Abstract
Although convincing evidence exists for the role of immunoglobulin G (IgG) antibodies in immunity to malaria, antibody titres do not usually predict protection. In this study we have assessed the interaction between Plasmodium falciparum-infected erythrocytes (PE), opsonized with immune serum containing different amounts of IgG antibody isotypes, with either THP-1 cells, ex-vivo human monocytes or IIAI.6 transfectant cells expressing FcγRIIa-Arg/Arg131 or –His/His131 allotypes. Our results show that PMA-treated THP-1 cells were capable of phagocytosing serum-opsonized PE by FcγRI (CD64) and FcγRIIa (CD32), acting synergistically. The known FcγRIIa polymorphism motivated us to examine its influence on IgG isotype-mediated phagocytosis of opsonized PE with human monocytes and the IIAI.6 transfectant cells expressing either allelic forms. Regardless of the cell type, PE phagocytosis with FcγRIIa-His/His131 was highest following opsonization with a predominantly IgG3-containing immune serum pool. In contrast, PE phagocytosis with FcγRIIa-Arg/Arg131 tended to be higher with an IgG1-containing pool. These results suggest a genetically determined influence of effector cell phenotype on IgG antibody–pathogen interaction in P. falciparum malaria.
Keywords: antibodies, FcγR, human, phagocytes, Plasmodium falciparum
INTRODUCTION
Antibody responses play a critical role in immune protection against asexual blood stages of Plasmodium falciparum. This has been demonstrated elegantly by passive transfer experiments using sera or purified immunoglobulins from adults resident in areas with hyperendemic malaria [1,2]. However, the mechanisms by which malaria-specific antibodies interfere with the development and/or multiplication of the asexual stages of human Plasmodia are still unclear. Druilhe and others have postulated that antibodies inhibit parasite growth in co-operation with either monocytes or neutrophils by antibody-dependent cellular inhibition (ADCI) [3,4] or immunophagocytosis [5] through cell-surface expressed Fc receptors after binding their parasite target.
The binding of the Fc portion of IgG to Fcγ receptors (FcγR) on the surfaces of monocytes, macrophages and neutrophils provokes biological functions such as antibody-dependent cellular cytoxicity (ADCC) (ADCI in malaria), phagocytosis, antigen presentation, release of infammatory mediators and enhancement of surface antigen expression, as well as generation of reactive oxygen species ([4], for a review see [6]). In humans, there are three classes of FcγR that bind cytophilic IgG (IgG1 and IgG3), namely: FcγRI (CD64), FcγRII (CD32) and FcγRIII (CD16). FcγRI is a high-affinity receptor capable of binding monomeric IgG1, IgG3 and IgG4. FcγRII and FcγRIII are low-affinity receptors, interacting with only IgG in complexed or aggregated form. Human FcγRII is a 40 kDa glycoprotein that is expressed on a variety of cells such as granulocytes, monocytes/macrophages, platelets, B cells, endothelial cells of the placenta and some T-cell subsets. The FcγRIIa subtype is expressed on neutrophils and monocytes/macrophages and initiates phagocytosis, ADCC and cellular activation. Recent studies provide evidence that a structural and functional polymorphism at position 131 of this receptor leads to a point mutation resulting in a switch from an arginine (R) to a histidine (H) residue in the proximal Ig-binding domain which greatly affects receptor affinity and specificity [7]. FcγRIIa-H131 exhibits an affinity for IgG2 not seen with the FcγRIIa-R131 [8] with consequent functional differences.
In vitro assays using immune sera seeking correlates for protection against malaria showed that FcγIIa receptors are involved [4]. Recent observations in two separate malaria studies showed that this polymorphism may have an influence on protection against this disease [9,10]. In all these studies, as well as in larger sero-epidemiological surveys, the quality of the antibody response, which is reflected in the distribution of the IgG isotype class(es), have been stressed [11–14]. Bouharoun-Tayoun and Druihle [11] observed differences in the distribution of Ig subclasses between clinically protected and non-protected individuals, with cytophilic isotypes (IgG1 and IgG3) being dominant in the protected individuals. In this context FcγRIIa-Arg/Arg, which binds IgG1 and IgG3 but not IgG2 [7], would be expected to be more efficient than the His/His allotype. FcγRIIa-His/His binds IgG1, IgG2 and IgG3, albeit with different affinities. Our own previous results show that ADCI in vitro is mediated predominantly by IgG3 [15], whereas Shi and colleagues [16], in a similar study, showed IgG1 to be more important.
