Abstract
The characteristics of the immunity induced by viral antigens or conferred by antiviral antibody via different routes of administration were evaluated comparatively. C57BL/6 mice were immunized via intranasal, intradermal or enteric routes with a live recombinant vaccinia virus expressing the respiratory syncytial virus (RSV) F glycoprotein (F·rVV) or RSV, and then challenged intranasally with RSV. Inhibition of RSV replication was observed in the lungs of all the mice; however, only intranasal immunization hindered virus replication in the nose. Lung inflammation, characterized by infiltration of neutrophils and of mononuclear cells was strongest in the intradermally immunized mice, but was observed in all F·rVV immunized mice to various degrees. Intranasal administration of a potently neutralizing human anti-RSV antibody Fab fragment to infected mice inhibited RSV replication in the nose and, when combined with intraperitoneal administration, protected both the lung and the nose in the absence of deleterious lung pathology. These data suggest that intranasal immunization with F·rVV reduces RSV replication in the respiratory tract, but still induces pathological lung inflammation, even though this is milder than that observed following intradermal immunization. Local neutralizing antibody is indispensable for protection in the nose.
Keywords: administration rote, antiviral antibody, F-glycoprotein, recombinant vaccinia virus, RSV
Introduction
Respiratory syncytial virus (RSV) causes serious respiratory infection in young children [1]. Although RSV has been recognized as a priority for vaccine development, a safe and effective vaccine is still not available for routine use [2,3]. In the 1960s, children vaccined with formalin-inactivated RSV showed a strong serological response against RSV, but were not protected from subsequent natural infection. Moreover, 80% of vaccinated children developed serious disease of the lower respiratory tract [4]. The mechanisms of vaccine-augmented disease have been investigated extensively, and yet the precise mechanism remains to be defined [5–8].
After the 1980s, it has been suggested that virus-induced immune responses, rather than the direct pathogenic effects of the virus, may underlie the pathogenesis of RSV disease. Openshaw et al. [9] have shown that mice immunized systemically with recombinant vaccinia virus carrying the G glycoprotein of RSV had reduced lung virus titres following subsequent challenge with RSV, but also an increase in the severity of lung pathology. Recent studies suggest that the production of Th2 cytokines is important in the pathogenesis of the exaggerated response to infection and in virus-induced airway hyperresponsiveness [10,11]; however, the role of Th1 cytokines, such as interferon-γ, during RSV infection is still not clear [12].
Parenteral administration of RSV-specific immunoglobulin preparations containing anti-RSV antibodies to patients suffering from bronchiolitis with RSV is now considered an effective immunoprophylaxis, particularly in those individuals with serious underlying diseases [13,14]. However, effective protection against RSV infection should include the upper respiratory tract, such as the nose, and not only the lower tract, because replicating virus in the nose spreads to the lower tract and may be transmitted readily to other individuals [15].
In this study, we have evaluated comparatively the immunity induced by RSV F glycoprotein or by anti-RSV antibody via mucosal routes.
Materials and methods
Viruses
RSV long strain (the prototype RSV group A strain) was grown in HEp-2 cells in minimal essential medium (MEM) supplemented with 2% fetal calf serum (FCS), 2 mm l-glutamine and antibiotics (penicillin and streptomycin, 100 U/ml), and preserved at − 70°C. For animal experiments, virus was partially purified by polyethylene glycol precipitation followed by centrifugation in a 35–65% discontinuous sucrose gradient, as described elsewhere [16]. Uninfected HEp-2 cells were processed similarly and used as controls.
Live recombinant vaccina virus expressing the RSV F glycoprotein (F·rVV) (provided by Dr R. A. Olsmsted [17]) and parent vaccina virus (strain WR) were grown on HEp-2 cells and infected cell lysates were sonicated for 2 min and centrifuged. The supernatant was layered over 25% sucrose in 10 mm Tris hydrochloride, pH 8·8. After centrifugation at 18 000 r.p.m. for 80 min at 4°C, the pelleted virus was resuspended in 10 mm Tris hydrochloride and stored at − 70°C until use.
