Abstract
Gram-positive bacteria, which lack lipopolysaccharide (LPS), produce a septic-shock-like condition, accompanied by release of pro-inflammatory cytokines. Various components of the bacteria may be responsible for this. We stimulated a whole blood system with heat-inactivated Streptococcus pneumoniae serotype 14 (S14) bacteria, with pneumococcal S14 capsular polysaccharide (PPS S14) and with PPS S14 coated on to latex beads, to compare interleukin 6 (IL-6) and tumour necrosis factor alpha (TNFα) production over a six hour period, to ascertain the contribution of PPS to the inflammatory response. This was compared with the response to LPS. After sonication of the bacteria, their PPS content was estimated by an enzyme-linked immunoabsorbent assay, to compare this with the concentration of free PPS needed to generate cytokine release. The whole bacteria elicited a much larger cytokine response than the equivalent amount of PPS alone, whereas the PPS-coated beads gave minimal response. The different cytokine responses to PPS and LPS suggest that there are differences in the receptors and/or signalling pathways for Gram-negative and Gram-positive bacteria. We conclude that the estimated amount of PPS in the bacteria is not enough to account for the large cytokine response we observed. Since PPS could not be shown to contribute significantly to cytokine induction, specific antibodies to PPS would not play any significant role in combating cytokine release associated with pneumococcal infection and possible septic shock. This needs to be considered in production of future vaccines.
Keywords: IL-6, TNFα, Streptococcus pneumoniae, capsular polysaccharide
INTRODUCTION
Streptococcus pneumoniae (S. pneumoniae), a Gram-positive bacterium, is the commonest cause of community-acquired pneumonia, accounting for up to 70% of cases in hospital [1]. There are more than 90 known serotypes of S. pneumoniae which differ in the structure of their polysaccharide capsule [2]. The capsule is not toxic itself [3], its virulence lies mainly in its antiphagocytic properties [4]. The chemical nature of the capsule, rather than its size, determines its level of virulence [3], impeding phagocytosis via complement inhibition.
Capsular polysaccharides of Gram-positive organisms are known to trigger inflammatory cytokine release [5,6] and with S. pneumoniae the more virulent the serotype the higher the level of secreted cytokine [7]. Tumour necrosis factor alpha (TNFα) levels as high as those induced by lipopolysaccharide (LPS) in Gram-negative bacteria have been reported [6] in isolated immune cell experiments in vitro. Whole blood experimental systems mimic conditions in vivo better because all the soluble factors that influence cytokine production are present. In one such study, it was found that 100–1000 fold more Gram-positive organisms were needed than Gram-negative to induce the same concentration of cytokine (interleukin 1β (IL-1β) and interleukin 6 (IL-6)) release [8]. However, this did not correlate with the severity of disease, indicating that different mechanisms are responsible for Gram-positive and Gram-negative sepsis. β2 integrins [9] and Toll-like receptor 2 (TLR2) [10,11] have been implicated in cytokine responses in Gram-positive infections, whereas CD14 and toll-like receptor 4 (TLR4) are instrumental in Gram-negative [8,12].
Our study concentrated on the possible contribution that pneumococcal capsular polysaccharide makes to cytokine production induced by heat-inactivated S. pneumoniae serotype 14 (S14) bacteria. We stimulated a whole blood system with heat-inactivated S. pneumoniae S14 bacteria, pneumococcal capsular polysaccharide (PPS) S14 and PPS S14 coated onto latex beads. S14 was the serotype of choice because it is the most virulent one in UK [3], is becoming antibiotic-resistant [13]. The free PPS was used to investigate whether this alone can stimulate cytokine production, or whether intact pneumococci are required. PPS S14 coated to latex beads, being similar in size to pneumococcal organisms, was also used as a stimulant to investigate whether size of particle is a contributing factor in cytokine release. LPS was used as a positive control, and unstimulated blood and uncoated beads as negative controls. A dose–response experiment was carried out with free PPS S14, LPS, and heat-inactivated S14 bacteria to ascertain the maximal concentrations of these antigens to use in the stimulation experiments, and one with cell-wall polysaccharide (CWPS) to see at what concentration this could make a significant contribution to cytokine release. The PPS content in a sonicate of S. pneumoniae S14 bacteria was measured by enzyme-linked immunoabsorbent assay (ELISA) to compare this with the concentration of free PPS needed to generate cytokine release. TNFα and IL-6 were selected as the cytokines to be measured because both are known to be secreted after stimulation with S14 bacteria, the TNFα reaching a peak after 6 h [14] and the IL-6 staying elevated much longer [15,16].
MATERIALS AND METHODS
Collection of samples
Blood samples were from five healthy, consenting volunteers of blood group O. Blood was collected into sterile tubes containing endotoxin-free lithium heparin to avoid cytokine induction occurring prematurely [17]. Experiments were done in a hooded cabinet with sterile pyrogen-free equipment, sterile solutions and reagents.
