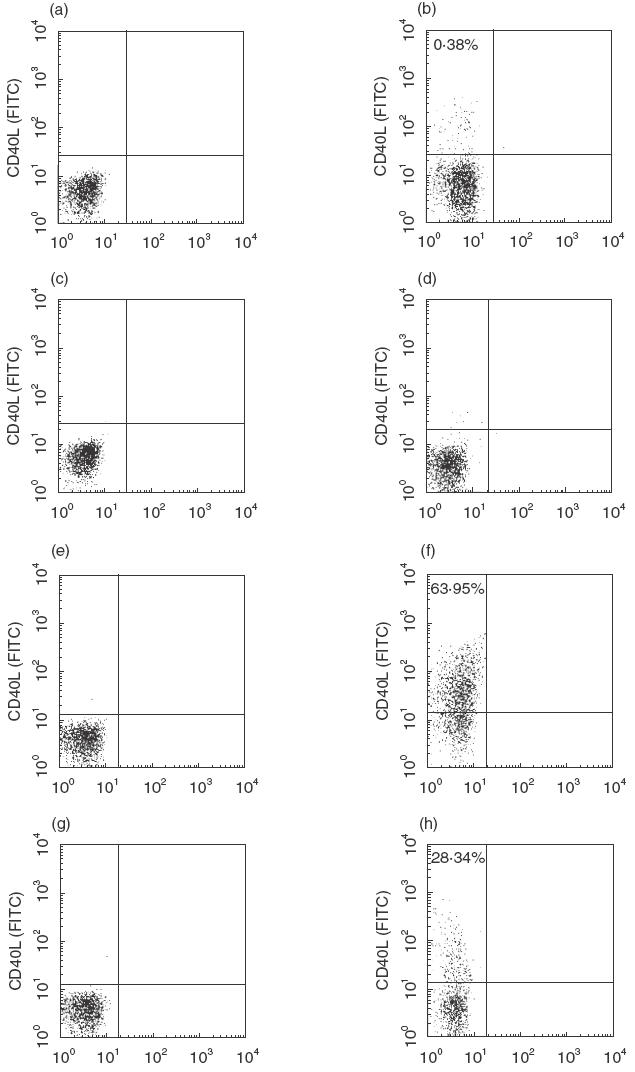
Representative dot-plots of CD40L expression on CD4+ T cells from control and ribavirin-treated patients following stimulation with HCV core antigen or mitogens. (a) Unstimulated cells from a ribavirin-treated patient; (b) cells from the same patient in (a) stimulated with 1 µg/ml HCV core antigen; (c) unstimulated cells from an untreated patients; (d) cells from the same patient in (c) stimulated with 1 µg/ml HCV core antigen; (e) unstimulated cells from a ribavirin-treated patients; (f) cells from the same patient in (e) stimulated with PMA-IO; (g) unstimulated cells from an untreated patient; (h) cells from the same patient in (g) stimulated with PMA-IO. The data are displayed as dot-plots.
Then, we tested whether differences in CD40L expression are found when T cells are stimulated in vitro in the presence of ribavirin. For this type of experiment PBMC from normal donors were stimulated in vitro with PMA-IO (20 ng/ml and 500 ng/ml, respectively), in the presence or absence of 1, 10 or 100 mm ribavirin. CD40L expression was consistently obtained in three out of three experiments performed using PBMC fron three different donors. However, no significant differences in CD40L expression were observed between controls and ribavirin-treated samples (mean percentage of CD40L-positive cells in untreated samples: 35·3 ± 11·4, mean percentage of CD40L-positive cells in 1, 10 or 100 mm ribavirin-treated samples: 34·7 ± 10, 30 ± 16·7 and 39 ± 13·3, respectively). These results suggest that the effect of ribavirin on CD40L expression may not be direct.
Cytokine production by peripheral T lymphocytes and monocytes
PBMC from ribavirin-treated patients and controls were stimulated by PMA-IO and analysed by FACS for IL-2, IFN-γ, IL-4 and IL-10 production. As shown in Table 3, the percentage of T cells positive for intracellular IL-2, IFN-γ, IL-4 and IL-10 production was not different statistically between the two groups. Table 3 also shows that production of IL-1β and tumour necrosis factor-α (TNF-α) by CD40L-stimulated peripheral monocytes was not different between ribavirin patients and OLT controls.