Fab anti-RSV antibody
Phage bearing RSV-specific Fab was selected via panning from antibody libraries expressed on the surface of filamentous phage prepared from bone marrow RNA of RSV seropositive donors as described previously [18]. Selected phages were converted to soluble Fab expression. The clone, Fab RSV 19, exhibited high neutralizing activity against RSV. Monoclonal IgG1 antibody (clone C2) to the F glycoprotein was prepared as described previously [19] and digested with pepsin to obtain F(ab′)2 fragments. Such fragments did not have RSV-specific neutralizing activity and were used as control.
Experimental design
Male C57BL/6 mice 8 weeks of age (Nippon Clea, Shizuoka, Japan) were immunized as follows: for enteric immunization, mice were anaesthetized with ketamine after overnight starvation, their abdomens were opened under aseptic conditions, and the small intestine was injected with 1 × 106 plaque-forming units (PFU) of F·rVV or control wild-type vaccinia virus (CVV) through a 27-gauge needle, then the abdomen was closed with silk sutures. For intranasal immunization, mice were anaesthetized by inhalation of methoxyflurane and injected with 1 × 105 PFU of F·rVV or CVV, or 1 × 106 PFU of RSV instilled into the noses under methoxyflurane anaesthesia. RSV challenge was performed intranasally in same way with 20 µl of RSV (1 × 106 PFU), and HEp-2 cells were used as controls. There were seven mice in each group.
Mice were sacrified by CO2 overdose and exsanguinated, then blood, bronchoalveolar lavage (BAL), nasal washings, lung tissue and nasal mucosa were collected. For BAL, the trachea was exposed and 0·7 ml of Hanks's balanced salt solution without Ca2+ and Mg2+ (HBSS) was injected gently into the trachea just below the larynx, expanding the lungs. The lavage was pulled back into the syringe and injected into the lungs before final withdrawal. For nasal washings, mice were pinned into a support tilted to elevate the head. HBSS (200 µl) was injected into the larynx and towards the nose. Nasal washings were collected in a tube as they exited the nares. Both fluids were clarified by low-speed centrifugation and stored at − 70°C for determination of antibody titres and cytokines by enzyme-linked immunosorbent assay (ELISA).
This laboratory animal study was approved by the local Animal Ethics Committee (Yamanashi Medical University), and conducted in conformity with their guidelines.
Virus assay
For the vaccinia virus assay, the small intestine, lung and nasal mucosa were homogenized in MEM containing 2% FCS and antibiotics. After repeated freeze-thawing, homogenates were clarified by centrifugation and the supernatant fluid was stored at − 70° for plaque assay. For RSV assay, lungs and nasal tissues were collected 4 days after challenge. Both tissues were weighed and homogenized in MEM containing 2% FCS and stored at − 70°C until assay.
Vaccinia virus and RSV were assayed by the plaque method on HEp-2 cells in 24-well microplates in triplicate. The overlay for the plaque assay consisted of MEM supplemented with 2% FCS, antibiotics and 1% methylcellulose. Plates were incubated for 7 days at 37°C. After the methylcellulose was removed, plaques were fixed with 10% formaldehyde and stained with 0·1% crystal violet.