Reagents
Phosphate-buffered saline (PBS) pH 7·4, carbonate-bicarbonate buffer capsules pH 9·6, sodium azide, bovine serum albumin, 0·8μ diameter polystyrene carboxylate-modified red fluorescent LATEX beads (catalogue number: L-3155), an equimolar mix of lipopolysaccharide from rough strains of Escherichia coli J5 and Salmonella minnesota Re 595, and 3,3′,5′,5-tetramethylbenzidine (TMB) were all purchased from Sigma Chemical Co. (Poole,UK). Lyophilized S. pneumoniae S 14 came from National Collection of Types and Cultures of the Public Health Laboratory; Colindale, London, and the PPS S14 was purchased from the American Type Culture Collection, Manassas, Virginia, USA. Trypticase soy broth came from Difco (Surrey, UK) and Todd Hewitt broth from Oxoid (Surrey, UK). Blood agar plates were obtained from the Microbiology Department at St. George's Medical School, London. Sterile Water for Irrigation was purchased from Baxter Healthcare (Newbury, UK) and was autoclaved twice. IL-6 and TNFα enzyme-linked immunosorbent assay sets, which included matched antibody pairs and recombinant standards, were purchased from Pharmingen (Becton Dickinson UK Ltd, Oxford, UK) The rabbit antiserum against PPS S14 and CWPS were purchased from the State Serum Institute (Copenhagen, Denmark) and the peroxidase-conjugated goat anti-rabbit immunoglobulin and FITC-conjugated rabbit anti-human immunoglobulin from DAKO (Ely, UK).
Preparation of antigens
S. pneumoniae S14 bacteria
The lyophilized S. pneumoniae S14 were reconstituted in 1 ml trypticase soy broth. The resulting suspension was plated onto Petri dishes containing blood agar, and grown for 18 h at 37°C in a 5% CO2 atmosphere. Colonies of characteristic appearance (diameter 1 mm, round, domed and surrounded by a zone of α haemolysis) [18] were chosen and spread on fresh blood agar Petri dishes for subculture. Following subculture, colonies of characteristic appearance were selected for transfer using a sterilized bacterial loop into containers with Todd Hewitt broth, and grown for 18 h at 37°C and 5% CO2. The bacteria were harvested by centrifuging the culture solution at 400 g for 30 min, and discarding the supernatant. The bacterial pellet was re-suspended in 3 ml PBS and this suspension recentrifuged at 400 g for 30 min The bacterial pellet was resuspended in 1 ml PBS. The bacterial suspension was inactivated by heating at 56°C in a water bath for 30 min and then centrifuged at 400 g for 30 min Inactivation of the bacteria was verified by subsequent sample plating, which was negative for growth. The supernatant was discarded and the bacterial pellet re-suspended in 1 ml PBS. 10 μl of bacterial suspension was added to 2 ml PBS. The diluted bacterial suspension was counted using a particle counter (Industrial D, Coulter Electronics, Bedfordshire, UK). The concentration was finally adjusted with PBS to give 10 μl aliquots containing 2·4 × 107 bacteria. These were stored at −40°C ready for use.
S. pneumoniae capsular polysaccharide S 14
PPS S14 was reconstituted with double autoclaved distilled water to a concentration of 1 mg/ml. Reconstituted PPS was stored at −70°C in aliquots in endotoxin-free Eppendorf tubes.
PPS S14-coated beads
All procedures for coating beads were done in dark conditions. Fifity μl of bead solution was coated overnight with 100 μg PPS in 500 μl of carbonate-bicarbonate coating buffer pH 9·6 at 4°C. The final solution was equivalent to 16·6 μg PPS/108 beads. Coated beads were washed twice with 1 ml PBS, pH 7·4, containing 0·05% Tween 20, centrifuged for 15 mins at 13 000 r.p.m. in a microcentrifuge at room temperature, then blocked by adding 500 μl of 1% Tween 20 in PBS and incubating at 37°C for 2 h. The beads were washed once, re-suspended with 6 ml PBS (1 × 108 beads/ml) and kept in the dark at 4°C, ready for use. Uncoated beads were suspended in PBS at 1 × 107 beads/ml as negative control. When comparing different coating concentrations of the beads, the same procedure was followed but the PPS adjusted to give estimated working concentrations of 16·6, 166 and 322 μg PPS/108 beads.
Lipopolysaccharide
LPS was reconstituted with sterile water to a concentration of 1 mg/ml and stored at −70°C in aliquots in endotoxin-free Eppendorf tubes.
Cell-wall polysaccharide
CWPS was reconstituted with sterile water to a concentration of 1 mg/ml and stored at −40°C in aliquots in endotoxin-free Eppendorf tubes.
Endotoxin was assessed in all of the above antigens by a limulus amoebocyte lysate method (Pyrogent®plus kit, Biowhittaker). PPS and CWPS were found to have undetectable levels, whereas a positive result (0·12 EU/ml) was found in a sample containing 1 × 107 heat-inactivated S14 bacteria. The concentration of 0·12 EU equals LPS of 0·012 ng (LAL Product technical support, BioWhittaker UK Ltd, Workingham, Berkshire, UK). The amount of endotoxin in the 1 × 107 heat-inactivated S14 bacteria per ml will not induce the cytokine responses according to our dose-dependent cytokine response to LPS (See the first paragraph in the result section). The role of endotoxin contamination of heat-inactivated S14 bacteria in the cytokine response can therefore be ignored.
