Abstract
Gp-340 is a glycoprotein belonging to the scavenger receptor cysteine rich (SRCR) group B family. It binds to host immune components such as lung surfactant protein D (SP-D). Recent studies found that gp-340 interacts directly with pathogenic microorganisms and induces their aggregation, suggesting its involvement in innate immunity. In order to investigate further its potential immune functions in the appropriate cell lines, the expression of gp-340 in four conventional immune cell lines (U937, HL60, Jurkat, Raji), and two innate immune-related epithelial cell lines (A549 derived from lung and AGS from stomach), was examined by RT-PCR and immunohistochemistry. The resting immune cell lines showed weak or no gp-340 mRNA expression; while the two epithelial cell lines expressed gp-340 at much higher level, which was differentially regulated by phorbol myristate acetate (PMA) treatment. In the A549 cells, gp-340 was up-regulated along with the PMA-induced proinflammatory expression of both IL-6 and IL-8. In AGS cells, PMA down-regulation of gp-340 was seen in parallel with an up-regulation of the two mature gastric epithelial specific proteins TFF1 (trefoil factor 1) and TFF2, which are implicated as markers of terminal differentiation. Analysis of the distribution of gp-340, together with the TFFs and SP-D in normal lung and gastric mucosa, supported further our in vitro data. We conclude that the differential regulation of gp-340 in the two epithelial cell lines by PMA indicates that gp-340 s involvement in mucosal defence and growth of epithelial cells may vary at different body locations and during different stages of epithelial differentiation.
Keywords: cytokine, epithelial cells, gp-340/DMBT1, SP-D, TFFs
Introduction
Gp-340 is a recently characterized 340-kDa glycoprotein that belongs to the SRCR group B protein family. It was first purified from bronchoalveolar lung washings of alveolar proteinosis patients, and shown to bind lung surfactant protein D (SP-D), a collectin involved in innate immunity [1,2] in a calcium-dependent manner [3]. The domain structure of gp-340 is composed of 13 SRCR domains, followed by two C1r/C1s Uegf Bmp1 (CUB) domains flanking the 14th SRCR and then a zona pellucida (ZP) domain. All these three types of domains have been implicated in mediating protein–protein interactions [4–6].
RT-PCR and immunohistochemistry studies showed that gp-340 is synthesized mainly by epithelial cells from the respiratory, alimentary and reproductive systems, although signals are also detectable in the brain [7]. It has also been found in association with cells from the immune system, such as alveolar macrophages [8].
Gp-340 has been cloned at cDNA level and identified to be an alternatively spliced form of the gene DMBT-1 (deleted malignant brain tumour-1) [7], which is located at chromosome 10q25.3–q26.1 [9]. DMBT-1 has been proposed as a candidate tumour suppressor gene for brain, gastrointestinal and lung cancer, because of its frequent homozygous deletion or lack of expression in these tumours [9–12], and its increased susceptibility to genomic instability [13].
Gp-340 is a large and highly glycosylated protein, present in various types of cells and showing a wide tissue distribution, which suggest multiple functions it may have. The majority of the known members of SRCR B family function in the body's immune system [14–16]. The high homology in structure between gp-340 and these molecules suggests that gp-340 may also play a role in the immune system. Mollenhauer and coworkers have shown that mRNA of the gp-340 encoding gene DMBT-1 is widely present in the human lymphoid organs such as lymph node, thymus and spleen [8]. Moreover, its chemokinetic effect on alveolar macrophages has been demonstrated [17]. The finding that gp-340 binds specifically to SP-D together with its expression in tissue macrophages, both intracellularly and in a plasma membrane associated form [7], suggested that gp-340 may act as an opsonin receptor for SP-D. However, recent data showed that gp-340 is identical to human salivary agglutinin, a soluble molecule that is able to bind and aggregate bacteria such as Streptococcus mutans and Helicobacter pylori [18,19], and to form hetero-complexes with IgA in mouth cavity [20]. These findings suggested a co-operative action between SP-D and soluble form of gp-340 in the defence against microorganisms, as shown recently in a viral model of aggregation and neutralization (Kevan L. Hartshorn et al. submitted for publication).
The known homologues of gp-340 in rat, mouse and rabbit, namely ebnerin, CRP-ductin and hensin, are all synthesized by epithelial cells at different body locations. They have been reported to play roles in various physiological processes, such as epithelial differentiation in kidney collecting ducts [21] and small intestine [22], or as part of the secretion of various glands [23]. Therefore similar functions for gp-340 in human systems are well worth being characterized.
At present, there is a lack of in vitro cell-culture systems which could be used to facilitate the investigation of the putative roles of gp-340 in both mucosal defence and in epithelial growth. To address such a need, we screened for gp-340 expression in six immune-related cell lines before and after PMA stimulation. After pronounced expression of gp-340 being found in the two epithelial cell lines, an effort was then made to correlate PMA regulation of gp-340 expression with the different epithelial responses it triggered, which might shed light on possible involvement of gp-340 in these cellular events. Our in vitro data were supported further by immunohistochemical localization of gp-340 in normal human lung and gastric mucosa.
Materials and methods
Antibodies
Monoclonal antibodies (MoAbs) against gp-340 (Hyb213-6) and SP-D (Hyb245-1) have been described previously [3,18,24]. Polyclonal antibodies against TFF1 (2239A) and TFF2 (2240B) are a generous gift from Dr Lars Thim, Novo Nordisk. A monoclonal antibody Ki-67 was obtained from Dako (Glostrup, Denmark).
Cell culture and stimulation
The T-cell leukaemia derived Jurkat cell line, the B lymphoblastoid Raji cell line, the monocytic cell line U937 and the promyelocytic cell line HL-60 (American Type Culture Collection, Rockville, MD, USA) were kept in RPMI-1640 culture medium (pH 7·4; Sigma, St Louis, MO, USA) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS), 50 µg/ml streptomycin and 50 IU/ml penicillin. The two epithelial cells, AGS (ATCC, Rockville, MD, USA), a poorly differentiated gastric adenocarcinoma cell line, and A549 (ATCC), a human pulmonary epithelial cell line which shows features of type II alveolar epithelial cells and produces surfactant, were grown in F-12K nutrient mix (Life Technology, Rockville, MD, USA), containing 10% (v/v) fetal bovine serum, 100 µg/ml streptomycin and 100 IU/ml penicillin. All cultures were maintained at 37°C in a humidified atmosphere containing 95% air and 5% CO2.