Antibody assay
Neutralizing antibody response to RSV was assessed by the plaque reduction method [20]. Briefly, 0·15 ml of serial fourfold-diluted heat-inactivated sample was mixed with an equal volume of RSV (200 PFU/0·1 ml) and incubated at room temperature for 1 h. Mixtures were then incubated on HEp-2 cells in 24-well microtitre plates and incubated for 7 days. The titre of neutralizing antibody was determined as the reciprocal of the sample dilution, which produced a 60% reduction of RSV plaques. IgG and IgA antibody response to RSV was measured by ELISA. Briefly, the wells of ELISA plates (Nunc Co. Ltd, Roskilde, Denmark) were coated with 100 ml of RSV or HEp-2 control preparations after dilution to 5 µg protein/ml in carbonate buffer (pH 9·6) overnight at 4°C. Plates were then washed three times with PBS containing 0·05% Tween 20 and incubated with PBS containing 10% FCS at room temperature for 2 h. After washing, wells were filled with 100 µl of twofold serial dilutions of samples in PBS containing 10% FCS and Tween 20. Plates were incubated for 2 h at 37°C and were again washed with PBS-Tween. To detect IgG, wells were incubated for 2 h at 37°C with 100 µl of peroxidase-conjugated, affinity-purified goat antimouse IgG (diluted 1 : 1000, ICN pharmaceuticals, Inc., CA, USA). To detect IgA, wells were incubated with 100 µl of peroxidase-conjugated, affinity-purified goat antimouse IgA (diluted 1 : 2000, ICN pharmaceuticals, Inc.). After being washed again, each well was developed for colour with 200 µl of o-phenylenediamine in citrate-phosphate buffer (pH 5·0) and O.D. at 490 nm was determined with an automated microplate reader. The ELISA titre was calculated by conventional positive-over-negative (P/N) in which the endpoint was the highest dilution that gave a P/N ratio equal or greater than 2. The antibody assay was carried out in duplicate.
Detection of cytokines
IL-2, IL-4, IL-5, IL-6, TNF-α and IFN-γ were analysed in duplicate based on the manufacture's manual (Genzyme, Cambridge, MA, USA).
Lung histopathology
Lungs were inflated in situ with buffered 10% formalin solution and fixed in the same reagent. Lung sections were embedded in paraffin and stained with haematoxylin–eosin and examined for peribronchial cellular inflammatory response and septal thickening. Coded lung sections were scored for lesions on an arbitrary scale of 0–3 with 1 representing infiltration involving one or two bronchioles or blood vessels and 3 representing lesions involving most of the bronchioles and blood vessels. In each mouse a minimum of three lobes was examined and scored double-blinded to avoid possible evaluator bias.
Anti-RSV antibody administration
RSV 19 antibody or control C2 antibody was administered to the mice 24 h after infection intraperitoneally (5 µg in 100 µl PBS)or intranasally (1 µg in 2 µl PBS). On the third day after antibody administration, RSV replication in the respiratory tract and lung pathology were examined as described above.
Statistical analysis
Comparison of experimental groups was by Student's t -test.
Results
Replication of F·rVV after immunization
After enteric immunization with 1 × 106 PFU of F·rVV, no infectious virus was recovered from lung or nose. Recovery from the intestine is shown in Fig. 1. Infectious F·rVV decreased and was not detected on day 7. Figure 2 shows recovery from the lung after intranasal immunization with 1 × 105 PFU of F·rVV; a peak was observed on day 3 and declined over 7 days. Virus was recovered from the nose for 10–12 days, but not detected on day 14. Infectious virus was not recovered from intestine after nasal immunization.
Fig. 1.
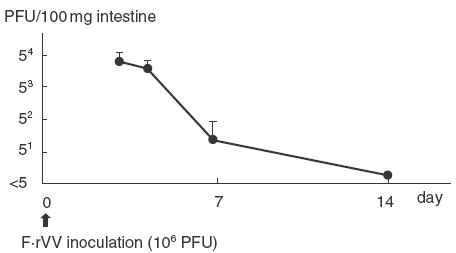
Recovery of infectious recombinant vaccinia virus after enteric immunization. The data represent the mean ± s.d. for five mice.
Fig. 2.