Dose-dependent cytokine response to whole bacteria, PPS, LPS and CWPS
One aliquot of heat-inactivated S14 bacteria containing 2·4 × 107 bacteria per 10 μl (stock solution) was thawed and 1 : 10, 1 : 100 and 1 : 1000 dilutions made using double autoclaved PBS in endotoxin-free Eppendorf tubes. Twenty ml of Group O blood was collected into a pyrogen-free tube containing endotoxin-free heparin and mixed well. 4 ml aliquots of this were pipetted into five 10 ml stoppered tubes and antigen added as shown in Table 1. The tubes were mixed well, 1 ml removed from each immediately into LP4- stoppered tubes and placed on ice to stop any reaction. The initial tubes were incubated at 37°C, with 5% CO2. Further 1 ml aliquots were removed at 6 h into LP4 tubes on ice, centrifuged at 200 g and 4°C for 5 min The supernatants were removed and stored at −40°C for subsequent cytokine analysis. A dose–response curve was plotted to ascertain the minimum bacterial concentration necessary to elicit a cytokine response after 6 h.
Table 1.
Dose–response experiment for whole bacteria. Dilution table for heat-inactivated bacteria to show the working concentrations used in the dose–response experiment (see Materials and methods)
| Tube | Contents | Working antigen concentration |
|---|---|---|
| 1 | 4 ml blood | Negative control |
| 2 | 4 ml blood + 16 μl stock S14 bacteria | 1 × 107 bacteria/ml |
| 3 | 4 ml blood + 16 μl 1 : 10 dilution of S14 bacteria | 1 × 106 bacteria/ml |
| 4 | 4 ml blood + 16 μl 1 : 100 dilution of S14 bacteria | 1 × 105 bacteria/ml |
| 5 | 4 ml blood + 16 μl 1 : 103 dilution of S14 bacteria | 1 × 104 bacteria/ml |
This experiment was repeated using PPS (stock solution 1 mg/ml) and making 1 : 10, 1 : 100 and 1 : 1000 dilutions. 80 μl of stock solution was added to 4 ml blood to give a final working PPS concentration of 20 μg/ml 40 μl of stock, 1 : 10, 1 : 100 and 1 : 1000 dilutions were added to further tubes containing 4 ml blood each to give final PPS working concentrations of 10, 1, 0·1, and 0·01 μg/ml. A dose–response curve was plotted to ascertain the minimum PPS concentration necessary to elicit a cytokine response after 6 h.
A similar experiment was done with LPS (stock solution 1 mg/ml). This was diluted with sterile PBS to a concentration of 1 μg/ml. Further dilutions with PBS were made (1 : 10, 1 : 100, 1 : 1000, 1 : 10 000) and 200 μl of each of these added to 4 ml blood to give final LPS working concentrations of 50, 5, 0·5, 0·05 and 0·005 ng/ml. A dose–response curve was plotted to ascertain the minimum LPS concentration necessary to elicit a cytokine response after 6 h.
The experiment was also repeated with CWPS. The stock solution (1 mg/ml) was diluted 1 : 10 and 1 : 100 with sterile PBS. 80 μl of stock solution was added to 4 ml blood to give a final CWPS working concentration of 20 μg/ml 40 μl of stock, 1 : 10, 1 : 100 and 1 : 1000 dilutions were added to tubes containing 4 ml blood each to give final CWPS working concentrations of 10, 1, 0·1 and 0·01 μg/ml. A dose–response curve was plotted after 6 hours’ stimulation to see if any possible contamination of the PPS preparation by CWPS could be responsible for observed cytokine release.
All these experiments included a negative control of blood incubated in the same experimental conditions including the same diluent, test tubes and handling procedure, but with the absence of stimulating antigens.
Rate of cytokine induction by whole bacteria, PPS and LPS
From the results of the previous experiments the most suitable concentration of the different antigens to be used in the stimulation experiments was chosen: 1 × 107 whole bacteria/ml blood, 10 μg PPS/ml blood, and 50 ng LPS/ml blood (Fig. 1). Similar experiments to those described above were then repeated on 8 occasions, using blood from 5 different blood group O subjects. Samples were taken after 0, 3, 4·5 and 6 hours’ incubation with the 3 antigens so that time-courses of cytokine production could be compared.
Fig. 1.