Thus, polymorphisms which may alter the relative antibody affinity of receptor(s) expressed on effector cells involved in antibody-mediated protection may ultimately influence disease outcome. Understanding the mechanism(s) of these interactions may help in the design of effective vaccines. To address this, we have designed experiments using a human monocytic cell line, THP-1, transfectant cell lines expressing the different allelic forms of FcγRIIa, as well as human monocytes in immunophagocytosis assays using well characterized sera from malaria exposed individuals.
MATERIALS AND METHODS
Serum donors
Serum samples were obtained from 23 semi-immune adults (18–54 years of age) from Lambaréné, a town in Gabon where P. falciparum malaria is hyperendemic [17]. As control, we used a pool of serum obtained from malaria non-exposed Europeans. Based on antibody quantification by ELISA described below we created different serum pools from malaria exposed and naive individuals: (i) P1: non-immune pool from non-exposed Europeans; (ii) P2: immune serum pool containing both IgG1 and IgG3; (iii) P3: immune serum pool containing predominantly IgG1; and (iv) P4: immune serum pool containing predominantly IgG3.
Monoclonal antibodies
The following monoclonal antibodies were obtained from Medarex (Annandale, NJ, USA): mouse anti-hFcRγI (CD64) MoAb 22 (mIgG1), mouse anti-hFcRγII (CD32) MoAb IV.3 (mIgG2b), mouse anti-hFcRγIII (CD16) MoAb 3G8 (purified Ig), FITC-labelled MoAb IV.3. Mouse anti-hFcRγII (CD32) MoAb AT10 was obtained from Dr Thomas Valerius (University of Erlangen-Nürnberg, Germany), and mouse anti-hFcγRI (CD64) clone 10·1 from Biozol, Germany. The mouse anti-hCD36 (mIgG1) clone CLB-IVC7 was obtained from Research Diagnostics INC, Flanders, NJ, USA. For inhibition studies, human monocytes and THP-1 cells were preincubated for 25 min at room temperature with anti-FcγR antibodies at the following concentrations: 10 µg/ml CD64 (anti-hFcγI clone 10·1); 5 µg/ml IV.3 (anti-hFcγRII); 0·5 µg/ml AT10 (hFcγRII); 10 µg/ml MoAb 3G8 (anti-hFcRγIII); 10 µg/ml isotype control MoAb (Sigma, Germany) were used as appropiate.
P. falciparum culture and antigen preparation
A P. falciparum isolate cys007, obtained from a child presenting with severe P. falciparum malaria at the Albert Schweitzer Hospital, Lambaréné, Gabon was adapted for in vitro culture according to the method of Trager and Jensen [18] using RPMI-1640 medium (Sigma, Germany) buffered with 25 mm Hepes, and supplemented with 25 mm sodium bicarbonate, 2 mm l-glutamine, 300 mm hypoxanthine and 10 µg gentamicin per ml (Gibco, Paisley, UK). Parasites were grown in culture medium supplemented with 10% non-immune sera (prescreened) in an atmosphere of 5% CO2, 5% O2 and 90% N2 and subcultured with O-positive erythrocytes depleted of lymphocytes (University Hospital, Tübingen, Germany).
To prepare crude schizont antigen for ELISA, the isolate was grown to a parasitemia of 3–5% with a majority of the parasites in the schizont stage. The cultures were enriched and synchronized by selective high-gradient magnetic sorting (MACS; Miltenyi Bio Tec, Bergisch Gladbach, Germany). Briefly, cultures were passed through a prewashed column (2% fetal calf serum (FCS) in phosphate-buffered saline (PBS)) in a magnetic field. Captured infected cells were eluted following removal of the column from the magnetic field. Synchronized and enriched parasites and uninfected erythrocytes used for culture were washed twice with PBS followed by controlled lysis with 0·1% saponin, 0·06 N NaCl, sonication in the presence of enzyme inhibitors and centrifugation at 10 000 g for 10 min at 4°C. The protein concentration of both the crude lysate and erythrocyte preparations were estimated by Bio-rad protein assay (Bio-rad laboratories GmbH, Munich, Germany).