Table 3.
FACS analysis of mitogen-induced production of IL-2, IFN-γ, IL-4 and IL-10, by T lymphocytes and TNF-α and IL-1β production by CD40L-stimulated monocytes, from ribavirin-treated patients and OLT controls
| Percentage of positive cells | ||
|---|---|---|
| Cytokine | Ribavirin | Controls |
| IFN-γ | 37 ± 11 | 39 ± 15* |
| IL-2 | 53 ± 19 | 56 ± 17* |
| IL-4 | 4 ± 3 | 3 ± 3* |
| IL-10 | 5 ± 3 | 5 ± 2* |
| TNF-α | 57 ± 21 | 53 ± 18* |
| IL-1β | 22 ± 9 | 19 ± 9* |
Peripheral blood mononuclear cells were stained with Cy-chrome-anti-CD3 or anti-CD14 monoclonal antibody for the determination of their surface phenotype. Intracellular cytokines were detected by staining with fluorescein isothiocyanate FITC-anti-IFN-γ, anti-IL-2, anti-IL-4, anti-IL-10, anti-TNF-α and anti-IL-1β monoclonal antibodies. CD3+ or CD14+-gated cells were analysed by FACS on FL1 (FITC) versus forward scatter (FSC) two-dimensional plots to discriminate positive cells.
P > 0·05. Data are means ± s.d.
DISCUSSION
Ribavirin may be more than a direct antiviral agent as it can be beneficial in the treatment of viral diseases with or without inhibiting viral replication [26]. Thus its in vivo utility may be ascribed to two distinct properties, a direct reduction in levels of circulating virus and the promotion of immunity against viral infection. Antiviral immunity is elicited predominantly by the action of cytotoxic T lymphocytes (CTL) and the expansion of the T helper (Th)1 cells which secrete Th1-type immunoregolatory cytokines such as IL-2 and IFN-γ. Based on studies in vitro and in animal models, several authors have suggested recently that the beneficial effect of ribavirin in chronic HCV infection, in particular its ability to enhance the antiviral activity of IFN-α, may be due to priming of T lymphocytes for an increased production of Th1 type cytokines [12–15]. Here we have addressed this possibility by analysing cytokine production by T lymphocytes and monocytes in patients treated with ribavirin for recurrent chronic hepatitis C after OLT. In agreement with previous data from our laboratory [27], the present findings do not support modulation of cytokine production as a mechanism for the anti-HCV activity of ribavirin in vivo. A possible explanation for the discrepancies between our results and those obtained in vitro and in animal models may be that, in humans, the differentiation of uncommitted T cell precursor toward the Th1 phenotype is regulated by a number of factors, including expression of co-stimulatory molecules on antigen-presenting cells, the type and the dose of the antigen and the cytokine milieu in which differentiation takes place [19,28,29]. These complex mechanisms of T cell differentiation are similar, but not identical, in animals [28]; moreover, they are completely lacking in in vitro systems. In addition, ribavirin treatment failed to prime patients monocytes for a reduced proinflammatory (IL-1β and TNF-α) response to CD40L stimulation. Release of proinflammatory mediators by intrahepatic monocyte-derived cells upon stimulation by CD40L expressing lymphocytes plays an important role in the pathogenesis of the hepatic inflammation process in chronic hepatitis C [30,31]. Thus, these data suggest that the ability of ribavirin to reduce aminotransferase levels (a reliable marker of hepatic inflammation) in HCV-infected patients may not be due to modulation of proinflammatory mediators production, as suggested previously [32].
The data presented here suggest that a novel mechanism may be involved in the anti-HCV activity of ribavirin. Indeed, we show that lymphocytes from patients treated with ribavirin responded with increased expression of CD40L to stimulation with both HCV core protein and mitogens, compared with cells from untreated controls. The expression of CD40L was significantly higher in the ribavirin patients that reduced their HCV RNA level of at least 0·5 log with respect to pretreatment values compared with those that did not. This suggests a direct relation between CD40L expression and the ability of the host to control HCV replication. Alternatively to mitogen stimulation, CD40L expression on CD4+ lymphocytes can be obtained by activation with anti-CD3 monoclonal antibodies. Anti-CD3 stimulates via TCR, therefore triggering all signalling pathways involved, some of which are bypassed using mitogens. Further experiments are needed to determine which stimulation procedure is more relevant for the in vivo situation.