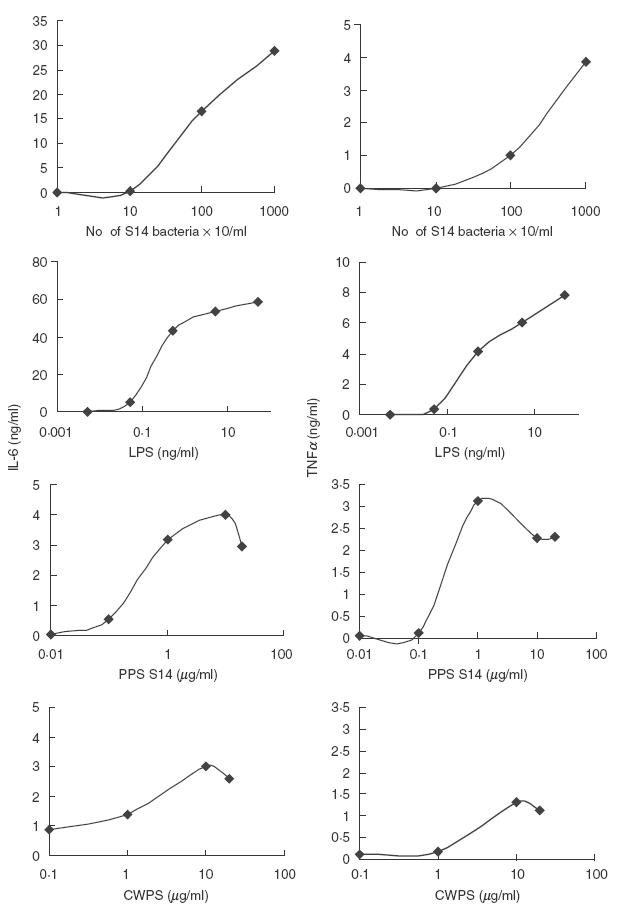
Dose–response analysis of heat-inactivated bacteria, LPS, PPS and CWPS on cytokine production. Whole blood from a healthy, blood group O subject was incubated with increasing amounts of heat-inactivated bacteria, LPS, PPS or CWPS for 6 h to ascertain the lowest concentration of each antigen to elicit a cytokine response. Each datum point represents the mean of duplicate determinations. Negative controls (unstimulated blood) are not shown because in all cases the cytokine level was <0·03 ng/ml.
Induction of cytokine release by PPS bound to beads
Further experiments were done using blood from three different blood group O subjects stimulated with 10 μg PPS/ml blood, 1 × 107 PPS-coated beads/ml blood, 1 × 107 uncoated beads/ml blood (negative control) and 50 ng LPS/ml blood (positive control). Samples were collected for cytokine analysis as described above. Blood incubated in the absence of stimulating antigen was also analysed as a negative control. The experiment was repeated once using a negative control, stimulation with free PPS (10 μg/ml) and 3 different coating concentrations of PPS on beads.
Assessment of coating of beads by fluorescence microscopy
Two μl of uncoated and coated beads were added to 10 μl of 1/16 diluted normal human serum, respectively, and incubated at 37°C for 2 h. After washing twice with 0·05% PBS-Tween 20, 10 μl of 1/20 diluted FITC conjugated rabbit anti-human immunoglobulin was added to each sample. A further 1 h incubation followed. After 2 more washes the samples were re-suspended in 0·5 ml PBS/0·1% BSA/0·05% sodium azide and observed under a fluorescent microscope at 525 nm.
Cytokine enzyme-linked immunoabsorbent assay (ELISA)
The samples were analysed for IL-6 and TNFα using a ‘sandwich’ ELISA assay following the manufacturer's instructions. (Pharmingen OPTEIA™ human IL-6 and TNFα Sets.) As a standard, recombinant human IL-6 and TNFα were used with a lower detection limit of 10 and 16 pg/ml, respectively.
Sonication of heat-inactivated S. pneumoniae S14 bacteria
1·4 ml of whole bacteria (prepared as described above) were added to 1·4 ml sterile PBS (under sterile conditions) and this suspension sonicated using a Jencons High Intensity Ultrasonic Processor for 1 minute. This was repeated seven times. The tube was centrifuged at 200 g and 4°C for 30 min, the supernatant removed and saved. The pellet was re-suspended in 2 ml sterile PBS and the sonication process repeated. The tube was centrifuged again and the supernatant added to the first one. The total volume of sonicate was made up to 5 ml with sterile PBS and aliquots stored at −40°C until analysed. The sonicate was equivalent to 6·7 × 108 bacteria per ml.
ELISA to estimate the PPS concentration of the heat-inactivated S14 bacteria used in the stimulation experiments
The wells of one half of a microtitre plate were coated with known concentrations of PPS to act as a standard curve (starting at a concentration of 1 μg/ml) and the other half coated with dilutions of sonicate (undiluted then double dilutions to 1 : 1024). The plate was incubated at 4°C overnight, washed, blocked and washed again. Rabbit antiserum against PPS S14 was added and the plate incubated at 37°C for 3 h. After further washing, peroxidase-conjugated goat antirabbit immunoglobulin was added and the plate incubated for 3 h at 37°C. Following further washing, the colour was developed using TMB substrate and read at 650 nm. This experiment was repeated using CWPS (starting at a concentration of 10 μg/ml) instead of PPS for coating, to investigate if there was any cross-reactivity from CWPS with the PPS S14 antiserum.