For immunophagocytosis assays, 3–5% parasite cultures containing mainly mature trophozoite or schizonts were sychronized by plasmagel flotation (Plasmagel, Laboratoire Roger Belon, France). Enriched and synchronized parasite preparations in some cases were trypsinized by exposure to 0·1 mg/ml trypsin-EDTA solution (Gibco, Paisley, UK) or by chymotryptic digestion with 0·1 mg/ml chymotrypsin solution (Sigma, Germany) at 37°C for 1 h. All preparations were washed twice in RPMI prior to assays.
ELISA
Detection of human IgG, IgG1, IgG2, IgG3 and IgG4 antibodies reacting with Cys007 P. falciparum schizont lysate was carried out as described by Aribot et al. [12], with some modifications. Briefly, 96-well plates (Corning Inc Costar®, Corning, NY, USA) were coated overnight at 4°C with 50 µl of P. falciparum antigen or erythrocyte extracts (5 µg/ml in carbonate buffer at pH 9·6). Following overnight incubation, plates were washed four times with PBS/0·5% Tween. 200 µl of 1% bovine serum albumin (BSA) in PBS was added to each well and incubated for 2 h at 37°C to block non-specific binding. The blocking step was followed by washing four times with PBS–Tween (0·5%). Serum samples diluted at 1 : 200 in PBS–Tween (0·5%) were applied (50 µl/well) in duplicate and incubated overnight at 4°C. In each plate, positive and negative control plasma pools were included, as well as four wells referred to as blank. In the blank wells only PBS–Tween (0·5%) was added. Following overnight incubation, plates were washed four times with PBS–Tween (0·5%). For total IgG determination, incubation after test samples was followed by application of 50 µl of horseradish peroxidase conjugated goat antihuman IgG (Fc-specific) (Sigma, St Louis, MO, USA) to individual wells and incubation in a waterbath at 25°C for 2 h.
For determination of IgG isotypes, 50 µl of antihuman IgG subclass-specific monoclonal antibodies (Caltag, San Francisco, CA, USA) diluted in PBS–Tween (0·5%) was dispensed into individual wells and incubated overnight at 4°C. The following antibodies were used: MoAb IgG1 (6069) 0·5 mg/ml (1/1000); IgG2 (6014) 0·5 mg/ml (1/2000); IgG3 (6047) 0·5 mg/ml (1/2000) and IgG4 (6023) 0·5 mg/ml (1/1000). Overnight incubation was followed by four washes in PBS–Tween and addition of 50 µl of horseradish peroxidase conjugated goat antimouse IgG (Sigma, St Louis, MO, USA) diluted at 1 : 10 000 in PBS−0·5% Tween to individual wells.
Incubation with conjugate (2 h at room temperature) was followed by development with 100 µl per well of a chromogen-containing substrate mixture (TMB, Kirkegaard & Perry, Gaithersburg, MD, USA). The reaction was stopped after 15 min by addition of 50 µl of 1 m phosphoric acid per well. The antibody response measured as optical density (O.D.) was determined using an ASYS Hitech DigiScan microplate reader with a 450-nm filter and a reference filter of 550 nm. The background (mock-cultured uninfected erythrocyte extract) for each serum was subtracted from the O.D. reading of the response to the parasite lysate.
Cells and determination of FcγRIIa genotypes and expression
THP-1 cells obtained from DMSZ (German Collection of Microorganisms and Cell Cultures GmbH, Braunschweig, Germany) were grown in RPMI-1640 supplemented with 10% heat-inactivated FCS, 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 25 mm HEPES, 1 mm sodium pyruvate (Sigma). Cells were differentiated to become adherent, elongated and macrophage-like cells by the addition of PMA (phorbol 12-myristate 13-acetate) at 20 ηm/ml for 72 h. FcγRIIa transfectants IIAI.6 Arg/Arg or His/His were a kind gift from Dr van de Winkel (University of Utrecht, the Netherlands). Cells were cultured as described by Rodriguez et al. [19] in RPMI-1640 supplemented with 10% heat-inactivated FCS, 2 mm l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 25 mm HEPES, 1 mm sodium pyruvate and 0·8 mg/ml Geneticin (G418, Life Technologies, Gibco BRL, Gaithersburg, MD). FcγRIIa expression was confirmed by flow cytometry using direct immunofluorescence with FITC-labelled MoAb IV.3. Transfectant FcγRIIa expression was observed to be stable during the course of experiments.
Human peripheral blood mononuclear cells (PBMC) were isolated from whole blood, from donors who gave informed consent, by standard Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation of heparin-treated blood from healthy, malaria non-exposed European donors. PBMC at 2 × 10/ml of culture medium plus 10% FCS were plated onto 8-well glass chambered slides (Nalge-Nunc, Naperville, IL, USA) and left to adhere for 2 h, after which non-adherent cells were washed off.
