Abstract
Antibodies specific for ribosomal P proteins (anti-P) are a hallmark of systemic lupus as anti-DNA antibodies are. It has been reported that anti-P antibodies are more frequently detected in anti-dsDNA positive sera. The aim of the present study was to verify the binding ability of anti-P antibodies towards polynucleotides and nucleosomes. We purified anti-P antibodies from 8 SLE patients' sera and we analysed them by ELISA on plates coated with DNA or nucleosomes. We performed also inhibition experiments in order to verify the specificity of the binding. All the purified anti-P antibodies bound DNA, but some anti-DNA binding activity remained among the non-anti-P antibodies in the flow through. Only half of the purified antibodies bound to nucleosomes, and anti-nucleosomal activity was demonstrated also in non anti-P antibody fraction. The inhibition experiments performed on two anti-P antibodies pointed out that only the homologous antigen inhibited the binding to either P peptide or DNA coated onto the solid phase. These results show that sera in which the two specificities coexist contain antibodies endowed with a double binding ability for DNA and the P peptide.
Keywords: Anti-DNA, anti-ribosomal antibodies, antinucleosomes, SLE
Introduction
Systemic lupus erythematosus (SLE) is characterized by the presence of several autoantibodies directed to various nuclear and cytoplasmic antigens [1]. Antibodies specific for ribosomal P proteins have been reported in SLE patients [2] in percentages ranging from 5 to 42%[3–5], possibly depending on the ethnic background of the population studied, being around 20% in Caucasian patients [6]. These antibodies are serological markers of SLE [7] as anti-Sm and anti-dsDNA antibodies are, as they are found only sporadically in other connective tissue diseases [8]. Besides the controversial correlation of anti-P antibodies with neuropsychological involvement [9–11], various reports suggested a possible correlation with disease activity [12], or with hepatic [13,14] and renal involvement [15] in SLE. From the serological point of view, anti-ribosomal antibodies have been detected more frequently in sera containing anti-Sm specificities [16,17], and indeed we have found that anti-P antibodies cross-react with SmD and SmB/B′ proteins [18]. It has recently been reported that anti-P antibodies are more frequently detected in anti-dsDNA positive sera and that they are related to renal disease activity [19,20].
These findings can depend on the coexpression of two autoantibodies in the same serum or may be related to a double specificity of the same antibody population. The aim of the present study is to analyse the fine specificity of anti-P antibodies affinity purified from SLE patient sera, studying their reactivity with polynucleotides and nucleosomes.
Materials and methods
Patient sera
Sera from a number of patients fulfilling the ARA criteria for the diagnosis of SLE [21] were tested for anti-P, anti-ssDNA and anti-dsDNA antibodies with the ELISA tests described below. Eight sera showing both anti-P and anti-DNA specificity were used for further experiments.
ELISA for anti-P protein antibodies
Anti-P antibodies were detected in sera or in eluates and flow throughs of the peptide column (see below) by a previously described ELISA assay [22]. Briefly, we used a multiple antigen peptide (MAP) bearing a lysine core and carrying four copies of the antigenic 13-mer C-terminal sequence shared by the three ribosomal P proteins as antigen for the coating of the plates (Nunc Maxi-Sorp F96, Denmark) at 1 µg/ml in PBS in an overnight incubation. Saturation was carried out with gelatine 1% in PBS. Serum samples diluted 1:300 and purified antibodies at different concentrations (from 20 µg/ml to 0·3 µg/ml) were diluted in PBS/gelatine 0·5%/Tween 20 0·05%. Anti-human IgG (Fab2 fragment) labelled with alkaline phosphatase (Sigma Chemical Co., St. Louis, MO, USA) was used as the second step antibody. Plates were developed with p-nitrophenyl-phosphate based substrate. Positive and negative controls were run in each assay.