Statistics
IL-6 and TNFα concentrations, after stimulation with different antigens, were presented as mean values of duplicate samples plus or minus the standard error of the mean. Statistical analysis and significance of group differences (Mann–Whitney U-tests) were performed using the SPSS for Windows (TM) Version 10 programme (SPSS, Chicago, IL, USA). P-values <0·05 were considered as statistically significant.
RESULTS
Dose–response results for heat-inactivated bacteria, PPS, LPS and CWPS
The relative efficiency of investigated concentrations of heat- inactivated bacteria, PPS S14, LPS and CWPS to stimulate IL-6 and TNFα after 6 h are shown in Fig. 1. From these results the concentration of each antigen to be used in further stimulation experiments was selected as follows: 1 × 107 bacteria/ml blood, 10 μg PPS/ml blood, and 50 ng LPS/ml blood. These selected concentrations are in general agreement with those used by other research groups in similar studies [4,11,13]. There was significant cytokine release after stimulation with PPS at a concentration of 1 μg/ml compared with unstimulated control (P < 0·05), whereas 10 times as much CWPS was needed to give a similar response. Therefore possible contamination of the PPS preparation with CWPS could not explain the cytokine production that was seen. Similarly C-reactive protein (CRP) was not considered to be a contributing factor because the measured CRP level of the blood used in these experiments was below our detection limit (<0·335 mg/l).
Stimulation experiments
Whole blood was stimulated on 8 separate occasions with heat-inactivated S14 bacteria, PPS and LPS at the selected concentrations. The time courses for cytokine stimulation are shown in Fig. 2(a,b). The time courses were terminated after 6 h because this was associated with maximal cytokine release before cell damage was apparent by microscopy. These results show that PPS induced significantly more IL-6 and TNFα than unstimulated blood (P = 0·001), heat-inactivated bacteria significantly more than PPS (P = 0·001) and LPS significantly more than heat- inactivated bacteria (P = 0·001). The relative stimulation of IL-6 and TNFα was compared. Heat-inactivated bacteria and LPS produced significantly higher concentrations of IL-6 than TNFα (P = 0·001 in each case), the ratios being IL-6/TNFα of 6·7 and 9·0, respectively, whereas with PPS the concentration of both cytokines was similar (P = 0·141) as shown in Fig. 3
Fig. 2.
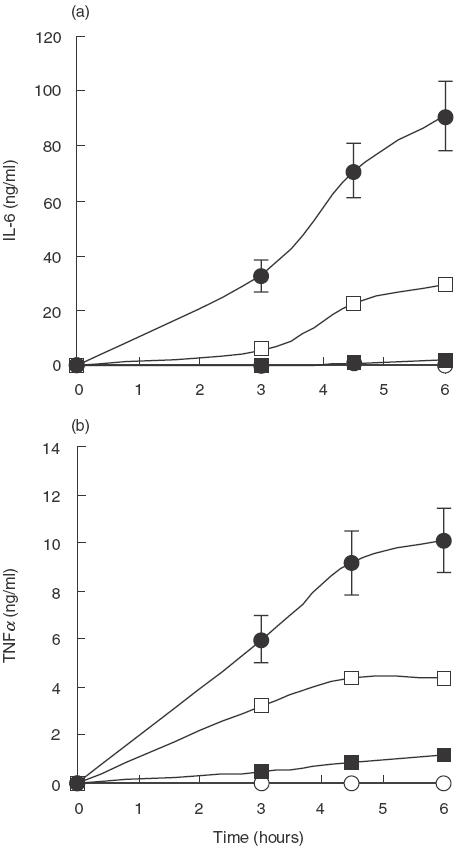
Time course analysis of cytokine production over a 6-h period after stimulation with different antigens. Whole blood from five healthy, blood group O subjects was incubated at 37°C with heat-inactivated bacteria (1 × 107 bacteria/ml blood), PPS (10 μg/ml blood), PPS-coated beads (1 × 107 beads/ml blood), uncoated beads (1 × 107 beads/ml blood), and LPS (50 ng/ml blood), sampled at 0, 3, 4·5 and 6 h and analysed for cytokine release. Unstimulated blood was used as a negative control. Results are plotted as the mean of duplicate determinations from the 8 experiments. ○ Control; ▪ PPS S14; • LPS; □ S14. Error bars represent the standard error of the mean
Fig. 3.