For stimulation, confluent monolayer of cells were cultured in 3·5 ml medium in 6-well tissue culture plates (Corning Costar, Cambridge, MA, USA). Phorbol 12-myristate 13-acetate (PMA, Calbiochem, Darmstadt, Germany) was added in a dose-range of 1–100 ng/ml for 24 h, after which cells were washed three times with PBS and harvested with trypsin (0·05% (w/v), Life Technology) and EDTA (0·02% w/v) for immunohistochemistry studies, or lysed directly in each well for total RNA isolation.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
The expressions of gp-340, SP-D, TFF1, TFF2, IL-6 and IL-8 mRNAs were determined by RT-PCR. Total RNA samples were isolated from various cultured cell lines using a RNeasy Protect Mini kit (Qiagen, West Sussex, UK). The respective total RNAs (5 µg) were converted into cDNAs using Oligo (dT)15 primers and Superscript II reverse transcriptase (Life Technology) and diluted with H2O to obtain 250 µl of the cDNA preparation. Five microlitres of this cDNA preparation was subjected to PCR. Sequences of all the primers used in the present study, sizes of PCR products, and PCR conditions are listed in Table 1. All the primers were designed to cross exon–intron borders, to exclude the amplification of genomic templates. Human transferrin receptor (TfR) was used to standardize the amount of templates for specific primers.
Table 1.
Primers and PCR conditions used for RT-PCR studies
| Primer sequences (product sizes) | PCR condition |
|---|---|
| Gp-340* (542 bp) | (94°C 30 s, 56°C 30 s, 72°C 22 s, 29cycles) |
| F: 5-AAATTCATCCTATGGTCTA-3 | |
| R: 5-GAGAGGGGAACTGCGGTAG-3 | |
| SP-D (466 bp) | (94°C 30 s, 61·5°C 30 s, 72°C 40 s, 35cycles) |
| F: 5′-AGCTGGGCCCAAAGGAGAAGTAGG-3′ | |
| R: 5′-AGCGGCAGAGCGTGGAGAGG-3′ | |
| TFF1† (146 bp) | (94°C 30 s, 60°C 30 s, 72°C 18 s, 25 cycles) |
| F: 5′-TTTGGAGCAGAGAGGAGGCAATG-3′ | |
| R: 5′-ACCACAATTCTGTCTTTCACGGGGG-3′ | |
| TFF2 (101 bp) | (94°C 30 s, 60°C 30 s, 72°C 18 s, 30 cycles) |
| F: 5′- GTGTTTTGACAATGGATGCTG − 3′ | |
| R: 5′- CCTCCATGACGCACTGATC-3′ | |
| IL-6 (353 bp) | (94°C 30 s, 58°C 30 s, 72°C 22 s, 28cycles) |
| F: 5′-AAAGAGGCACTGGCAGAAAACAAC-3′ | |
| R:5′-TTAAAGCTGCGCAGAATGAGAATGA-3′ | |
| IL-8 (612 bp) | (94°C 30 s, 54°C 30 s, 72°C 40 s, 27 cycles) |
| F: 5′-CACCGGAAGGAACCATCTCA-3′ | |
| R: 5′-CCCGTGCAATATCTAGGAAAATC-3′ | |
| HTfR* (300 bp) | (94°C 30 s, 56°C 30 s, 72°C 22 s, 23 cycles) |
| F: 5′-GTCAATGTCCCAAACGTCACCAGA-3′ | |
| R: 5′-ATTTCGGGAATGCTGAGAAAACAGACAGA-3′ |
The PCR products of gp-340, SP-D, TFF1, TFF2 and TfR obtained from several amplifications were pooled together for each molecule, subjected to agarose gel electrophoresis, extracted using QIAEX II gel extraction kit (Qiagen) and sequenced.
Immunohistochemistry
Sections, 4 µm, were cut from paraffin-embedded blocks of tissue and cell pellets from cultured epithelial cells fixed in neutral-buffered formaldehyde. Sections were mounted on ChemMate Capillary Gap Slides (Dako, Glostrup, Denmark), dried at 60°C, deparaffinized and hydrated. Antigen retrieval was performed using microwave heating in Target Retrieval Solution (Dako). Three Tissue-Tek containers (Miles Inc., Elkhart, USA), each with 24 slides in 250 ml buffer, were placed on the edge of a turntable inside the microwave oven. Slides were heated for 11 min at full power (900 W), then for 15 min at 400 W and then left in buffer for another 15 min. Any endogenous biotin was then blocked with a Biotin-Blocking System (Dako), and the slides were incubated with a monoclonal antibody against gp-340 (Hyb213-6, 8·5 µg/ml), a monoclonal antibody against SP-D (Hyb245-1, 2·5 µg/ml), a monoclonal antibody against Ki-67 antigen (Dako, 20 µg/ml) and polyclonal antibodies against TFF1 (2239A) and TFF2 (2240B) in 1 : 500 dilution, for 25 min at room temperature. Immunostaining was performed with the ChemMate HRP/DAB detection kit (K5001, Dako) using automated equipment (TechMate 1000, Dako), and was followed by brief nuclear counterstaining in Mayer's haematoxylin. Cover slips were mounted with Aquatex (Merck, Darmstadt, Germany). The specificity of immunostaining was verified by replacing the primary antibodies with an isotype MoAb or irrelevant polyclonal antibody to the same concentration.
Flow cytometry
Cells were permeabilized using a Cytofix/Cytoperm Kit (Pharmingen, San Diego, CA, USA), following the instruction of the manufacture. A549 cells were then incubated with 2 µg/ml primary MoAb against human SP-D (Hyb245-1), and AGS cells with a polyclonal antibody against TFF1 (2239A, 1 : 100 dilution) in wash buffer (PBS, 1% (v/v) heat-inactivated FCS, 0·09% (w/v) sodium azide, pH 7·4–7·6) for 1 h at 4°C. Control experiments were carried out using mouse IgG1 (Dako) or normal rabbit immunoglobulin fraction (Dako) at the same concentration as specific antibodies. Thereafter the cells were washed twice and incubated with FITC-conjugated goat antimouse F(ab′)2 or FITC-conjugated swine antirabbit F(ab′)2 secondary antibody (1 : 20 dilution; Dako), for 45 min at 4°C in darkness. Stained cells were washed twice and subjected to immediate analysis on a FACScan flow cytometer (Becton Dickinson, San Diego, CA, USA). Data were analysed using CellQuest Software (Becton Dickinson).