Purification of anti-P protein antibodies
The ribosomal MAP peptide (16 mg/g resin) was conjugated to CNBr activated-Sepharose (Sigma Chemical Co., St. Louis, MO, USA). Immunoglobulins from 8 sera containing anti-P antibodies from 8 different SLE patients were precipitated with ammonium sulphate; the precipitates were dissolved in PBS buffer, dialysed, and applied to the column. For each serum the flow-through was collected, and the antibodies binding to the column were eluted by 0·1 m glycine buffer pH 2·8 containing 3 m NaCl. The content of anti-P antibodies in eluates and flow throughs was tested by ELISA. The flow throughs were applied to the column until the anti-P antibody activity was removed.
Isolation of anti-DNA antibodies
The IgG fraction, obtained from 4 patients sera (patients 1, 2, 5, 7) by affinity chromatography on a Protein A column, was absorbed on a polydT column until no more proteins were eluted by elution buffer. The eluted anti-DNA antibodies were concentrated on a Centricon (Amicon Division, Grace Co., Beverly, MA, USA) and dialysed against PBS.
DNA-binding activity
Anti-DNA activity was measured as previously described with minor modifications [23]. Briefly, polystyrene plates (Greiner, Germany) were precoated for 30 min with poly lysine 50 µg/ml, washed with TBS (TRIS buffered saline), and were then coated with 10 µg/ml of ssDNA or dsDNA in TBS 10 mm EDTA. After overnight incubation at room temperature, the residual positive charges of lysine were blocked by the addition of polyglutamate 50 µg/ml and incubated one hour at room temperature. The plates were again washed with TBS, and 3% BSA, 5% FCS in PBS was added. After one hour the antibodies diluted in 1% BSA, 2·5% FCS, 0·05% Tween in PBS (diluting buffer) were added at the chosen concentration and the plates were incubated for 3 h at room temperature. The plates were then washed once with 1% Tween in PBS and twice with PBS. Alkaline phosphatase-conjugated goat anti-human IgG (Sigma) in diluting buffer was added and the solution was incubated overnight at 4°C. After washings, the bound enzymatic activity was measured with p-nitrophenyl-phosphate based substrate. Positive and negative controls were run in each assay.
Inhibition experiments
For the inhibition experiments, the amount of anti-P antibodies that gave 50% of the maximum binding on the solid phase was determined. This amount was then pre-incubated with different amounts of synthetic peptides or nucleic acids for one hour at room temperature, before being transferred to antigen-coated plates. The assay was carried out as for the direct binding assay. LKM1 sequence (aa 254–271 of the cytochrome P-450 monooxygenase) synthesized on a MAP core identical to the ribosomal MAP was used as control peptide in inhibition experiments. Double stranded synthetic polynucleotides (polydGpolydC and polyApolyU) were used as inhibitors on DNA coated plates.
Anti-nucleosomal activity
Anti-nucleosomal antibodies were detected by a commercial kit (INOVA Diagnostic, QUANTA Lite Chromatin ELISA). Eluates and flow throughs of the peptide column were tested at 10 µg/ml according to the manufacturer's instructions. Immunoglobulins from normal human sera were included in the test as negative controls.
Results
Purified anti-P antibodies and non anti-P antibodies from SLE patients were tested on ssDNA and dsDNA coated plates (Figs 1 and 2). All the eluates of the P column (anti-P antibodies) bound ssDNA and 6/8 bound dsDNA. Results are shown in Table 1.
Fig. 1.