The FcγRIIa-R-H131 genotype of THP-1 cells and human monocytes was determined by a nested sequence-specific primer-polymerase chain reaction (SSP-PCR) on genomic DNA as described by Carlsson et al. [20].
Phagocytosis assay
Immunophagocytosis assays were performed as described by McGilvray et al. [21], with some modifications. Differentiated THP-1 cells, monocytes or transfectant cells adhered to 8-well glass chambered slides (Nalge-Nunc) were employed to study phagocytosis. First, we determined the optimal serum concentration for opsonizing P. falciparum-infected erythrocytes (PE) by incubating the PEs with different concentrations of the heat-inactivated human serum pool, P2 (0, 5, 10, 20 and 50%) for 1 h at 37°C. In blocking assays, the FcγR I and II of THP-1 cells were blocked for 25 min with saturating amounts of anti-FcγR MoAb at room temperature. Following blocking of Fc receptors, cells were washed twice with RPMI-1640.
To study antibody-mediated phagocytosis mediated by Fcγ receptors, opsonized PE following treatment with 50% serum (heat-inactivated for 30 min at 55°) for 1 h at 37°C or mock-opsonized parasites (PE) were resuspended in 500 µl of RPMI-10% FCS-l-glutamine and added to different cells at a PE : cell ratio of 25 : 1 in duplicate. For each assay, non-infected erythrocytes (UE) were included and plates rotated gently for 4 h at 37°C, 5% CO2. At the end of the 4-h incubation period, non-adherent PE or UE were washed thrice with RPMI-1640, and adherent but non-internalized PE or UE were lysed in ice-cold distilled water for 30 s. All cell preparations were fixed with methanol and stained with Giemsa. Phagocytosis was assessed by light microscopy by counting of 200–500 cells. Only erythrocytes within the phagocytic cell outline were considered in the estimation of phagocytosis. The average phagocytosis index was calculated as the percentage of effector cells with clear evidence of internalized PE measured in duplicate wells.
Statistical analyses
Differences between continuous variables were assessed using the nonparametric Mann–Whitney U-test, where a two-tailed P < 0·05 was considered significant.
RESULTS
We evaluated the role of FcγRI and FcγRIIa in IgG-mediated immunophagocytosis of P. falciparum in vitro using the human monocytic cell line, THP-1, genotyped human monocytic cells and a transfectant cell line IIAI.6, expressing different allelic forms of the FcγRIIa. In our initial experiments, we used the human monocytic cell line, THP-1, that has previously been shown to kill P. falciparum parasites in vitro [22]. First, we performed phagocytic assays to determine the phagocytic capacity and optimal serum concentration for opsonization. These cells proved able to internalize opsonized PE and the process was observed to be dependent on the serum concentration (Fig. 1). UEs were not ingested by cells (data not shown).
Fig. 1.
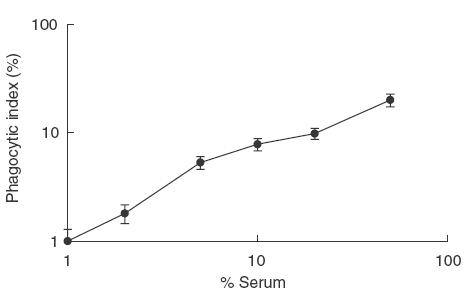
Effect of the concentration of opsonizing immune serum on phagocytosis of P. falciparum-infected erythrocytes (PE). PMA-treated THP-1 cells were exposed to PE opsonized with 0, 5, 10, 20, 40 and 50% heat-inactivated immune serum pool, P2, for 4 h at 37°C. Values on the x- and y-axes are log-transformed.
PE parasites expressing the variant surface antigen, PfEMP1 (P. falciparum erythrocyte membrane protein 1) are susceptible to CD36-mediated nonopsonic phagocytosis [21]. We examined the role of P. falciparum parasite surface antigens in antibody-mediated phagocytosis. Treatment of PEs with trypsin or chymotrypsin greatly reduced phagocytosis of PEs (Fig. 2). In the presence of immune serum this trypsin-mediated reduction was less marked, suggesting that trypsin-insensitive surface expressed parasite antigens may persist.
Fig. 2.