Results
Distribution of gp-340 in immune and epithelial cell lines
Expression of gp-340 mRNA in the four immune cell lines and two epithelial cell lines was analysed by RT-PCR. The expression profile shown in Fig. 1a was obtained after 42 cycles of PCR amplification. Samples from different treatments were standardized by the intensity of the PCR products using transferrin receptor (TfR) specific primers. No signal was detected from the B lymphoblastoid Raji cell, the monocytic cell line U937 and the promyelocytic cell line HL-60. A weak signal from the T-cell leukaemia-derived Jurkat cells was clear and repeatable. Strong signals with slightly different strength were found in the two adenocarcinoma cell lines, A549 derived from alveolar type II cells, and AGS derived from gastric epithelial cells (Fig. 1a). The PCR products obtained from both cell lines were isolated and sequenced, and were found to be identical to the corresponding sequences published for gp-340 and TfR, confirming that the correct transcripts were being amplified in the PCR.
Fig. 1.
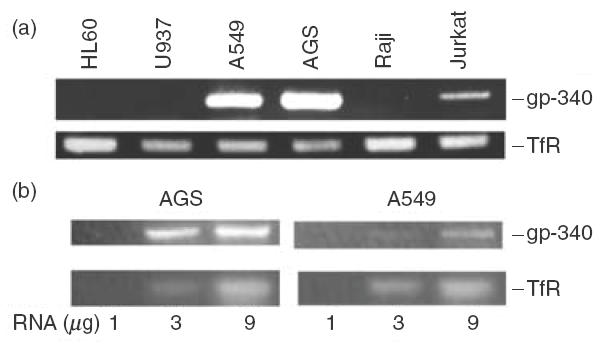
RT-PCR analysis of gp-340 mRNA expression in different cell lines (a) and the levels of the gp-340 transcripts in the gastric epithelial cell line AGS and pulmonary epithelial cell line A549 (b). A transferrin receptor (TfR) specific product from the cDNAs of all the cells was used as an internal control. The results shown are representatives of three independent experiments.
When the number of PCR cycles was reduced to 35 and a range of RNA concentrations were tested, gp-340 levels appeared much higher in AGS cells than in A549 cells (Fig. 1b). Further studies showed that approximately seven more cycles of PCR were needed for A549 cells to obtain a band with similar intensity as that of AGS cells. This quantitative difference in gp-340 expression, seen between the two epithelial cell lines, was also demonstrated by the immunohistochemical staining, using the monoclonal antibody Hyb213-6 against gp-340, whose specificity has been proved previously by Western blot [3]. About 5% of the AGS cells were stained positive (Fig. 2a,i) and fewer in the whole population of A549 cells, although the signals were repeatable and very clear (Fig. 2b,i). Images at high magnification allowed identification of strong granular staining for gp-340 with even intracellular distribution in AGS (Fig. 2c,i,ii) and A549 (Fig. 2c,iii,iv) cells, and no membrane-attached form of gp-340 could be seen.
Fig. 2.
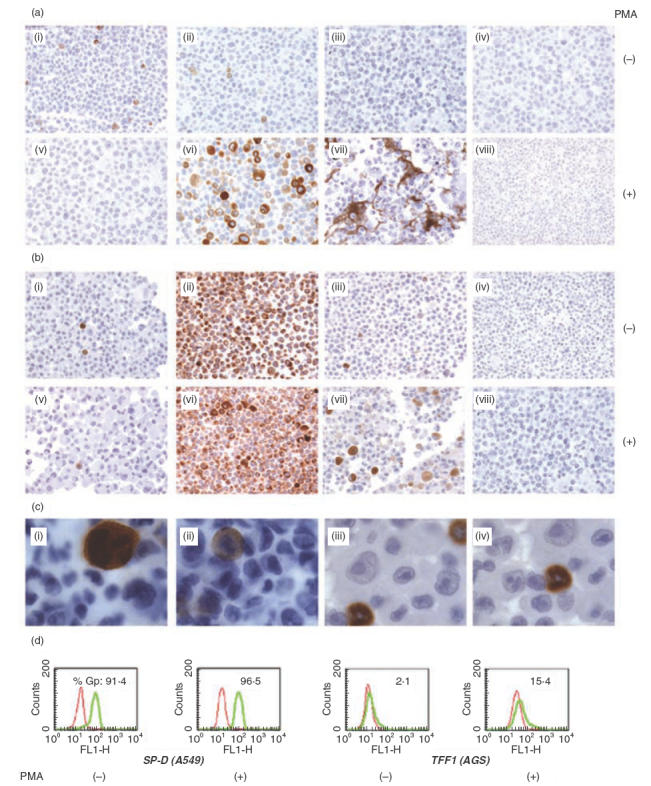
Analysis of protein synthesis for gp-340, TFF1, TFF2 and SP-D using immunohistochemistry (a, b and c) and flow cytometry (d), in unstimulated and PMA stimulated (100 ng/ml) AGS (a) and A549 cells (b): i and v, gp-340; ii and vi, TFF1 (a) or SP-D (b); iii and vii, TFF2 (a) or TFF1 (b); iv and viii, control staining using isotype monoclonal antibody Hyb99–1 (IgG1) to substitute the specific antibodies at the same concentration. (c) Intracellular distribution of gp-340 in AGS cells (i and ii) and A549 cells (iii and iv). Original magnifications: (a) and (b) ×200; (c) ×1000. The results displayed represent two independent experiments. (d) FACS analysis demonstrates the PMA effect on the percentage of cells gated positive (%Gp) for SP-D in A549 cells and for TFF1 in AGS cells. The histograms in red represent background staining using control antibodies, as explained in Materials and methods. Data are representative of three independent experiments.
Differential regulation of gp-340 expression by PMA
PMA is a highly potent inflammatory stimulus for a variety of immune-related cell types. Considering the strong evidence for gp-340 involvement in body's immunity, an attempt was made to induce gp-340 expression from any of the six cell lines by PMA stimulation. No effect of such was seen in the four immune cell lines, after PMA addition at 100 ng/ml for up to 48 h, a duration that has been shown to be long enough to activate Raji and Jurkat cells, and to differentiate HL60 [25–27] (data not shown). In A549 cells however, gp-340 transcripts were up-regulated by PMA (Fig. 3a), but no increase at protein level could be detected (Fig. 2b,v). Post-transcriptional block of protein synthesis has been reported for both keratin and the integrin β subunit in A549 carcinoma cell line [25,26]. Gp-340 was clearly present in type II cells in normal lung (Fig. 4a) but largely absent in A549 cells (Fig. 2b,i), suggesting that blockage at such a level may also apply for gp-340, causing the difficulty in detecting its basal level synthesis and the PMA up-regulation in A549 cells. This is not surprising, as abnormality of gp-340 expression has been well documented and related to its possible role in tumour suppression, although attention so far has only been focused on the possible mechanisms at genomic and transcriptional level. Unexpectedly, 24 h of PMA treatment remarkably down-regulated gp-340 mRNA in AGS cells in a dose-dependent manner (Fig. 3b), and abolished its protein synthesis almost completely at the same time-point, as detected by immunohistochemistry (Fig. 2a,v).