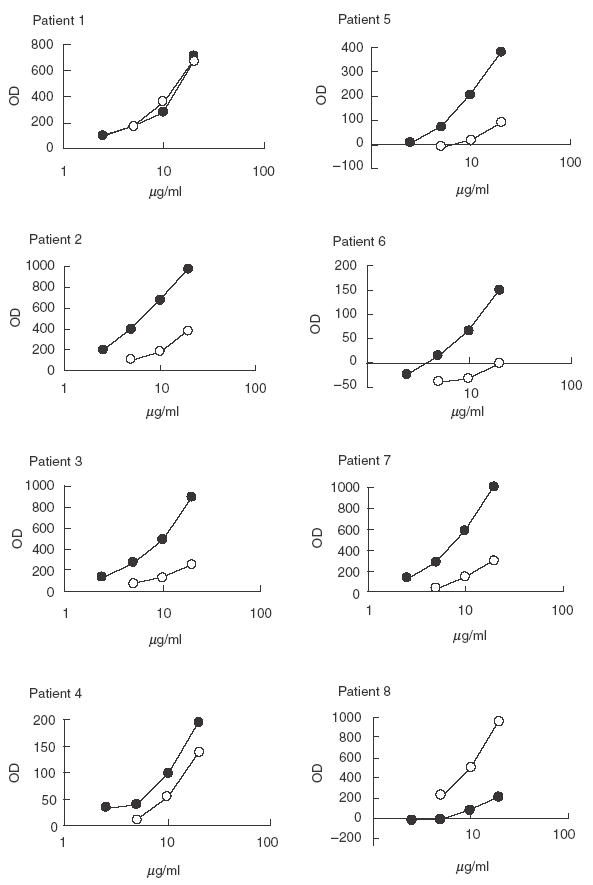
Binding of purified anti-P antibodies (•) and of the flow throughs (○) to ssDNA coated plate in ELISA. Purified anti-P antibodies were tested at concentration of 20, 10, 5 and 2·5 µg/ml. Antibodies of the flow throughs were tested at concentration of 20, 10 and 5 µg/ml.
Fig. 2.
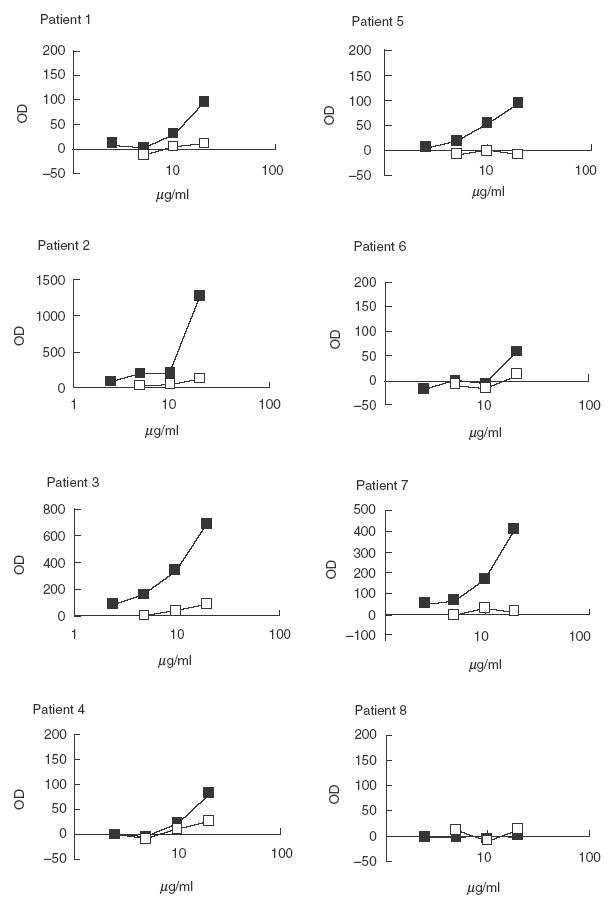
Binding of purified anti-P antibodies (▪) and of the flow throughs (□) to dsDNA coated plate in ELISA. Purified anti-P antibodies were tested at concentration of 20, 10, 5 and 2·5 µg/ml. Antibodies of the flow throughs were tested at concentration of 20, 10 and 5 µg/ml.
Table 1.