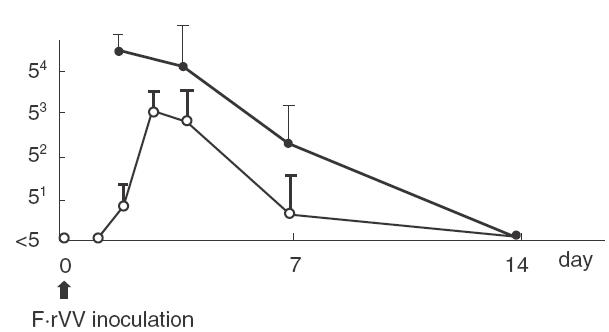
Recovery of infectious recombinant vaccinia virus in lung and in nasal mucosa after intranasal administration with 1 × 105 PFU in 5 µl. The data represents the mean ± s.d. for five mice. —, PFU/100 mg nasal tissue; —, PFU/100 mg lung tissue.
RSV replication in the respiratory tract after intranasal challenge
In non-immunized mice, after nasal inoculation with 1 × 106 PFU of RSV, peak RSV replication in the lung was 103 PFU/100 mg of wet tissue (total lung weight is approximately 1 g) observed on day 4, and declined until day 7. From nasal mucosa, RSV was recovered for 12 days after inoculation (data not shown).
Figure 3 shows recovery of RSV from the respiratory tract of immunized mice 4 days after RSV challenge on day 21. RSV replication in the lung was reduced (less than 50 PFU/100 mg of tissue) in all immunized groups of mice except in the CVV immunized group. In nasal mucosa, RSV recovered appoximately 2 × 103 PFU/100 mg of tissue in intradermal and enteric F·rVV-immunized mice and HEp-2 administered mice. The protection in the nose was observed only in the intranasal immunization groups.
Fig. 3.
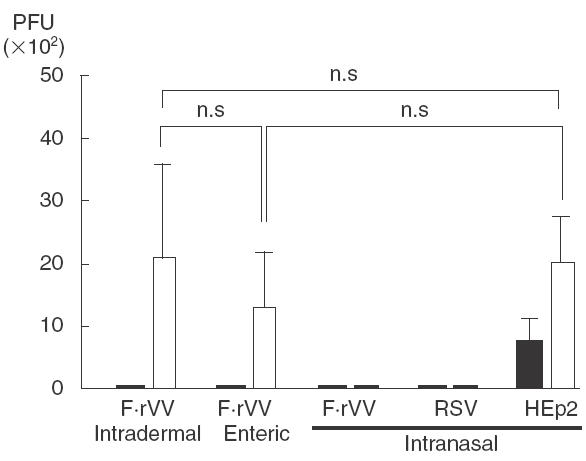
Protective efficacy after immunization with vaccinia virus-RSV recombinant virus or RSV. The data represent mean ± s.d. for seven mice; n.s.: not significant. ▪, Lung; □, nasal; mucosa. n = 7.
Anti-RSV antibody response in serum, BAL and nasal wash samples
IgG antibody in serum was detected in all mice on day 24, except in the CVV immunization group. As shown in Fig. 4, IgG antibody titre was highest in the RSV immunization group and F·rVV intradermal immunization groups and was lowest in the intranasal immunization group.
Fig. 4.
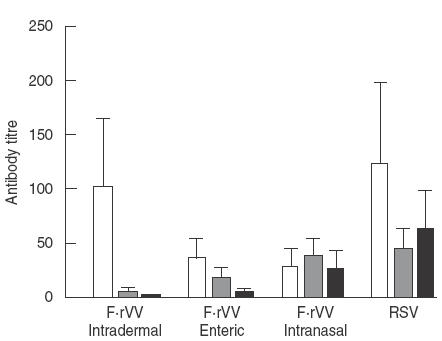
Antibody response to RSV in serum, BAL and nasal wash samples 4 days after RSV challenge. IgG in serum: F·rVV intradermal versus F·rVV enteric or F·rVV intranasal: P < 0·05, RSV versus F·rVV enteric or F·rVV intranasal: P < 0·05. IgA in BAL: F·rVV intradermal versus F·rVV enteric, F·rVV intranasal or RSV: P < 0·01, F·rVV enteric versus F·rVV intranasal or RSV: P < 0·01.IgA in nasal wash: F·rVV intradermal versus F·rVV enteric, F·rVV intranal or RSV: P < 0·01, F·rVV enteric versus F·rVV intranasal or RSV: P < 0·01, F·rVV intranasal versus RSV: P < 0·05. □, IgG in serum; ░, IgA in BAL; ▪, IgA in nasal wash. n = 7.