Cell-to-cell interactions involving a variety of cell types are required for an effective immune response. In particular, CD40–CD40L interactions are critical for the development of the CD4 T cell-dependent cell-mediated immune response at multiple levels [18,19]. Lack of the CD40–CD40L interaction results in reduced activation of CD4 T cells, inefficient priming and expansion of antigenic specific T cells, inappropiate release of IL-12 and polarization of the immune response toward Th2 response [33–36]. Furthermore, CD40–CD40L interactions are important for the effector stage at the site of inflammation and for the secretion of inflammatory mediators [20–22]. In this context, the ability of ribavirin to enhance the expression of CD40L may contribute to up-regulate HCV-specific CTL responses and potentiate the immunomediated anti-HCV capacity of IFN-α which increases MHC II molecules expression of hepatocyte surface [37]. Finally, recent data also suggest that CD40L may have direct antiviral activity [38]. This was demonstrated by rapid clearance of recombinant vaccinia viruses expressing CD40L from a variety of immune-deficient mice. The mechanism of CD40L-mediated direct antiviral activity remains unclear; however, the above-mentioned study suggests that this antiviral activity is independent of other antiviral host defenses.
It has been reported that PBMC from transplant patients receiving cyclosporin A failed to express CD40L upon stimulation [39]. Because the subjects studied here were assuming cyclosporin A, the possibility can be raised that ribavirin may simply interfere with cyclosporin A activity. This is improbable, however, as activation-induced CD40L expression was not different between healthy subjects and control OLT patients, suggesting that cyclosporin A was not affecting CD40L expression substantially under these experimental conditions. More importantly, recent experimental data indicate that the targeting of CD40L may offer an opportunity for tolerance induction in transplant recipients [40]. Here we found that although ribavirin increases CD40L expression, this was not associated with an increased rate of rejection. This can be due either to the concomitant use of cyclosporin A, which may inhibit CD40L MoAb-induced allograft survival [40] or to the ability of ribavirin to reduce the cytotoxic effect of immune cells via inhibition of their proliferation [41].
In conclusion, the data reported in this study suggest a possible explanation to the ability of ribavirin to affect HCV replication and to potentiate the anti-HCV activity of IFN-α. However, one must be cautious in extrapolating the results with OLT recipients to other conditions related to chronic HCV infection. Further studies in different in vivo setting may help to confirm and extend the present findings.
REFERENCES
- 1.Hosoya M, Shigheta S, Ishii T, Suzuki H, De Clercq E. Comparative inhibitory effects of various nucleoside and nonnucleoside analogues on replication of influenza virus types A and B in vitro and in vivo. J Infect Dis. 1993;168:641–6. doi: 10.1093/infdis/168.3.641. [DOI] [PubMed] [Google Scholar]
- 2.Shigeta S, Mori S, Baba M, et al. Antiviral activities of ribavirin, 5-ethynyl-1-beta-Dribofuranosyl-imidazole-4carboxamide, and 6′-(R)-6′-C-methylneoplanocin A against several ortho-and paramyxoviruses. Antimicrobial Agents Chemother. 1992;36:435–9. doi: 10.1128/aac.36.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Bisceglie AM, Shindo M, Fong TL, et al. A pilot study of ribavirin therapy for chronic hepatitis C. Hepatology. 1992;16:649–54. doi: 10.1002/hep.1840160307. [DOI] [PubMed] [Google Scholar]
- 4.Dusheiko G, Main J, Thomas H, et al. Ribavirin treatment for patients with chronic hepatitis C: results of a placebo-controlled study. J Hepatol. 1996;25:591–8. doi: 10.1016/s0168-8278(96)80225-x. [DOI] [PubMed] [Google Scholar]