The table summarizes the results obtained with anti-P and non anti-P antibodies tested on DNA and nucleosomes
| Anti- ssDNA | Anti- dsDNA | Anti- nucleosomes | |
|---|---|---|---|
| Patient 1 | |||
| Purified anti-P | Positive | Positive | Positive |
| Flow through | Positive | Negative | Negative |
| Patient 2 | |||
| Purified anti-P | Positive | Positive | Positive |
| Flow through | Positive | Negative | Negative |
| Patient 3 | |||
| Purified anti-P | Positive | Positive | Negative |
| Flow through | Positive | Negative | Negative |
| Patient 4 | |||
| Purified anti-P | Positive | Positive | Positive |
| Flow through | Positive | Negative | Positive |
| Patient 5 | |||
| Purified anti-P | Positive | Positive | Negative |
| Flow through | Positive | Negative | Negative |
| Patient 6 | |||
| Purified anti-P | Positive | Negative | Negative |
| Flow through | Negative | Negative | Negative |
| Patient 7 | |||
| Purified anti-P | Positive | Positive | Positive |
| Flow through | Positive | Negative | Positive |
| Patient 8 | |||
| Purified anti-P | Positive | Negative | Negative |
| Flow through | Positive | Negative | Positive |
Flow throughs were quite heterogeneous. In some patients the anti-P column removed most anti-ssDNA and dsDNA binding activity (patients 2, 3, 5), and most anti-dsDNA binding activity in patients 1, 4 and 7. Anti-ssDNA were equally distributed between eluate and flow through in patients 1 and 4 while in patient 7 the flow through was almost devoid of anti-ssDNA activity; on the contrary in patient 8 the flow through contained more anti-ssDNA antibodies than the eluate.
Conversely anti-DNA antibodies obtained by purification on a polydT column from patients 1, 2, 5 and 7 showed binding activity on ribosomal MAP (data not shown).
To further analyse the cross-reactivity of anti-P antibodies, inhibition experiments were performed on the antibodies purified from patients 3 and 7 sera that display high anti-DNA binding ability. The results obtained for the two patients are shown in Figs 3 and 4. In both cases only the ribosomal peptide inhibited the binding of anti-P antibodies to the P peptide in a dose-dependent way. Similarly, the binding of anti-P antibodies to solid phase DNA was not inhibited by the P or the control peptide while the nucleic acids exerted a dose-dependent inhibition, and among them the most effective were ssDNA and polydGdC.
Fig. 3.
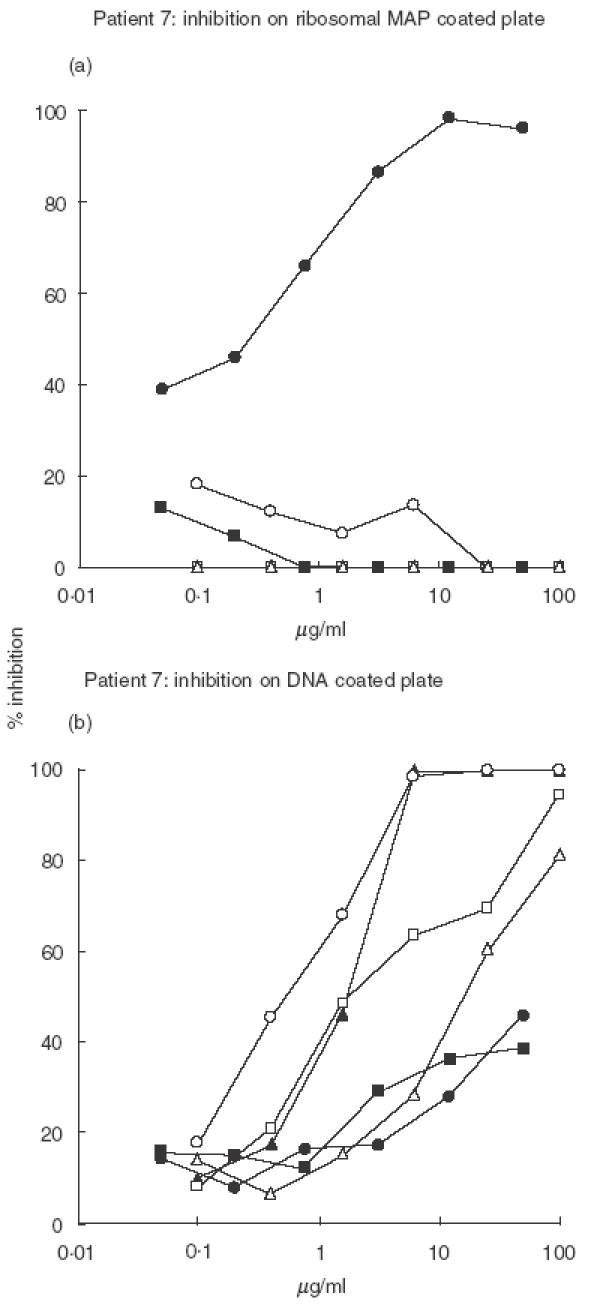
Purified anti-P antibodies from Pt 7 were preincubated with various inhibitory agents at concentrations between 100 and 0·05 µg/ml, then dispensed to (a) ribosomal MAP or (b) DNA coated plate in order to verify the residual binding activity. See Materials and methods for details. • Ribosomal MAP, □ MAP LKM, □ dsDNA, ▴ polydGdC, ○ ssDNA, ▵ polyApolyU.