IgA antibody response in BAL and in nasal washing samples was highest in the RSV immunization group, then in the F·rVV intranasal > enteric > intradermal immunization group, respectively. In the intradermal immunization group a low IgA titre was detected in BAL, but not in nasal washing samples. IgA antibody titres (mean ± s.e.) in serum range was 43·4 ± 5·9, 11·4 ± 1·4, 6·9 ± 0·6 and 82·3 ± 12·0 for F·rVV intradermal, F·rVV enteric, F·rVV intranasal and whole RSV administered mice, respectively.
Detection of cytokines in BAL
As shown in Table 1, various cytokines were detected in BAL. In F·rVV immunization groups, Th1 cytokines such as IFN-γ, and IL-2 were dominant and their concentration was higher in the intradermal immunization group than in the enteric or intranasal immunization group. Low levels of Th2 cytokines such as IL-4 and IL-5 were detected only in the intradermal immunization group.
Table 1.
Concentration of cytokines in BAL 4 days after RSV challenge (mean ± s.d. pg/ml)
| Cytokines | |||||
|---|---|---|---|---|---|
| Virus and immunization route | IL-2 | IL-4 | IL-5 | IL-6 | IFN-r |
| F·rVV intradermal | 17·5 ± 8·5 | 8·9 ± 6·3 | 14·1 ± 6·8 | 140·1 ± 67·4 | 464·3 ± 224·0 |
| F·rVV enteric | 12·6 ± 4·9 | <12 | <12 | 74·2 ± 30·7 | 292·9 ± 166·3 |
| F·rVV intranasal | 18·3 ± 7·4 | <12 | <12 | 82·5 ± 38·2 | 292·9 ± 166·3 |
| RSV intranasal | <12 | <12 | <12 | 68·6 ± 22·1 | 399·3 ± 210·4 |
n = 7
Lung pathology
No obvious inflammation was observed in the lung of any group of mice on day 21 before RSV challenge. Four days after challenge, infiltration of neutrophils and of mononuclear cells and thickness of alveolar walls were recognized at various levels (Fig. 5). Inflammation was most severe in the intradermal immunization group, less severe in the intranasal > enteric F·rVV immunization groups and very mild in the RSV intranasal immunization group (Table 2).
Fig. 5.
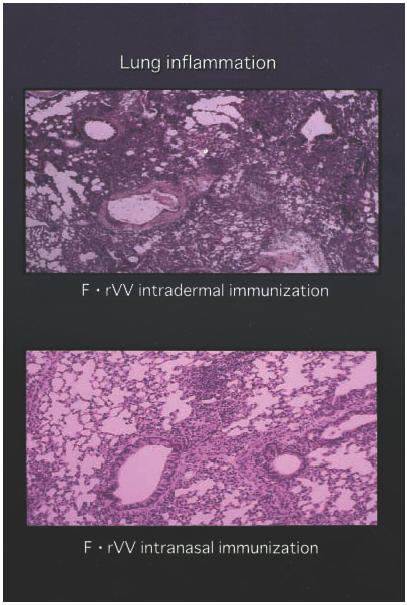
Lung sections were examined for peribronchial cellular inflammatory response and septal thickening. Inflammation was most severe in the intradermal immunization group and less severe in the intranasal group.
Table 2.