- 5.Di Bisceglie AM, Conjeevaram HS, Fried MW, et al. Ribavirin as therapy for chronic hepatitis C. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 6.Hoofnagle JH, Lau D, Conjeevaram H, et al. Prolonged therapy of chronic hepatitis C with ribavirin. J Viral Hep. 1996;3:247–52. doi: 10.1111/j.1365-2893.1996.tb00050.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee JH, von Wagner M, Roth WK, et al. Effect of ribavirin on virus load and quasispecies distribution in patients infected with hepatitis C. J Hepatol. 1998;29:29–35. doi: 10.1016/s0168-8278(98)80175-x. [DOI] [PubMed] [Google Scholar]
- 8.Poynard T, Marcelin P, Lee SS, et al. Randomised trial of interferon-α-2b plus ribavirin for 48 weeks or for 24 weeks versus interferon-α-2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. Lancet. 1998;352:1426–32. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 9.Brouwer JT, Hansen BE, Niesters HGM, et al. Early prediction of response in interferon monotherapy and in interferon-ribavirin combination therapy for chronic hepatitis C: HCV RNA at 4 weeks versus ALT. J Hepatol. 1999;30:192–8. doi: 10.1016/s0168-8278(99)80061-0. [DOI] [PubMed] [Google Scholar]
- 10.Bell H, Hellum K, Harthug S, et al. Treatment with interferon-alpha2a alone or interferon-alpha2a plus ribavirin in patients with chronic hepatitis C previously treated with interferon-alpha2a. Scand J Gastroenterol. 1999;2:194–8. doi: 10.1080/00365529950173087. [DOI] [PubMed] [Google Scholar]
- 11.Barbaro G, Di Lorenzo G, Soldini M, et al. Interferon-α-2b and ribavirin in combination for chronic hepatitis C patients not responding to interferon-α alone: an Italian multicenter, randomized, controlled, clinical study. Am J Gastroenterol. 1998;93:2445–51. doi: 10.1111/j.1572-0241.1998.00702.x. [DOI] [PubMed] [Google Scholar]
- 12.Hultgren C, Milich DR, Weiland O, et al. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune response. J General Virol. 1998;79:2381–91. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 13.Tam RC, Pai B, Bard J, et al. Ribavirin polarizes human T cell responses toward a type 1 cytokine profile. J Hepatol. 1999;30:376–82. doi: 10.1016/s0168-8278(99)80093-2. [DOI] [PubMed] [Google Scholar]
- 14.Martin J, Navas S, Quiroga JA, et al. Effects of the ribavirin-interferon a combination on cultured peripheral blood mononuclear cells from chronic hepatitis C patients. Cytokine. 1998;10:635–44. doi: 10.1006/cyto.1997.0333. [DOI] [PubMed] [Google Scholar]
- 15.Ning Q, Brown D, Parodo J, et al. Ribavirin inhibits viral-induced production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine response. J Immunol. 1998;160:3487–93. [PubMed] [Google Scholar]
- 16.Hollenbaugh D, Grosmaire LS, Kullas CD, et al. The human cell antigen gp39, a member of the TNF family, is a ligand for the CD40 receptor: expression of a soluble form of gp39 with B cell co-stimulatory activity. EMBO J. 1992;11:4313–21. doi: 10.1002/j.1460-2075.1992.tb05530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vey E, Zhang JH, Dayer JM. IFN-gamma and 1,25 (OH) 2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J Immunol. 1992;149:2040–6. [PubMed] [Google Scholar]
- 18.Grewal IS, Flavell RA. CD40–CD40L interactions in T cell activation. Immunol Rev. 1996;153:85–106. doi: 10.1111/j.1600-065x.1996.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 19.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–35. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 20.Alderson MR, Armitage RJ, Tough TW, Strockbine L, Fanslow WC, Spriggs MK. CD40 expression by human monocytes: regulation by cytokines and activation of monocytes by the ligand for CD40. J Exp Med. 1993;178:669–74. doi: 10.1084/jem.178.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiener PA, Moran-Davis P, Rankin BM, Wahl AF, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble gp 39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–25. [PubMed] [Google Scholar]