Fig. 4.
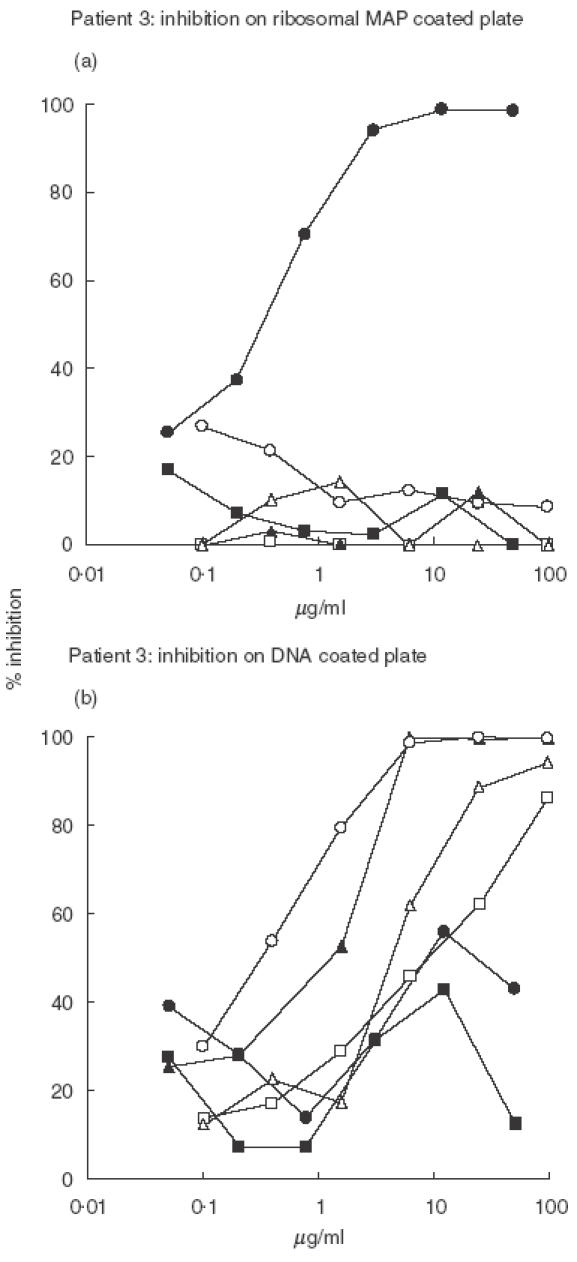
Purified anti-P antibodies from Pt 3 were preincubated with various inhibitory agents at concentrations between 100 and 0·05 µg/ml, then dispensed to ribosomal MAP or DNA coated plate in order to verify the residual binding activity. See Materials and methods for details. • Ribosomal MAP, ▪ MAP LKM, □ dsDNA, ▴ polydGdC, ○ ssDNA, ▵ polyApolyU.
Purified anti-P antibodies and non anti-P antibodies from SLE patients were also tested for anti-nucleosomal binding capacity (see Table 1). Anti-P from patients 1, 2, 4 and 7 bound nucleosomes, and only in patients 4 and 7 anti-nucleosomal activity was present also in the non anti-P antibody fraction. In patient 8 anti-P antibodies did not bind dsDNA and anti-nucleosomal activity was detected only in the flow through. patients 3, 5 and 6 did not show any anti-nucleosomal activity, either in the anti-P fraction or in the flow through.