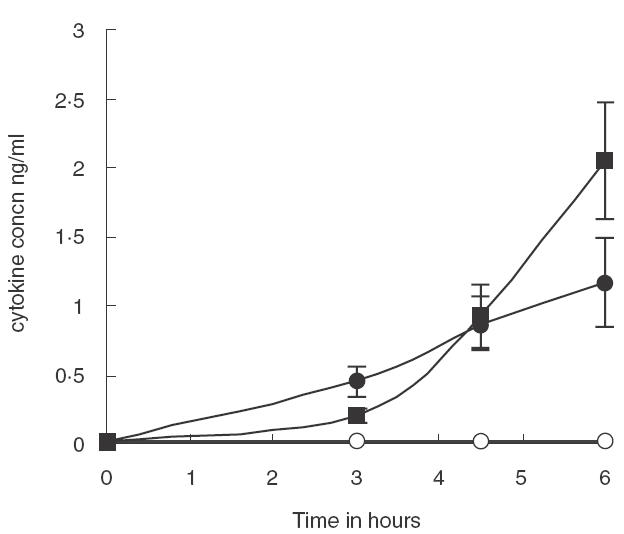
Time course analysis of cytokine production over a 6-h period after stimulation with PPS Whole blood from five healthy, blood group O subjects was incubated at 37°C with PPS (10 μg/ml blood, sampled at 0, 3, 4·5 and 6 h and analysed for cytokine release. Unstimulated blood was used as a negative control. Results are plotted as the mean of duplicate determinations from the 8 experiments. ○ Control; ▪ IL6; • TNFα. Error bars represent the standard error of the mean
A similar experiment to that described for the other antigens was done twice with PPS-coated beads and uncoated beads. The time courses for these are not presented as cytokine stimulation could not be detected (IL-6 and TNFα values were all less than 0·3 ng/ml), whereas free PPS, included as a control, induced a similar amount of IL-6 and TNFα as in the first experiments.
Comparison of different coating concentrations of beads
Fluorescence microscopy showed that binding had occurred, as shown in Fig. 4. However, no attempt was made to measure quantitatively the concentration of PPS which had bound to the latex beads. We calculated that even if all the polysaccharide had been bound to the beads in these stimulation experiments this would equate to a PPS concentration of approximately 80 ng/ml in the final incubation mixture. This concentration of free PPS would represent the beginning of the dose–response curve for induction of both IL-6 and TNFα, so another experiment was carried out using higher coating concentrations of PPS for the beads. The results are presented in Table 2. No measurable increase was detected at the higher coating concentrations, but again the amount of PPS bound to the beads was not measured.
Fig. 4.
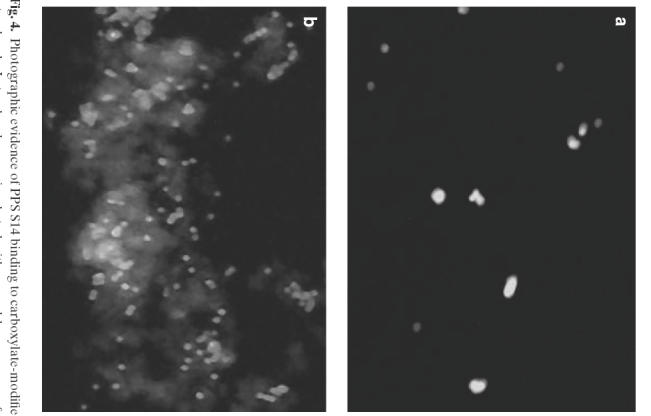
Photographic evidence of PPS S14 binding to carboxylate-modified latex beads. Latex beads were incubated with normal human serum for 1 h, washed and incubated with FITC labelled antihuman IgG. No binding was observed under the Olympus BX40 system microscope. Latex beads coated with PPS (as described in Methods section) were incubated with normal human serum for 1 h, washed and incubated with FITC labelled antihuman IgG. The light shaded areas (fluorescence) indicate the IgG binding to the beads
Table 2.
Effect on cytokine release of increasing the coating concentration of PPS coated onto latex beads. Blood sample from a healthy, blood group O subject was incubated at 37°C with PPS (10 μg/ml), PPS-coated beads at concentrations of 16·6 μg, 166 μg and 332 μg/108 beads and uncoated beads (1 × 108 beads/ml blood), sampled at 0, 3, 6 and 8 h and analysed cytokine release. The values shown represent the mean of duplicate determinations
| 0 h | 3 h | 6 h | 8 h | |
|---|---|---|---|---|
| IL-6 pg/ml | ||||
| Control (blood) | 12 | 13 | 31 | 43 |
| PPS.S14 | 12 | 62 | 1371 | 2829 |
| Beads only | 15 | 20 | 38 | 90 |
| 16·6 μg PPS/108 beads | 9 | 14 | 36 | 58 |
| 166 μg PPS/108 beads | 9 | 18 | 42 | 62 |
| 332 μg PPS/108 beads | 10 | 23 | 60 | 84 |
| TNFα pg/ml | ||||
| Control (blood) | 22 | 25 | 24 | 24 |
| PPS.S14 | 14 | 472 | 1085 | 1118 |
| Beads only | 34 | 23 | 30 | 35 |
| 16·6 μg PPS/108 beads | 11 | 23 | 41 | 28 |
| 166 μg PPS/108 beads | 15 | 24 | 37 | 23 |
| 332 μg PPS/108 beads | 12 | 32 | 32 | 30 |
ELISA to estimate the PPS concentration of the heat-inactivated bacteria used in the stimulation experiments
An ELISA was done, after sonication of the whole bacteria, using a rabbit antiserum against PPS, so that the amount of PPS in the bacterial cells could be estimated. The mean value obtained (n = 4) was 1·34 μg PPS/ml of solution containing 1 × 107 bacteria. This is an order of magnitude lower than the concentration of free PPS that we used in our stimulation experiments, supporting the role of other factors playing an important role in the release of inflammatory cytokines in response to S. pneumoniae infection. There was no cross-reactivity from the CWPS. (Absorbance <0·1).