Comparison of P. falciparum antibody-mediated phagocytosis following different enzymatic treatment. P. falciparum-infected erythrocytes (PE) were subjected to treatment with trypsin or chymotrypsin or untreated for 1 h at 37°C. Following enzymatic treatment, PE were either opsonized with P2 or mock-opsonized and incubated with monocytes for 4 h at 37°C.
We next examined the potential role of IFN-γ in antibody-mediated phagocytosis by first exposing PMA-treated THP-1 cells for 24 h to recombinant human IFN-γ. For opsonization, we used pools of sera from non-exposed and malaria semi-immune individuals (see Materials and methods and Table 1). Our results show a non-significant trend for enhanced phagocytic activity in stimulated cells with the different pools (Fig. 3), suggesting that IFN-γ may be important for the induction of antibody-dependent phagocytosis of P. falciparum-infected erythrocytes. Several studies [23–25] have shown that IFN-γ stimulation enhances FcγRI expression but had no effect on FcγRII expression. The enhanced phagocytosis observed following stimulation could be the result of FcγI up-regulation which we also observed by flow cytometry (data not shown). The highest phagocytic indices were observed with pool P4, but the differences with P2 or P3 did not reach statistical significance.
Table 1.
Anti-schizont specific IgG isotype antibodies in sera from non-exposed and malaria-exposed individuals
| Serum pool* | IgG | IgG1 | IgG2 | IgG3 | IgG4 |
|---|---|---|---|---|---|
| P1 | 0·1 | 0 | 0·1 | 0·1 | 0·1 |
| P2 | 0·6 | 0·2 | 0·1 | 0·3 | 0 |
| P3 | 0·7 | 0·6 | 0·2 | 0·1 | 0·1 |
| P4 | 0·7 | 0·2 | 0·3 | 1·3 | 0 |
Pools were created as described in Materials and methods. Values shown are mean optical densities (O.D.) of individual sera included in pools.
Fig. 3.
Influence of interferon-gamma (IFN-γ) treatment on PMA-treated THP-1 cells on antibody-mediated phagocytosis of P. falciparum-infected erythrocytes (PE). PMA-treated THP-1 cells were stimulated with IFN-γ at 100 U/ml for 24 h and phagocytosis performed using PE opsonized with different IgG serum pools as described in Materials and methods. □, Unstimulated;  IFN-γ-stimulated.
IFN-γ-stimulated.
We next assessed the relative contribution of the different FcγR (I or II) classes to the IgG-mediated phagocytosis of PE. Blocking experiments were performed with or without saturating amounts of different anti-FcγR MoAbs (Fig. 4). Phagocytosis of serum-opsonized PE was reduced by blocking of THP-1 cells with whole CD32 or whole CD64-blocking antibodies, whereas internalization of non-opsonized PE was unaffected. In the presence of both blocking antibodies, phagocytosis was reduced to background levels. Thus, IgG-induced phagocytosis proved dependent upon both monocyte FcγRI and FcRγIIa. This result suggests a role for both FcγRI and IIa in antibody-mediated phagocytosis of opsonized PE. FcγRI has no polymorphic forms and is expressed constitutively in monocytes/macrophages only. FcγRIIa, on the other hand, is expressed on a range of different phagocytic cells and there is evidence that a point mutation in position 131 is implicated in receptor function. Hence, FcγRIIa, the more abundant of the two receptors, might be expected to play a bigger role in antibody-mediated protection in vivo. We examined the influence of this polymorphism on immunophagocytosis of PE in vitro by assessing the phagocytic capacities of a transfectant cell line, IIAI.6, expressing either the RR or the HH polymorphic forms as well as of monocytes from individuals expressing homozygous FcγRIIa-R131 or IIa-H131 with no previous malaria exposure. We used well-characterized sera in an attempt to show the influence of cytophilic IgG antibody isotypes in the receptor–parasite interaction (Table 1). Transfectants expressing FcγRIIa-H/H131 as well as monocytes from FcγRIIa-H/H131 donors were significantly more efficient in phagocytosis of P4 opsonized parasites than were FcγIIa-R/R131 transfectants or FcγIIa-R/R131 human donors’ monocytes (Fig. 5a,b). The serum pool P4 contains predominantly IgG3 antibodies with specificity for crude schizont extract of the parasite used in immunophagocytosis. Phagocytosis of PE opsonized with the P3 immune serum pool, containing predominantly anti-schizont IgG1, was higher in the presence of cells expressing FcγRIIa-R/R131 rather than FcγRIIa-H/H131, but significantly so only in the case of transfectants (Fig. 5a,b).