Discussion
The results of the present study indicate that anti-ribosomal antibodies display both anti-ssDNA and/or anti-dsDNA activity; in half of the cases they recognize epitopes exposed also on DNA complexed with histones and nonhistonic proteins in nucleosomes.
The anti-ribosomal antibodies were purified using a MAP where a core of polyLys carries 4 copies of C-terminal sequence of P proteins. These antibodies bind in ELISA the ribosomal MAP and in immunoblotting the three ribosomal P proteins [22]. The anti-P antibodies did not react with the unrelated sequence synthesized as a MAP in direct binding or in inhibition experiments, thereby excluding that the eluates from P column contain antibodies reacting with the MAP core structure.
The ability to bind ssDNA seems to be a general property of anti-P antibodies, and the binding to dsDNA is present in most anti-P preparations. Similarly to most anti-DNA antibodies [24], anti-P antibodies recognize the backbone of polydeoxynucleotides. In fact, analysing their binding properties by inhibition assays, we found that ssDNA and polydGdC are the best inhibitors for anti-P antibody binding to DNA, but also the other nucleic acids exert a good inhibition. The RNA analogue polyApolyU is less effective, indicating a preferential recognition of polydeoxynucleotides.
The cross-inhibition experiments showed that the binding to the solid phase antigen could be inhibited only by the homolo-gous antigen: the P peptide does not inhibit the binding to DNA and conversely DNA does not inhibit the binding to the P peptide.
These results suggest that the antibody binding site can accommodate both antigens, as the binding of one antigen does not restrict the binding ability for the other antigen. This property, already observed with the anti-P binding to Sm antigen [18] seems to be a distinctive feature of anti-ribosomal autoantibodies.
The anti-P and anti-DNA specificities are only partially overlapped since the anti-P column removes all the anti-dsDNA activity but only part of the anti-ssDNA activity. Moreover, antibodies binding nucleosomal antigens are present both in the anti-P fraction and in the flow through in four cases and exclusively in the flow through in one case. It is presently unclear if the anti-nucleosomal binding activity retained in the flow through is due to antibodies specific for nucleosomal proteins or for DNA-protein complexes.
The cross-reactivity of anti-DNA antibodies with ribosomal P proteins was first reported by Sun et al. [25]. They observed that purified anti-dsDNA antibodies, able to bind to the membrane of cells and to exert a cytostatic effect, reacted with ribosomal P proteins, and their dsDNA binding activity was partially inhibited by the C-terminal peptide of P proteins or the recombinant P1 protein.
In a subsequent paper, Sun et al. [26] reported that purified anti-dsDNA antibodies exerted their cytostatic activity on several cell types through their binding to P proteins expressed on the surface of the cells, and they suggested that this binding may have a pathogenic potential, i.e. may mediate tissue damage in SLE patients.
Takeda et al. [27] also examined the cross-reactions of anti-P and anti-DNA antibodies. Similarly to our findings, they reported that anti-P activity was present in most of the anti-dsDNA antibodies they tested. However, they were unable to obtain anti-P antibodies applying anti-DNA antibodies on a P peptide-coupled column and they concluded that anti-P activity was not a property but rather a contaminant of anti-DNA antibodies. The selection of anti-DNA antibodies reacting exclusively with dsDNA and the purification procedure for anti-P antibodies may explain these results, different from the ones reported by Sun and in the present paper. However, in another recent report [28] the same authors suggest a wide overlap of anti-P and anti-DNA antibodies and describe similar immunochemical features of the two autoantibodies in the different phases of systemic lupus.
In conclusion it is known that anti-DNA antibodies are an extremely heterogeneous group of antibodies. It is likely that the population of anti-DNA with anti-P activity corresponds to a small fraction of anti-DNA antibodies. On the other hand, anti-P antibodies are overlapped with anti-Sm antibodies. Our findings suggest a complex interplay of the 3 autoantibody specificities that represent serological markers of SLE. Their serum coexpression may be due to expansion of clones with double reactivity and it is presently unknown if the double reactivity offers a selective advantage to the clones. Nevertheless the presence of anti-P antibodies in renal eluates [29] from nephritic lupus kidneys and the higher frequency of these antibodies in active renal disease suggest an important pathogenic role of these autoantibodies in lupus nephritis.