Fig. 3.
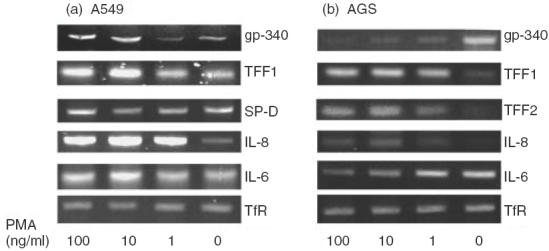
RT-PCR analysis of mRNA expression of the molecules gp-340, SP-D, TFF1, TFF2, IL-6 and IL-8 in epithelial cell lines A549 (a) and/or AGS (b). RNA templates were extracted from unstimulated and PMA stimulated A549 and AGS cells, converted to cDNAs using oligo (dT)15 primers. Molecules of interest were amplified using specific primer pairs, as described in Table 1. TfR-specific product was used as an internal control.
Fig. 4.
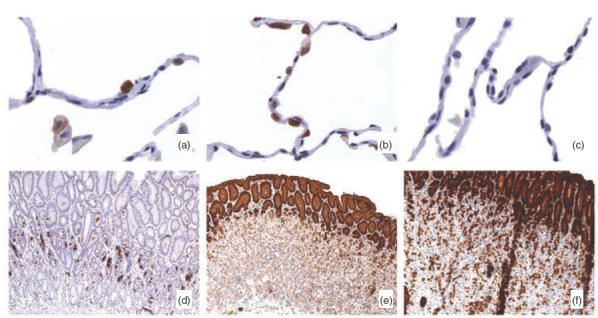
Immunohistochemical localization of gp-340, TFF1, TFF2 and SP-D in normal human lung and/or gastric mucosa. Tissue sections were processed and stained as described in Materials and Methods. Images from high magnification (×400) show the specific expression of gp-340 (a) and SP-D (b) in type II cells but absent in type I cells in epithelial cells lining around alveolar sac. Expression of TFF1 was missing in normal human distal lung (c). Images from low magnification (×50) demonstrate the complete thickness of the epithelial layer of the gastric mucosa, and strong granular signals of gp-340 (d) dominantly in the neck region, but barely detectable toward luminal and bottom side; TFF1 (e) and TFF2 (f) were found primarily in the superficial mucin-producing epithelial cells. The profiles represent staining results from at least three different normal tissue samples.
PMA regulation of expressions of the protein markers in AGS and A549 cells
The ability of PMA to regulate gp-340 expression differentially in the two epithelial cell lines, suggested that PMA might trigger the cellular responses other than the inflammatory type. In order to clarify the nature of the cellular events in the two epithelia following PMA treatment, a number of molecule markers were analysed. These included the two proinflammatory cytokines IL-8 and IL-6, gp-340 binding protein SP-D and the markers for gastric epithelial differentiation TFF1 and TFF2.
The chemokine IL-8 was induced significantly in A549 cells upon addition of 1 ng/ml PMA. Only a slight increase of IL-8 was detected in AGS after PMA treatment, reaching a level corresponding to the basal level in A549 (Fig. 3a,b). Expression of IL-6 was also enhanced in A549, but to a lesser extent compared to IL-8 (Fig. 3a). Interestingly, down-regulation of IL-6 was obtained in PMA-treated AGS cells in a dose-dependent manner (Fig. 3b). In A549 cells, PMA merely changed the intensity of mRNA or protein signal for SP-D, which showed strong basal expression (Figs 3a and 2b,ii,vi), in more than 90% of the resting cells shown by FACS analysis (Fig. 2d). Mucin-associated polypeptide TFF1 was expressed weakly and only in a small percentage of A549 and AGS cells (Fig. 3a,b,Fig. 2a,ii,b,iii,d). In both types of cells, PMA remarkably up-regulated TFF1 mRNA (Fig. 3a,b). The protein synthesis was also induced significantly, as quantified by the number of cells gated as positive in AGS cells, by FACS analysis (Fig. 2d), and signal intensity demonstrated by the image of the immunostaining (Fig. 2a,vi,b,vii). Meanwhile, TFF2 was also induced markedly by PMA in AGS cells (Fig. 3b), although the peptide was in a secreted form rather than being retained intracellularly (Fig. 2a,iii,vii), as reported previously that TFF2 immunoreactivity was seen abundantly in the mucus layer covering the gastric mucosa in normal rat, indicating its luminal secretion [27]. The PCR products for SP-D, TFF1 and TFF2 obtained from both cell lines were sequenced, and found to be identical to the corresponding sequences published for these molecules.
PMA effects on morphology of AGS and A549
Phase contrast microscopy revealed that most AGS cells changed into an asymmetric shape with an elongated cytoplasm extending toward one end, in response to 6 h PMA treatment (Fig. 5b), which returned to normal shape after 24 h stimulation (Fig. 5c). This phenomenon was not seen in A549 cells, which remained an even distribution of cytoplasm and round in appearance with stretching edges, indicating normal spreading of the cells during PMA stimulation until a confluent state was reached (Fig. 5e,f).
Fig. 5.
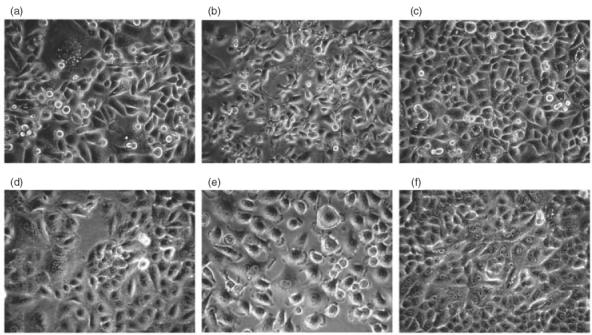
Phase contrast microscopy showing the effects of PMA on the morphology of AGS and A549 cells. AGS cells (a) and A549 cells (d) before PMA addition. Morphological changes were observed in most AGS cells after PMA stimulation for 6 h, with asymmetric shape and elongated cytoplasm extending toward one end of the cells (b). The same type of change was not observed in A549 cells, which maintained an even distribution of cytoplasm with stretching edges toward all directions (e). 24 h after PMA addition, the morphology of AGS cells returned to normal (c); A549 cells obtained well-defined borders along cellular junctions in confluence (f). Original magnification, × 200. The images were identical in five independent experiments.