Fig. 4.

Effect of FcγR-blocking monoclonal antibodies (MoAbs) on antibody-mediated phagocytosis of P. falciparum-infected erythrocytes (PE) by THP-1 monocytic cells. PMA-treated THP-1 cells were incubated with either mock-opsonized PE, trypsinized PE or P2 opsonized PE or cells were blocked with saturating amounts of either FcγRI and/or FcγRll blocking MoAbs and exposed to P2 opsonized PE. Data are mean ± s.d. of a representative of three different experiments.
Fig. 5.

Phagocytosis of P. falciparum by IIAI.6 transfectants and ex vivo monocytes. (a) IIAI.6 cells expressing FcγRlla-R131 or IIa-H131 were used as effector cells with different pools of sera-opsonized P. falciparum-infected erythrocytes (PE). Data are mean ± s.d. of two different experiments carried out in duplicate. (b) Ex vivo monocytes from FcγRlla-R131 or IIa-H131 human subjects simulating experiments in (a). Data in (b) are mean ± s.d. of three individuals in each group carried out in duplicate. *P < 0·05 for comparison of phagocytic indices of cells expressing Arg-Arg versus His-His. □, Arg-Arg;  , His-His.
, His-His.
DISCUSSION
In this study we have investigated the role of Fcγ receptors (FcγR) in antibody-mediated phagocytosis using a human monocytic cell line, THP-1, human monocytes and IIAI.6 transfectant cells expressing the different FcγRIIa-Arg/His131 allelic forms. First, we confirmed that opsonization of P. falciparum-infected erythrocytes (PE) with immune serum enhances phagocytosis by PMA-treated THP-1 cells and that this process depends principally on trypsin-sensitive antigen(s) expressed on the PE surface [21,22]. We speculate that the residual amount of phagocytosis of PE we observed following trypsinization and opsonization in these experiments may reflect the involvement of trypsin-insensitive parasite-derived antigens, such as the rifins [26]. Turrini et al. [27] showed previously that monocyte-mediated phagocytosis of P. falciparum-infected human red blood cells involves both immune and nonimmune determinants as well as parasite factors. In addition, and as our results confirm, non-opsononized PE expressing PfEMP1 can be internalized by monocytes and macrophages [21], The enhancement of THP-1-mediated PE phagocytosis by IFN-γ pretreatment confirms earlier findings by Kumaratilake et al. [22] and implies the involvement of FcγRI, expression of which is selectively increased following monocyte stimulation [23–25]. On the other hand, the highest levels of phagocytosis were seen consistently after opsonization of PE with a serum pool containing predominantly parasite-specific IgG3, the isotype favoured by FcγRII. It should also be noted that our own genotypic analysis showed that the THP-1 cell line we used expresses the His/His allele of FcγRIIa, which could influence the level of phagocytosis (see below). Receptor blockade experiments, nevertheless, indicated that the phagocytosis we observed here depended on both FcγRI and FcγRII.
Because, as noted above, THP-1 cells express FcγRIIa-His/His131, we undertook more detailed investigations of FcγR–IgG isotype interactions by using a transfectant cell line expressing either FcγRIIa-His/His131 or FcγRIIa-Arg/Arg131 as well as human monocytes expressing the two alleles in their homozygous form. Regardless of the cell type, PE phagocytosis with FcγRIIa-His/His131 was significantly higher following opsonization with the IgG3-containing immune serum pool, while there was a trend for higher phagocytic indices for the combination of FcγRIIa-Arg/Arg131-expressing cells with the IgG1 pool. For both receptor types, the hierarchy of the affinity of Fc binding places IgG3 first, followed by IgG1, the major difference between the two concerning IgG2 which is bound by the His/His type with an affinity equal to that of IgG1, but much less so by the Arg/Arg type [28]. Our results could therefore be an indication of a difference in the relative affinities for IgG1 and IgG3 of the two FcγRIIa types. A possible influence of the differing amounts of parasite-specific IgG2 present in the different pools (see Table 1) cannot be excluded, however, as this would be predicted to influence the profile seen with the His/His131 type. In any case, the profile of PE phagocytosis observed with the transfected cells was not altered by the presence of FcγRI on the monocytes used. Other studies have shown that inhibition of the growth of P. falciparum blood-stage parasites in vitro is mediated by co-operation between monocytes and malaria-specific IgG1 and IgG3, but not IgG2, via the FcγRIIa [4]. Controversies surround the role of IgG2 in protection against malaria. IgG2 can bind FcγRIIa-H131 but has always been regarded as a non-cytophilic antibody [29] and has been reported to be associated with a delay in acquisition of protective responses against malaria [11]. Contrastingly, studies by Aucan and colleagues [9] as well as Deloron and colleagues [30] have shown that parasite-specific IgG2 is associated with protection from malaria. In addition, among the antibodies directed to the PE surface during acute P. falciparum infections, IgG2 predominates (AE Tebo, unpublished observations), which may be of particular relevance to our findings here.