Further studies are needed to elucidate the mechanisms involved in the induction of nephritis and the specific role of individual autoantibody populations in mediating organ damage.
REFERENCES
- 1.Steinberg AD, Klinman DM. Pathogenesis of systemic lupus erythematosus. Rheum Dis North Am. 1988;14:25–41. [PubMed] [Google Scholar]
- 2.Elkon KB, Parnassa AB, Foster CL. Lupus antibodies target ribosomal P proteins. J Exp Med. 1985;162:459–71. doi: 10.1084/jem.162.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonfa E, Elkon KB. Clinical and serological associations of the antiribosomal P protein antibodies. Arthritis Rheum. 1986;29:981–5. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- 4.Schneebaum AB, Singleton JD, West SG, Blodjett JK, Allen LG, Cheronis JC, Kotzin BL. Association of psychiatric manifestations with antibodies to ribosomal P proteins in systemic lupus erythematosus. Am J Med. 1991;90:54–62. doi: 10.1016/0002-9343(91)90506-s. [DOI] [PubMed] [Google Scholar]
- 5.Teh LS, Lee MK, Wang F, et al. Antiribosomal P protein antibodies in different populations of patients with systemic lupus erythematosus. Br J Rheumathol. 1993;32:663–5. doi: 10.1093/rheumatology/32.8.663. [DOI] [PubMed] [Google Scholar]
- 6.Arnett FC, Reveille JD, Moutsopoulos HM, Georgescu L, Elkon KB. Ribosomal P autoantibodies in systemic lupus erythematosus. Frequencies in different ethnic groups and clinical and immunogenetic association. Arthritis Rheum. 1996;39:1833–9. doi: 10.1002/art.1780391109. [DOI] [PubMed] [Google Scholar]
- 7.Gordon J, Towbin H, Roshental M. Antibodies directed against ribosomal protein determinants in the sera of patients with connective tissue diseases. J Rheumatol. 1982;9:247–52. [PubMed] [Google Scholar]
- 8.Bonfa E, Elkon KB. Clinical and serologic associations of the antiribosomal P protein antibody. Arthritis Rheum. 1986;29:981–5. doi: 10.1002/art.1780290806. [DOI] [PubMed] [Google Scholar]
- 9.Bonfa E, Golombek SJ, Kaufman LD, Skelly S, Weissbach H, Brot N, Elkon KB. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987;317:265–71. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- 10.Nojima Y, Minota S, Yamada A, Takaku F, Aotsuka S, Yokohari R. Correlation of antibodies to ribosomal P protein with psychosis in patients with systemic lupus erythematosus. Ann Rheum Dis. 1992;51:1053–5. doi: 10.1136/ard.51.9.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teh LS, Isenberg DA. Antiribosomal P protein antibodies in systemic lupus erythematosus. A reappraisal. Arthritis Rheum. 1994;37:307–15. doi: 10.1002/art.1780370303. [DOI] [PubMed] [Google Scholar]
- 12.Sato T, Uchiumi T, Ozawa T, et al. Autoantibodies against ribosomal proteins found with high frequency in patients with systemic lupus erythematosus with active disease. J Rheumatol. 1991;18:1681–4. [PubMed] [Google Scholar]
- 13.Arnett FC, Reichlin M. Lupus hepatitis: an under-recognized disease feature associated with autoantibodies to ribosomal P. Am J Med. 1995;99:465–72. doi: 10.1016/s0002-9343(99)80221-6. [DOI] [PubMed] [Google Scholar]
- 14.Koren E, Schnitz W, Reichlin M. Concomitant development of chronic active hepatitis and antibodies to ribosomal P proteins in a patient with systemic lupus erythematosus. Arthritis Rheum. 1993;36:1325–8. doi: 10.1002/art.1780360917. [DOI] [PubMed] [Google Scholar]