Gp-340 distribution in mucosal epithelia of normal lung and stomach
Tissue distribution of gp-340, TFF1, TFF2 and SP-D was studied by immunohistochemistry. In lung sections, positive staining for gp-340 was obtained in the round-shaped type II cells, but was absent in the flat-shaped type I cells lining the alveolar sacs (Fig. 4a). Its presence was also noted in the neighbouring alveolar macrophages, although with a lower intensity (Fig. 4a). Strong staining for SP-D was seen in all type II cells around the alveolar cavities (Fig. 4b). This is consistent with the previous findings that human lung is the major body site where this surfactant protein is synthesized [24]. TFF1 was undetectable in the same serial tissue sample of the distal lung (Fig. 4c).
In gastric mucosa, strong granular staining was obtained in part of the cells within the neck region when the monoclonal antibody Hyb213-6 against gp-340 was applied (Fig. 4d). This region corresponds well to a special gastric mucosal zone, where stem cells and different lineages of progenitor cells are located and actively proliferating, illuminated by the monoclonal antibody against Ki-67 antigen, a marker for proliferating cells [31] (Fig. 6). Gp-340 reactivity tended to disappear toward the matured superficial or basal gastric epithelia, although random signals can be seen underneath the neck region (Figs 4d, 6a,c). On the other hand, both TFF1 and TFF2 reactivity was found primarily in the surface epithelial cells adjacent to the gastric lumen (Fig. 4e,f). Consistent with the previous report [36], such specific location indicates that in gastric mucosa, TFF1 and TFF2 are synthesized mainly by terminally differentiated superficial epithelia, although TFF2 could also be found in deep glands in a scattered presence next to the surface epithelia zone (Fig. 4f). Control antibodies detected no signal in both types of histological samples (data not shown).
Fig. 6.
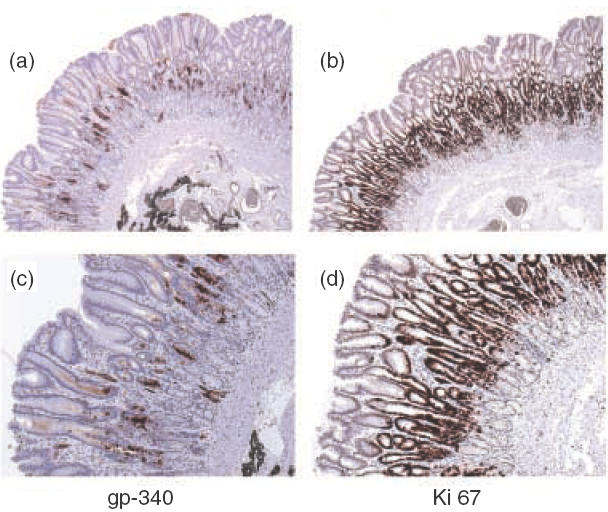
Immunohistochemical analysis of the localizations for gp-340, and a proliferation marker Ki-67 illustrated epithelial proliferation zone, in the gastric mucosa. Serial sections of normal human gastric mucosa were stained with monoclonal antibodies Hyb213–6 (a and c), and monoclonal antibody Ki-67 (b and d). Original magnifications: (a) and (b) ×100; (c) and (d) × 200.
Discussion
Increasing data have accumulated, suggesting a direct involvement of gp-340 in innate immunity, by its interaction with both pathogens [18,19] and the body's immune components such as tissue macrophages [17], IgA [20] and the collectin SP-D [3,7,32]. It is therefore worth identifying the immune-related cells that express gp-340, which will facilitate studies of its involvement in the pathogen–host interaction at cellular level. For this purpose, we screened four types of immune cell lines by RT-PCR and found, as reported previously, low expression of gp-340 in Jurkat T-cell line [8] but not in the Raji B-cell line, the monocytic U937 or the promyelocytic HL-60 cell lines. Even after 48 h PMA treatment, no up-regulation of gp-340 was seen in these cells using a variety of detections (data not shown). The presence of gp-340 in the T-cell line is potentially interesting, as SP-D has been reported to inhibit T lymphocyte proliferation and its IL-2 production [33,34], indicating a possible role for gp-340 in mediating these effects. However, we could not detect gp-340 signal in B cell line Raji, as demonstrated in a previous study [8]. This might result from the difference in PCR amplification approaches being used: Mollenhauer and co-workers performed net PCR in two steps, whereas in the present study one PCR with 42 cycles was used which was regarded as sufficient in representing the basal level of mRNA expression. Both studies suggested, if any, an extremely low level of gp-340 transcripts in Raji cells. Immunostaining revealed gp-340 presence in mature tissue macrophages, sometimes even co-localized with SP-D in subcellular compartments [7,8]. It was therefore of interest to assess whether macrophage precursor cell lines, such as HL60 and U937 lying along different stages of differentiation, could also expression gp-340. This could help to identify whether gp-340 expression is also differentiation stage specific in the immune system, as has been shown for gp-340 and its rodent homologues in epithelial cells at different body locations [21,22,35], and whether it could play a role in the differentiation process of any immune cells. The lack of basal gp-340 synthesis in these two cell lines and after 48 h PMA stimulation suggested that gp-340 expression might only be related to the final stage of macrophage maturation. Studies addressing these aspects are being carried out.
When the two epithelial cell lines were analysed, both the pulmonary type II epithelial cell line A549 and gastric epithelial cell line AGS showed much higher mRNA expression for gp-340, compared with the other four immune cell lines. This observation caught our immediate attention, as it is the first demonstration of the expression of gp-340 at a high level in epithelial cell lines. Epithelial cells have been regarded as first-line defence components in the immune system to protect mucosal surface, by actively secreting several proinflammatory cytokines [36–38] and becoming antigen-presenting cells [39,40] upon microbial challenges. Salivary agglutinin/gp-340, was found to be synthesized by the glandular epithelia in oral cavity, and was able to bind and aggregate S. mutans [41]. Moreover, staining of histological samples revealed production of gp-340 in both pulmonary and gastric epithelial cells (Fig. 4), which fits into the wide mucosal epithelia distribution of its binding protein, collectin SP-D [24,42]. In particular, this study localized the expression of both gp-340 and SP-D in type II alveolar epithelial cells, in vivo and in vitro. Collectins and SRCR domain-containing molecules are known for their ‘pattern recognition’ ability, which allows them to distinguish self structures from the nonself ones, by recognizing different patterns of glycoconjugates or lipid components on the surfaces of microorganisms [43–45]. A complex formed between SP-D and gp-340 may combine the preferences of each molecule in pattern recognition, illustrating one possible mechanism by which the body's first-line defence system can adapt to increase both specificity and affinity of interactions with invading pathogens. In addition, synthesis of the two types of innate immune molecules by the neighbouring or even the same epithelia lining along the inner surface of the body might facilitate the synchronous regulation of their joint action against potential infection, thus further strengthening the ability of epithelial cells in mucosal defence.