In conclusion, our study confirms the potential utility of THP-1 cells for the study of both FcγRI- and FcγRIIa-mediated immune phagocytosis of PE in vitro. Their importance in studying FcγRIIa-dependent phagocytosis may be limited because they express FcγRIIa-H131, which binds IgG3 and both IgG2 and IgG1. As an alternative, as we have demonstrated, transfected cell lines expressing FcγR provide a reliable and useful substitute for assessing parasite antigen-specific antibody functions. Our data provide evidence that the level of phagocytic activity directed against plasmodium-infected human erythrocytes may depend not only on the IgG isotype profile of the opsonizing serum but also on effector cell receptor polymorphisms.
Acknowledgments
This study received financial support from the EU INCO-DEV Programme, contract number IC18CT980359. A.E.T. acknowledges support by the DAAD (German Academic Exchange Agency).
REFERENCES
- 1.Cohen S, Mcgregor LA, Carrington SC. Gammaglobulin and acquired immunity to malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 2.Sabchareon A, Burnof T, Ouattar AD, et al. Parasitological and clinical response to immunoglobulin administration in Plasmodium falciparum malaria. Am J Trop Med Hyg. 1991;45:297–308. doi: 10.4269/ajtmh.1991.45.297. [DOI] [PubMed] [Google Scholar]
- 3.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druihle P. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–41. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druihle P. Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med. 1995;182:409–18. doi: 10.1084/jem.182.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante A, Kumaratilake LM, Rzepczyk CM, Dayer J-M. Killing of Plasmodium falciparum by cytokine activated effector cells (neutrophil and macrophages) Immunol Lett. 1990;25:179–88. doi: 10.1016/0165-2478(90)90112-4. [DOI] [PubMed] [Google Scholar]
- 6.van der Pol WL, van de Winkel JGJ. IgG receptor polymorphisms: risk factors for disease. Immunogenetics. 1998;48:222–32. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 7.Warmerdam PAM, van de Winkel JGJ, Vlug A, Westerdaal NAC, Capel PJA. A single amino acid in the second Ig-like domain of the human Fc gamma receptor II is critical for human IgG2 binding. J Immunol. 1991;147:1338–43. [PubMed] [Google Scholar]
- 8.Parren PW, Warmerdam PA, Boeije LC, et al. On the interaction of IgG subclasses with the low affinity Fc gamma RIIa (CD32) on human monocytes, neutrophils, and platelets. Analysis of a functional polymorphism to human IgG2. J Clin Invest. 1992;90:1537–46. doi: 10.1172/JCI116022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aucan C, Traore Y, Tall F, et al. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect Immun. 2000;68:1252–8. doi: 10.1128/iai.68.3.1252-1258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi YP, Nahlen BL, Kariuki S, et al. Fcy receptor IIa (CD32) polymorphism is associated with protection of infants against high-density Plasmodium falciparum infection. VII. Asembo Bay Cohort project. J Infect Dis. 2001;184:107–11. doi: 10.1086/320999. [DOI] [PubMed] [Google Scholar]
- 11.Bouharoun-Tayoun H, Druihle P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may responsible for delayed acquisition of protective immunity. Infect Immun. 1992;60:1473–81. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aribot G, Rogier C, Sarthou JL, et al. Age- and transmission- dependent immunoglobulin isotype responses to Plasmodium falciparum blood stage antigens in individuals living in a holoendemic area of Senegal (Dielmo, West Africa) Am J Trop Med Hyg. 1997;54:449–57. doi: 10.4269/ajtmh.1996.54.449. [DOI] [PubMed] [Google Scholar]