DISCUSSION
Responses to bacterial antigens in a whole blood system result from interaction of multiple plasma factors (immunoglobulins, complement, binding proteins) and multiple cell-surface receptors (Fc receptors, scavenger receptors, toll-like receptors, CD14, etc.). In this study we have used the measurement of the cytokines IL-6 and TNFα as an indicator of the overall pattern of inflammatory cytokine release after stimulation with an intact bacterium and isolated bacterial antigens. The inflammatory cytokine release following stimulation of human blood in vitro with whole bacteria was significantly greater than that of PPS alone (P = 0·001). Also, we estimated that the concentration of PPS needed to induce cytokine release was much higher than that present in the total number of bacteria used in these experiments.
The immune response is not only dependent on the structure of the antigen but on its size too. Thus, it is possible that free PPS could behave differently from the particulate form found in the bacteria. To test this we bound PPS onto latex beads (which are the same size as intact bacteria) and investigated the cytokine release from blood stimulated with these. PPS-coated beads did not elicit a response at all. The amount of polysaccharide used to coat the beads was of the same order of magnitude as that calculated to be in the whole bacteria. However, this failed to elicit a cytokine response, again indicating that other factors contained in the bacteria must be responsible for the cytokine release, e.g. CWPS, surface proteins, pneumolysin. Although we did not quantify the concentration of PPS on the beads microscopic evidence showed that the beads were coated with PPS and that they underwent phagocytosis.
Polysaccharide preparations may be contaminated with CWPS and it is possible that the cytokine release seen with PPS was due to contaminating CWPS. We investigated the relative efficiency of PPS and CWPS for releasing cytokines and found that PPS was 10 times more efficient on a weight basis. Even if the PPS preparation contained as much as 10% contamination with CWPS this would not be expected to contribute to the cytokine release observed.
Different patterns of cytokine release are associated with different receptor recruitment. PPS produced similar concentrations of IL-6 and TNFα, whereas LPS and whole bacteria elicited large quantities of IL-6 compared to TNFα (ratios of 9·0 and 6·7, respectively). This predominant IL-6 response, in the case of LPS particularly, is in agreement with recent studies which indicate that IL-6 may play a significant role in host defence against bacterial infection [16] whereas previously it was considered merely a marker for the severity of the bacterial challenge.
The larger IL-6/TNFα ratio observed with LPS (i.e. Gram-negative bacteria) compared with that from Gram-positive bacteria can probably be attributed to the activation of different primary toll-like receptors initially. TLR4 has been identified as the primary receptor for enteric LPS whereas TLR2 is implicated as the receptor for Gram-positive cell wall components [11]. Many models are being suggested currently as to how different TLR proteins, in conjunction with additional receptors, stimulate macrophages in defence against specific organisms [10,12,19–21]. Cauwells et al. [4], working with a whole blood system and comparing LPS, whole, unencapsulated R6x S. pneumoniae and purified pneumococcal cell wall concluded that as many as two receptors, in addition to CD14, appear to lead to cytokine production by Gram positive bacteria, but not by Gram-negative organisms. However these different signalling pathways might converge intracellularly.
Specific antibodies to the capsular polysaccharide play an important role in fighting infection and enhancing phagocytosis at a local level. Once infection becomes systemic the inflammatory response leading to sepsis and death is a major problem and antibodies to PPS may have an insignificant role in influencing this. In thinking about the design of future pneumococcal vaccines our results would support the idea that the inclusion of other antigens besides capsular polysaccharide could render a vaccine more effective in protection during systemic infection. This is particularly relevant in that recent evidence-based analysis of vaccine trials concluded that the present polysaccharide-vaccine is of no benefit to ‘at risk’ groups [22].
Acknowledgments
The authors wish to acknowledge the financial support from the COGENT Trust.