Therefore, it is of great interest to carry on the studies of the relationship between gp-340 and epithelial cells in the context of the local innate immunity. As a first step, the inflammatory stimulate PMA was used to treat AGS and A549 cells, because the small percentage of gp-340 positive cells in the two resting epithelial cell lines suggested a potential for this molecule to be further induced. Indeed, PMA increased transcripts of gp-340 in the A549 cells, although it failed to enhance protein synthesis of gp-340, due possibly to a post-transcriptional block in this carcinoma cell line, which has been implicated for other molecules [28,29]. In the striking contrast, PMA abolished gp-340 signal almost completely in AGS cells, which seemed inconsistent with a mucosal inflammation related function for this molecule.
So far, there are at least two distinct functions being proposed for gp-340 and its homologues: its involvement in mucosal defence [18,19] and its regulation of epithelial development [21,22]. Both processes could be manipulated by PMA in vitro, because PMA has been shown to induce a variety of cellular responses including proliferation, differentiation and apoptosis, as well as the well-known inflammatory type of responses, through its activation of different PKC isoforms [46–48]. Therefore, we characterized further the PMA triggered cellular events in AGS and A549 cells. The results from assessing the expression of a range of molecule markers indicated that PMA induced inflammatory response from A549 cells, but initiated differentiation in AGS cells.
A549 is a relatively differentiated tumour cell line, featuring the phenotype of alveolar type II cells by its surfactant-producing ability. In accordance with this, we found the mRNA expression of SP-D and the uniform immunostaining in nearly the whole population of A549 cells. This high level of SP-D basal expression might explain the inability of its up-regulation after PMA treatment. As we and others could not detect TFF1 expression in type II cells in the normal lung by immunohistochemistry, its up-regulation in A549 cells could not be related to a differentiation process as it could in AGS cells. Although TFF1 is produced dominantly in gastrointestinal tract to promote mucosal defence and wound healing [49], its pulmonary synthesis has recently been found in inflammatory situations such as cystic fibrosis (CF) and bronchitis [49,50], where it has been shown to bind to major pathogenic bacteria present in the airways [49]. Thus, increased expression of TFF1 suggested a PMA-triggered defence response in the A549 cells. This conclusion was supported by the remarkable induction of the two proinflammatory cytokines IL-8 and IL-6. Up-regulation of gp-340 transcripts in such a background, indicated its possible involvement in the epithelial proinflammatory response in human lungs, in agreement with its chemokinetic effect on alveolar macrophages [17].
As a multi-functional molecule, TFF1 is co-localized with TFF2 at high levels in the gastric surface epithelia throughout the stomach [51], and has been reported to be essential for normal differentiation of gastric mucosa, because deficient mice develop antropyloric adenomas [52]. Our immunohistochemical staining also located both TFF1 and TFF2 predominantly in the terminally differentiated surface epithelial cells of the gastric mucosa, but not in the neck region underneath, where gp-340 was detected primarily and the proliferation/differentiation of progenitor cells occurs. Moreover, PMA induced IL-8 only to its constitutive level in AGS cells, instead of the remarkable increase seen in A549 cells. Taken together, up-regulation of these molecules indicated a terminal differentiation in AGS cells initiated by PMA, which led to the loss of gp-340 expression. A higher level of IL-6 expression has been reported to accompany malignancy in colon cancer and to promote proliferation of tumour cells [53,54]. Its down-regulation here (Fig. 3b) is also consistent with an ongoing process of differentiation and reduction of malignancy.
Further evidence lies in the PMA-induced morphological changes of AGS cells. Phorbol esters are known to be capable of converting actin from globular to filamentous form, leading to its assembly in cultured cells [55,56]. Our observation of the asymmetric cell polarity in AGS cells in response to PMA fits into such a dramatic reorganization of the cytoskeleton, which was reported to be related closely to motility, endocytosis, cell division and differentiation [57]. A similar change was not observed in A549 cells. These results indicated that AGS adenocarcinoma cells might represent an undifferentiated form of gastric epithelia. Gp-340 was present only in resting AGS cells, but no longer detectable after their differentiation triggered by PMA, suggesting that its expression was associated with less differentiated epithelial progenitors. This view was confirmed by our tissue distribution studies, localizing gp-340 within the proliferation zone in gastric mucosa, where it might be involved in the development of the stem/progenitor cells.
In conclusion, the presence of gp-340 has been demonstrated for the first time in epithelial cell lines, together with its binding protein SP-D. Differential regulation of gp-340, expression by PMA in the two types of epithelial cells, supported the involvement of this molecule in both innate immunity and epithelial growth. Supported by the analysis of histological samples from normal lung and gastric mucosa, our in vitro observations indicate further that these functions might be body location-related and epithelial differentiation stage-specific.
Acknowledgments
This work was supported by the Alfred Benzon Foundation, EU grant no. QLK2-CT 2000–00325, the Novo Nordic Foundation and the Danish Medical Research Council grant no. 52-00-0962. Weiqun Kang appreciates the financial support from the K.C Wong scholarship from Hong Kong and the Scatcherd Scholarship from Oxford University. We thank Dr Helen White-Cooper for skilful practical assistance.