- 22.Caux C, Massacrier C, Vanbervliet B, et al. Activation of human dendritic cells through CD40 cross-linking. J Exp Med. 1994;180:1263–72. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borrow P, Tishon A, Lee S, et al. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–42. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitemire JK, Slifka MK, Grewall IS, Flavell RA, Ahamed R. Primary immune response to a viral infection in CD40 ligand-deficient mice. J Virol. 1996;70:8375–81. doi: 10.1128/jvi.70.12.8375-8381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oxenius A, Campbell KA, Maliszewski CR, et al. CD40–CD40 ligand interactions are critical in T–B cooperation but not for other anti-viral CD4+ T cell functions. J Exp Med. 1996;183:2209–18. doi: 10.1084/jem.183.5.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall CB, McBride JT, Walsh EE, et al. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. N Engl J Med. 1983;308:1443–7. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- 27.Bergamini A, Bolacchi F, Cepparulo M, et al. Treatment with ribavirin and interferon-α reduces interferon-γ expression in patients with chronic hepatitis C. Clin Exp Immunol. 2001;123:459–65. doi: 10.1046/j.1365-2249.2001.01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann TR, Sad S. The expanding universe of T cell subset: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 29.Del Prete G, Maggi E, Romagnani S. Human Th1 and Th2 cells: functional properties, mechanisms of regulation, and role in diseases. Laboratory Invest. 1994;70:299–306. [PubMed] [Google Scholar]
- 30.Zylberberg H, Rimaniol AC, Pol S, Masson A, et al. Soluble tumor necrosis factor receptors in chronic hepatitis C. a correlation with histological fibrosis and activity. J Hepatol. 1999;30:185–91. doi: 10.1016/s0168-8278(99)80060-9. [DOI] [PubMed] [Google Scholar]
- 31.Itoh Y, Okanoue T, Ohnishi N, et al. Serum levels of soluble tumor necrosis factor receptors and effects of interferon therapy in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1999;94:1332–40. doi: 10.1111/j.1572-0241.1999.01083.x. [DOI] [PubMed] [Google Scholar]
- 32.Hultgren C, Milich DR, Weiland O, Sallberg M. The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J General Virol. 1998;79:2381–91. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 33.Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–20. doi: 10.1038/378617a0. [DOI] [PubMed] [Google Scholar]
- 34.Koch F, Stanzl U, Jennewein P, et al. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-10. J Exp Med. 1996;184:741–6. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rincon M, Anguita J, Nakamura T, Fikrig E, Flavell RA. Interleukin (IL)-6 directs the differentiation of IL-4 producing CD4+ T cells. J Exp Med. 1997;185:461–9. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blotta MH, Marshall JD, DeKruyff RH, Umetsu DT. Cross-linking of the CD40 ligand on human CD4+ T lymphocytes generates a costimulatory signal that upregulates IL-4 synthesis. J Immunol. 1996;156:3133–40. [PubMed] [Google Scholar]
- 37.Reheman B, Chang KM, McHutchison JG, Kokka R, Houghton M, Chisari FV. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J Clin Invest. 1996;98:1432–40. doi: 10.1172/JCI118931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruby J, Bluethmann H, Aguet M, Ramshaw IA. CD40 ligand has potent antiviral activity. Nat Med. 1995;1:437–41. doi: 10.1038/nm0595-437. [DOI] [PubMed] [Google Scholar]
- 39.Fuleihan R, Ramesh N, Horner A, et al. Cyclosporin A inhibits CD40 ligand expression in T lymphocytes. J Clin Invest. 1994;93:1315–20. doi: 10.1172/JCI117089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smiley ST, Csizmadia V, Gao W, Turka LA, Hancock WW. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement for NFkappaB: implication for tolerance induction. Transplantation. 2000;70:415–9. doi: 10.1097/00007890-200008150-00005. [DOI] [PubMed] [Google Scholar]
- 41.Heagy W, Crumpacker C, Lopez PA, Finberg RW. Inhibition of immune functions by antiviral drugs. J Clin Invest. 1991;87:5512–20. doi: 10.1172/JCI115217. [DOI] [PMC free article] [PubMed] [Google Scholar]