- 15.Hulsey M, Goldstein R, Scully L, Surbeck W, Reichlin M. Anti-ribosomal P antibodies in systemic lupus erythematosus. a case-control study correlating hepatic and renal disease. Clin Immunol Immunopathol. 1995;74:252–6. doi: 10.1006/clin.1995.1037. [DOI] [PubMed] [Google Scholar]
- 16.Elkon KB, Bonfa EL, Lovet R, Eisenberg RA. Association between anti-Sm and anti-ribosomal P protein autoantibodies in human systemic lupus erythematosus and MRL/lpr mice. J Immunol. 1989;143:1549–54. [PubMed] [Google Scholar]
- 17.Nojima Y, Minota S, Yamada A, Takaku F. Identification of an acidic ribosomal protein reactive with anti-Sm autoantibody. J Immunol. 1989;143:1915–20. [PubMed] [Google Scholar]
- 18.Caponi L, Bombardieri S, Migliorini P. Anti-ribosomal antibodies bind the Sm proteins D and B/B′. Clin Exp Immunol. 1998;112:139–43. doi: 10.1046/j.1365-2249.1998.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chindalore V, Neas B, Reichlin M. The association between anti-ribosomal P antibodies and active nephritis in systemic lupus erythematosus. Clin Immunol Immunopathol. 1998;87:292–6. doi: 10.1006/clin.1998.4541. [DOI] [PubMed] [Google Scholar]
- 20.Monova D, Argirova T, Monov S. Antiribosomal P antibodies in patients with lupus glomerulonephritis. Clin Nephrol. 2001;55:425–6. [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Caponi L, Pegoraro S, Di Bartolo V, Rovero P, Revoltella R, Bombardieri S. Autoantibodies directed against ribosomal P proteins. use of a multiple antigen peptide as the coating agent in ELISA. J Immunol Meth. 1995;179:193–202. doi: 10.1016/0022-1759(94)00285-5. [DOI] [PubMed] [Google Scholar]
- 23.Puccetti A, Madaio MP, Bellese G, Migliorini P. Anti-DNA antibodies bind to DNAse I. J Exp Med. 1995;181:1797–804. doi: 10.1084/jem.181.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz RS, Stollar BD. Origins of anti-DNA autoantibodies. J Clin Invest. 1985;75:321–7. doi: 10.1172/JCI111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun KH, Liu WT, Tsai CY, Tang SJ, Han SH, Yu CL. Anti-dsDNA antibodies cross-react with ribosomal P proteins expressed on the surface of glomerular mesangial cells to exert a cytostatic effect. Immunology. 1995;85:262–9. [PMC free article] [PubMed] [Google Scholar]
- 26.Sun KH, Liu WT, Tang SJ, Tsai CY, Hsieh SC, Wu TH, Han SH, Yu CL. The expression of acidic ribosomal phosphoproteins on the surface membrane of different tissues in autoimmune and normal mice which are the target molecules for anti-double-stranded DNA antibodies. Immunology. 1996;87:362–71. [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda I, Rayno K, Wolfson-Reichlin M, Reichlin M. Heterogeneity of anti-dsDNA antibodies in their cross-reaction with ribosomal P protein. J Autoimmun. 1999;13:423–8. doi: 10.1006/jaut.1999.0330. [DOI] [PubMed] [Google Scholar]
- 28.Takeda I, Rayno K, Movafagh FB, Wolfson-Reichlin M, Reichlin M. Dual binding capabilities of anti-double-stranded DNA antibodies and anti-ribosomal phosphoprotein (P) antibodies. Lupus. 2001;10:857–65. doi: 10.1191/096120301701548508. [DOI] [PubMed] [Google Scholar]
- 29.Reichlin M, Wolfson-Reichlin M. Evidence for the participation of anti-ribosomal P antibodies in lupus nephritis. Arthritis Rheum. 1999;42:2728–9. doi: 10.1002/1529-0131(199912)42:12<2728::AID-ANR34>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]