REFERENCES
- 1.Lawson PR, Reid KB. The roles of surfactant proteins A and D in innate immunity. Immunol Rev. 2000;173:66–78. doi: 10.1034/j.1600-065x.2000.917308.x. [DOI] [PubMed] [Google Scholar]
- 2.Crouch E, Wright JR. Surfactant proteins a and d and pulmonary host defense. Annu Rev Physiol. 2001;63:521–54. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- 3.Holmskov U, Lawson P, Teisner B, et al. Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule. J Biol Chem. 1997;272:13743–9. doi: 10.1074/jbc.272.21.13743. [DOI] [PubMed] [Google Scholar]
- 4.Bodian DL, Skonier JE, Bowen MA, et al. Identification of residues in CD6 which are critical for ligand binding. Biochemistry. 1997;36:2637–41. doi: 10.1021/bi962560+. [DOI] [PubMed] [Google Scholar]
- 5.Bauskin AR, Franken DR, Eberspaecher U, et al. Characterization of human zona pellucida glycoproteins. Mol Hum Reprod. 1999;5:534–40. doi: 10.1093/molehr/5.6.534. [DOI] [PubMed] [Google Scholar]
- 6.Gal P, Zavodszky P. Structure and function of the serine-protease subcomponents of C1: protein engineering studies. Immunobiology. 1998;199:317–26. doi: 10.1016/S0171-2985(98)80036-3. [DOI] [PubMed] [Google Scholar]
- 7.Holmskov U, Mollenhauer J, Madsen J, et al. Cloning of gp-340, a putative opsonin receptor for lung surfactant protein D. Proc Natl Acad Sci USA. 1999;96:10794–9. doi: 10.1073/pnas.96.19.10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mollenhauer J, Herbertz S, Holmskov U, et al. DMBT1 encodes a protein involved in the immune defense and in epithelial differentiation and is highly unstable in cancer. Cancer Res. 2000;60:1704–10. [PubMed] [Google Scholar]
- 9.Mollenhauer J, Wiemann S, Scheurlen W, et al. DMBT1, a new member of the SRCR superfamily, on chromosome 10q25.3–26.1 is deleted in malignant brain tumours. Nat Genet. 1997;17:32–9. doi: 10.1038/ng0997-32. [DOI] [PubMed] [Google Scholar]
- 10.Somerville RP, Shoshan Y, Eng C, et al. Molecular analysis of two putative tumour suppressor genes, PTEN and DMBT, which have been implicated in glioblastoma multiforme disease progression. Oncogene. 1998;17:1755–7. doi: 10.1038/sj.onc.1202066. [DOI] [PubMed] [Google Scholar]
- 11.Wu W, Kemp BL, Proctor ML, et al. Expression of DMBT1, a candidate tumor suppressor gene, is frequently lost in lung cancer. Cancer Res. 1999;59:1846–51. [PubMed] [Google Scholar]
- 12.Mori M, Shiraishi T, Tanaka S, et al. Lack of DMBT1 expression in oesophageal, gastric and colon cancers. Br J Cancer. 1999;79:211–3. doi: 10.1038/sj.bjc.6690035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mollenhauer J, Holmskov U, Wiemann S, et al. The genomic structure of the DMBT1 gene: evidence for a region with susceptibility to genomic instability. Oncogene. 1999;18:6233–40. doi: 10.1038/sj.onc.1203071. [DOI] [PubMed] [Google Scholar]
- 14.Buechler C, Ritter M, Orso E, et al. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 15.Calvo J, Places L, Padilla O, et al. Interaction of recombinant and natural soluble CD5 forms with an alternative cell surface ligand. Eur J Immunol. 1999;29:2119–29. doi: 10.1002/(SICI)1521-4141(199907)29:07<2119::AID-IMMU2119>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Starling GC, Whitney GS, Siadak AW, et al. Characterization of mouse CD6 with novel monoclonal antibodies which enhance the allogeneic mixed leukocyte reaction. Eur J Immunol. 1996;26:738–46. doi: 10.1002/eji.1830260403. [DOI] [PubMed] [Google Scholar]
- 17.Tino MJ, Wright JR. Glycoprotein-340 binds surfactant protein-A (SP-A) and stimulates alveolar macrophage migration in an SP-A-independent manner. Am J Respir Cell Mol Biol. 1999;20:759–68. doi: 10.1165/ajrcmb.20.4.3439. [DOI] [PubMed] [Google Scholar]
- 18.Ligtenberg TJ, Bikker FJ, Groenink J, et al. Human salivary agglutinin binds to lung surfactant protein-D and is identical with scavenger receptor protein gp-340. Biochem J. 2001;359:243–8. doi: 10.1042/0264-6021:3590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakobphol A, Xu F, Hoang VM, et al. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J Biol Chem. 2000;275:39860–6. doi: 10.1074/jbc.M006928200. [DOI] [PubMed] [Google Scholar]
- 20.Oho T, Yu H, Yamashita Y, Koga T. Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans. Infect Immun. 1998;66:115–21. doi: 10.1128/iai.66.1.115-121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Awqati Q, Vijayakumar S, Takito J, et al. Phenotypic plasticity and terminal differentiation of the intercalated cell: the hensin pathway. Exp Nephrol. 2000;8:66–71. doi: 10.1159/000020650. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H, Bjerknes M, Chen H. CRP-ductin: a gene expressed in intestinal crypts and in pancreatic and hepatic ducts. Anat Rec. 1996;244:327–43. doi: 10.1002/(SICI)1097-0185(199603)244:3<327::AID-AR5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita F, Miyawaki A, Mikoshiba K. Vomeroglandin/CRP-ductin is strongly expressed in the glands associated with the mouse vomeronasal organ: identification and characterization of mouse vomeroglandin. Biochem Biophys Res Commun. 2000;268:275–81. doi: 10.1006/bbrc.2000.2104. [DOI] [PubMed] [Google Scholar]
- 24.Madsen J, Kliem A, Tornoe I, et al. Localization of lung surfactant protein D on mucosal surfaces in human tissues. J Immunol. 2000;164:5866–70. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- 25.Aoki Y, Kao PN. Erythromycin inhibits transcriptional activation of NF-KappaB, but not NFAT, through calcineurin-independent signaling in T cells. Antimicrob Ag Chemother. 1999;43:2678–84. doi: 10.1128/aac.43.11.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Das D, Pintucci G, Stern A. MAPK-dependent expression of p21 (WAF) and p27 (Kip1) in PMA-induced differentiation of HL60 cells. FEBS Lett. 2000;472:50–2. doi: 10.1016/s0014-5793(00)01416-2. [DOI] [PubMed] [Google Scholar]
- 27.Robichon A, Sreedharan SP, Yang J, et al. Induction of aggregation of Raji human-B lymphoblastic cells by vasoactive intestinal peptide. Immunology. 1993;79:574–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Meleady P, Clynes M. Bromodeoxyuridine induces integrin expression at transcriptional (alpha2 subunit) and post-transcriptional (beta1 subunit) levels, and alters the adhesive properties of two human lung tumour cell lines. Cell Adhes Commun. 2001;8:45–59. doi: 10.3109/15419060109080706. [DOI] [PubMed] [Google Scholar]
- 29.Meleady P, Clynes M. Bromodeoxyuridine increases keratin 19 protein expression at a posttranscriptional level in two human lung tumor cell lines. In Vitro Cell Dev Biol Anim. 2001;37:536–42. doi: 10.1290/1071-2690(2001)037<0536:BIKPEA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Jeffrey GP, Oates PS, Wang TC, et al. Spasmolytic polypeptide: a trefoil peptide secreted by rat gastric mucous cells. Gastroenterology. 1994;106:336–45. doi: 10.1016/0016-5085(94)90590-8. [DOI] [PubMed] [Google Scholar]