- 13.Sarthou JL, Angel G, Aribot G, et al. Prognostic value of anti-Plasmodium falciparum specific IgG3, cytokines, and their soluble receptors in West African patients with severe malaria. Infect Immun. 1997;65:3271–6. doi: 10.1128/iai.65.8.3271-3276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oeuvray C, Theisen M, Rogier C, Trape J-F, Jepsen S, Druilhe P. Cytophilic immunoglobulin responses to Plasmodium falciparum glutamate-rich protein are correlated with protection against clinical malaria in Dielmo. Senegal Infect Immun. 2000;68:2617–20. doi: 10.1128/iai.68.5.2617-2620.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tebo AE, Kremsner PG, Luty AJ. Plasmodium falciparum: a major role for IgG3 in antibody-dependent monocyte-mediated cellular inhibition of parasite growth in vitro. Exp Parasitol. 2001;98:20–8. doi: 10.1006/expr.2001.4619. [DOI] [PubMed] [Google Scholar]
- 16.Shi YP, Udhayakumar V, Oloo AJ, Nahlen BL, Lal AA. Differential effect and interaction of monocytes, hyperimmune sera, and immunoglobulin G on the asexual stage Plasmodium falciparum parasites. Am J Trop Med Hyg. 1999;60:135–41. doi: 10.4269/ajtmh.1999.60.135. [DOI] [PubMed] [Google Scholar]
- 17.Wildling E, Winkler S, Kremsner PG, Brandts C, Jenne L, Wernsdorfer WH. Malaria epidemiology in the province of Moyen Ogooue. Gabon Trop Med Parasitol. 1995;46:77–82. [PubMed] [Google Scholar]
- 18.Trager W, Jensen JB. Cultivation of erythrocytic stages. Bull World Health Organ. 1977;155:363–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez ME, van der Pol W-L, Sanders LAM, van de Winkel JGJ. Crucial role of FcgammaRIIa (CD32) in assessment of functional anti-Streptococcus pneumoniae antibody activity in human sera. J Infect Dis. 1999;179:423–33. doi: 10.1086/314603. [DOI] [PubMed] [Google Scholar]
- 20.Carlsson LE, Santoso S, Baurichter G, et al. Heparin-induced thrombocytopenia: new insights into the impact of the FcγRIIa -R-H131 polymorphism. Blood. 1998;92:1526–31. [PubMed] [Google Scholar]
- 21.McGilvray ID, Serghides L, Kapus A, Rotstein OD, Kain KC. Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood. 2000;96:3231–40. [PubMed] [Google Scholar]
- 22.Kumaratilake LM, Ferrante A, Jaeger T, Morris-Jones SD. The role of complement, antibody, and tumor necrosis factor alpha in the killing of Plasmodium falciparum by the monocytic cell line THP-1. Infect Immun. 1997;65:5342–5. doi: 10.1128/iai.65.12.5342-5345.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guyre PM, Morganelli PM, Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983;72:393–7. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perussia B, Dayton ET, Lazarus R, Fanning V, Trinchieri G. Immune interferon induces the receptors for monomeric IgG1 on human monocytic and myeloid cells. J Exp Med. 1983;158:1092–113. doi: 10.1084/jem.158.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleit HB, Kobasiuk CD. The human monocyte-like cell line THP-1 expresses Fc gamma RI and Fc gamma RII. J Leukoc Biol. 1991;49:556–65. doi: 10.1002/jlb.49.6.556. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez V, Hommel M, Chen Q, Hagblom P, Wahlgren M. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J Exp Med. 1999;190:1393–404. doi: 10.1084/jem.190.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turrini F, Ginsburg H, Bussolino F, Pescarmona GP, Serra MV, Arese P. Phagocytosis of Plasmodium falciparum-infected human red blood cells by human monocytes: involvement of immune and non-immune and parasite developmental stages. Blood. 1992;80:801–8. [PubMed] [Google Scholar]
- 28.Pleass RJ, Woof JM. Fc receptors and immunity to parasites. Trends Parasitol. 2001;17:545–51. doi: 10.1016/s1471-4922(01)02086-4. [DOI] [PubMed] [Google Scholar]
- 29.Ferrante A, Rzepczyk CM. Atypical IgG subclass antibody responses to Plasmodium falciparum asexual stage antigens. Parasitol Today. 1997;13:145–8. doi: 10.1016/s0169-4758(97)89812-2. [DOI] [PubMed] [Google Scholar]
- 30.Deloron P, Dubois B, Le Hesran JY, et al. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin Exp Immunol. 1997;110:212–8. doi: 10.1111/j.1365-2249.1997.tb08319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