REFERENCES
- 1.Boulnois GJ. Pneumococcal proteins and the pathogenesis of disease caused by Streptococcus pneumoniae. J General Microbiol. 1992;138:249–59. doi: 10.1099/00221287-138-2-249. [DOI] [PubMed] [Google Scholar]
- 2.Catterall JR. Streptococcus pneumoniae. Thorax. 1999;54:929–37. doi: 10.1136/thx.54.10.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruyn GAW, Zegers BJM, van Furth R. Mechanisms of host defence against infection with Streptococcus pneumoniae. Clin Infect Dis. 1992;14:251–62. doi: 10.1093/clinids/14.1.251. [DOI] [PubMed] [Google Scholar]
- 4.Cauwels A, Wan E, Leisman M, Tuomanen E. Co-existence of CD-14 dependent and independent pathways for stimulation of human monocytes by gram-positive bacteria. Infect Immun. 1997;65:3255–60. doi: 10.1128/iai.65.8.3255-3260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soell M, Diab M, Haan-Archipoff G, Beretz A, Herbelin C, Poutrel B, Klein JP. Capsular polysaccharide types 5 and 8 of Staphylococcus aureus bind specifically to human epithelial (KB) cells, endothelial cells and monocytes and induce release of cytokines. Infect Immun. 1995;63:1380–6. doi: 10.1128/iai.63.4.1380-1386.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson SQ, Singh R, Bice DE. Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumour necrosis factor α production by murine macrophages. Am J Respir Cell Mol Biol. 1994;10:284–9. doi: 10.1165/ajrcmb.10.3.8117447. [DOI] [PubMed] [Google Scholar]
- 7.Arva E, Andersson B. Induction of phagocyte-stimulating and Th1-promoting cytokines by in vitro stimulation of human peripheral blood mononuclear cells with Streptococcus pneumoniae. Scand J Immunol. 1999;49:417–23. doi: 10.1046/j.1365-3083.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 8.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor 4 mediates LPS-induced signal transduction. J Biol Chem. 1999;274:10689–92. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 9.Cuzzola M, Mancuso G, Beninati C, et al. β integrins are involved in cytokine responses to whole gram-positive bacteria. J Immunol. 2000;164:5871–6. doi: 10.4049/jimmunol.164.11.5871. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting Edge. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 11.Dekkers PEP, Juffermans NP, ten Hove T, de Jonge E, van Deventer SJH, van der Poll T. Endotoxin down-regulates monocyte and granulocyte IL-6 receptors without influencing gp 130 expression in humans. J Infect Dis. 2000;181:1055–61. doi: 10.1086/315356. [DOI] [PubMed] [Google Scholar]
- 12.Matsuguchi T, Musikacharoen T, Ogawa T, Yoshikai Y. Gene expression of toll-like receptor 2, but not toll-like receptor 4, is induced by LPS and inflammatory cytokines in mouse macrophages. J Immunol. 2000;165:5767–72. doi: 10.4049/jimmunol.165.10.5767. [DOI] [PubMed] [Google Scholar]
- 13.Freiling JTM, Mulder JA, Hendriks T, Curfs JHAJ, van der Linden CJ, Sauerwein RW. Differential induction of pro- and anti- inflammatory cytokines in whole blood by bacteria: effects of antibiotic treatment. Antimicrob Agents Chemother. 1997;41:1439–43. doi: 10.1128/aac.41.7.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayringer I, Reindl M, Berger T. A critical comparison of frequently used methods for the analysis of tumour necrosis factor-α expression by human immune cells. J Immunol Meth. 2000;235:33–40. doi: 10.1016/s0022-1759(99)00208-2. [DOI] [PubMed] [Google Scholar]
- 15.Schultz MJ, Speelman P, Zaat S, Sander J, van Deventer H, van der Poll T. Erythromycin inhibits tumour necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob Agents Chemother. 1998;7:1605–9. doi: 10.1128/aac.42.7.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Poll T, Keogh CV, Guirao X, Buurman WA, Kopf M, Lowry SF. Interleukin-6 gene-deficient mice show impaired defence against pneumococcal pneumonia. J Infect Dis. 1997;176:439–44. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 17.Riches P, Millar BC, Rowbottom AW. Influence of collection and separation of blood samples on plasma IL-1, IL-6 and TNFα concentrations. J Immunol Meth. 1992;153:125–31. doi: 10.1016/0022-1759(92)90314-j. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood D, Slack R, Peutherer J. Pathogenesis, Immunity, Laboratory Diagnosis and Control. 14. Edinburgh: Churchill Livingstone; 1994. Medical Microbiology: a Guide to Microbial Infections. [Google Scholar]
- 19.Underhill DM, Ozinsly A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–5. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 20.Means TK, Golenbock DT, Fenton MJ. The biology of toll-like receptors. Cytokine Growth Factor Rev. 2000;11:219–32. doi: 10.1016/s1359-6101(00)00006-x. [DOI] [PubMed] [Google Scholar]
- 21.Perera PY, Mayadas TN, Takeuchi O, Akira S, Zaks-Zilberman M, Goyert SM, Vogel SN. CD11b/CD18 acts in concert with CD14 and toll-like receptor (TLR) 4 to elicit full lipopolysac- charide and taxol-inducible gene expression. J Immunol. 2001;166:574–81. doi: 10.4049/jimmunol.166.1.574. [DOI] [PubMed] [Google Scholar]
- 22.Moore RA, Wiffen PJ, Lipsky BA. Are the pneumococcal polysaccharide vaccines effective? Meta-analysis of the prospective trials. BMC Family Prac. 2001;1:1–12. doi: 10.1186/1471-2296-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