- 31.Brown DC, Gatter KC. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990;17:489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 32.Hirsch-Lerner D, Barenholz Y. Hydration of lipoplexes commonly used in gene delivery: follow-up by laurdan fluorescence changes and quantification by differential scanning calorimetry. Biochim Biophys Acta. 1999;1461:47–57. doi: 10.1016/s0005-2736(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang JY, Shieh CC, You PF, et al. Inhibitory effect of pulmonary surfactant proteins A and D on allergen-induced lymphocyte proliferation and histamine release in children with asthma. Am J Respir Crit Care Med. 1998;158:510–8. doi: 10.1164/ajrccm.158.2.9709111. [DOI] [PubMed] [Google Scholar]
- 34.Borron PJ, Crouch EC, Lewis JF, et al. Recombinant rat surfactant-associated protein D inhibits human T lymphocyte proliferation and IL-2 production. J Immunol. 1998;161:4599–603. [PubMed] [Google Scholar]
- 35.Mollenhauer J, Herbertz S, Helmke B, et al. Deleted in malignant brain tumors 1 is a versatile mucin-like molecule likely to play a differential role in digestive tract cancer. Cancer Res. 2001;61:8880–6. [PubMed] [Google Scholar]
- 36.Abreu MT, Arnold ET, Thomas LS, et al. TLR4 and MD-2 expression are regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002 doi: 10.1074/jbc.M110333200. [DOI] [PubMed] [Google Scholar]
- 37.Cockwell P, Calderwood JW, Brooks CJ, et al. Chemoattraction of T cells expressing CCR5, CXCR3 and CX3CR1 by proximal tubular epithelial cell chemokines. Nephrol Dial Transplant. 2002;17:734–44. doi: 10.1093/ndt/17.5.734. [DOI] [PubMed] [Google Scholar]
- 38.Schaller M, Mailhammer R, Grassl G, et al. Infection of human oral epithelia with Candida species induces cytokine expression correlated to the degree of virulence. J Invest Dermatol. 2002;118:652–7. doi: 10.1046/j.1523-1747.2002.01699.x. [DOI] [PubMed] [Google Scholar]
- 39.Barrera CA, Almanza RJ, Ogra PL, et al. The role of the invariant chain in mucosal immunity. Int Arch Allergy Immunol. 1998;117:85–93. doi: 10.1159/000023994. [DOI] [PubMed] [Google Scholar]
- 40.Barrera C, Espejo R, Reyes VE. Differential glycosylation of MHC class II molecules on gastric epithelial cells. Implications in local immune responses. Hum Immunol. 2002;63:384–93. doi: 10.1016/s0198-8859(02)00386-5. [DOI] [PubMed] [Google Scholar]
- 41.Bikker FJ, Ligtenberg AJ, van der Wal JE, et al. Immunohistochemical detection of salivary agglutinin/gp-340 in human parotid, submandibular, and labial salivary glands. J Dent Res. 2002;81:134–9. [PubMed] [Google Scholar]
- 42.Murray E, Khamri W, Walker M, et al. Expression of surfactant protein D in the human gastric mucosa and during Helicobacter pylori infection. Infect Immun. 2002;70:1481–7. doi: 10.1128/IAI.70.3.1481-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brinker KG, Martin E, Borron P, et al. Surfactant protein D enhances bacterial antigen presentation by bone marrow-derived dendritic cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1453–63. doi: 10.1152/ajplung.2001.281.6.L1453. [DOI] [PubMed] [Google Scholar]
- 44.Wright JR, Borron P, Binker KG, et al. Surfactant protein A: regulation of innate and adaptive immune responses in lung inflammation. Am J Respir Cell Mol Biol. 2001;24:513–17. doi: 10.1165/ajrcmb.24.5.f208. [DOI] [PubMed] [Google Scholar]
- 45.Dunne DW, Resnick D, Greenberg J, et al. The type I macrophage scavenger receptor binds to Gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–7. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreton K, Turner RA, Paton A, et al. Changes in PKC subspecies protein expression as C6 cells reach G (0) by contact inhibition in the presence of serum. Biochem Soc Trans. 1995;23:446S. doi: 10.1042/bst023446s. [DOI] [PubMed] [Google Scholar]
- 47.Frey MR, Clark JA, Leontieva O, et al. Protein kinase C signaling mediates a program of cell cycle withdrawal in the intestinal epithelium. J Cell Biol. 2000;151:763–78. doi: 10.1083/jcb.151.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kiley SC, Welsh J, Narvaez CJ, et al. Protein kinase C isozymes and substrates in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 1996;1:177–87. doi: 10.1007/BF02013641. [DOI] [PubMed] [Google Scholar]
- 49.dos Santos Silva E, Ulrich M, Doring G, et al. Trefoil factor family domain peptides in the human respiratory tract. J Pathol. 2000;190:133–42. doi: 10.1002/(SICI)1096-9896(200002)190:2<133::AID-PATH518>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 50.Wiede A, Jagla W, Welte T, et al. Localization of TFF3, a new mucus-associated peptide of the human respiratory tract. Am J Respir Crit Care Med. 1999;159:1330–5. doi: 10.1164/ajrccm.159.4.9804149. [DOI] [PubMed] [Google Scholar]
- 51.Hanby AM, Jankowski JA, Elia G, et al. Expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in Barrett's metaplasia and the native oesophageal epithelium: delineation of epithelial phenotype. J Pathol. 1994;173:213–9. doi: 10.1002/path.1711730303. [DOI] [PubMed] [Google Scholar]
- 52.Tomasetto C, Masson R, Linares JL, et al. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology. 2000;118:70–80. doi: 10.1016/s0016-5085(00)70415-x. [DOI] [PubMed] [Google Scholar]
- 53.Piancatelli D, Romano P, Sebastiani P, et al. Local expression of cytokines in human colorectal carcinoma: evidence of specific interleukin-6 gene expression. J Immunother. 1999;22:25–32. doi: 10.1097/00002371-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Wong WM, Wright NA. Cell proliferation in gastrointestinal mucosa. J Clin Pathol. 1999;52:321–33. doi: 10.1136/jcp.52.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dlugosz AA, Tapscott SJ, Holtzer H. Effects of phorbol 12-myristate 13-acetate on the differentiation program of embryonic chick skeletal myoblasts. Cancer Res. 1983;43:2780–9. [PubMed] [Google Scholar]
- 56.Melamed I, Downey GP, Aktories K, et al. Microfilament assembly is required for antigen-receptor-mediated activation of human B lymphocytes. J Immunol. 1991;147:1139–46. [PubMed] [Google Scholar]
- 57.Mann VH, Law MH, Watters D, et al. The effects of bistratene A on the development of Plasmodium falciparum in culture. Int J Parasitol. 1996;26:117–21. doi: 10.1016/0020-7519(95)00073-9. [DOI] [PubMed] [Google Scholar]
- 58.Nogueira AM, Machado JC, Carneiro F, et al. Patterns of expression of trefoil peptides and mucins in gastric polyps with and without malignant transformation. J Pathol. 1999;187:541–8. doi: 10.1002/(SICI)1096-9896(199904)187:5<541::AID-PATH283>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]