Lung pathology 4 days after RSV challenge
| Virus and immunization route used for immunization | Lesion score (mean ± s.d.) |
|---|---|
| F·rVV intradermal | 2·7 ± 0·49a |
| F·rVV enteric | 1·1 ± 0·38b |
| F·rVV intranasal | 1·7 ± 0·49c |
| RSV intranasal | 0·4 ± 0·53d |
a versus b,a versus c,a versus d: P < 0·01; b versus c, b versus d, c versus d: P < 0·05.
Antibody administration
RSV replication in the respiratory tract 3 days after administration of antibody to mice infected intranasally with RSV 1 day earlier is shown in Fig. 6. C-2 antibody, which does not neutralize RSV, did not protect against viral replication at all. Fab 19 antibody prepared by phage library technology with high neutralizing activity reduced viral recovery only in the lung, but not in the nose when administered intraperitoneally. When 1 µg of 19 antibody was administered to the nose of infected mice, RSV replication in the nose was dramatically reduced. Combined nasal/intraperitoneal administration protected both the lung and nose. Neither 19 antibody nor C2 antibody augmented lung pathology (data not shown).
Fig. 6.
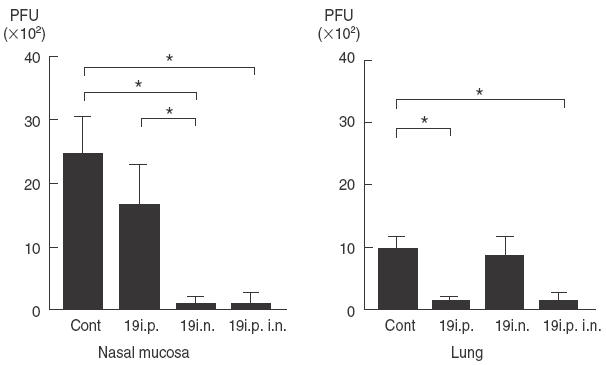
Recovery of infectious RSV in lung and in nasal mucosa on the third day after RSV 19 antibody administration to RSV-infected mice intraperitoneally (i.p.) or intranasally (i.n.). The data represent mean ± s.d. for 11 mice. n = 11; *P < 0·05.
Discussion
Our findings indicate that RSV-specific antibody titres in the respiratory tract correlate more closely with resistance to respiratory infection than levels of anti-RSV antibodies in serum [21,22]. The bulk of these local antibodies, which are of the IgA isotype, are induced by mucosal immunization, exemplified by intranasal or enteric routes [23]. Intranasally administered antigen induces the differentiation of local B lymphocytes into IgA-secreting plasma cells. Enteric administration stimulates precursor B cells in gut-associated lymphoid tissues such as Peyer's patches, which enter the blood stream and specifically migrate to the submucosa of the respiratory tract, where final differentiation into IgA secreting plasma cells occurs. This migration of IgA plasma cell precursors to distant mucosal membranes has contributed to the concept of a common mucosal immune system [24]. To activate the mucosal immune system effectively, multiple administrations of large amounts of antigen are needed. Several strategies, such as microencapsulation, incorporation in liposomes and coupling to cholera toxin and transfecting to recombinant virus, have been considered for enhancing the mucosal immune response [25,26].
In this study, we evaluated comparatively immunization with F·rVV via intradermal, enteric and intranasal routes. Intranasal immunization provides protection from RSV replication after intranasal inoculation in both the lung and nose, whereas intradermal and enteric immunization protect only in the lung, not in the nose. Poor induction of local IgA anti-RSV response in the intradermal immunization group may account for the poor protection of nasal tissues [27]. In the enteric immunization group, significant IgA anti-RSV response was observed in nasal wash samples, although the response may not have been large enough for protection. Previous studies demonstrated that cell-mediated cytotoxic activity could be induced in the respiratory tract of cotton rats after intranasal inoculation with RSV, but not after parenteral immunization [28]. The appearance of cell-mediated immunity on the surface of mucosal membrane may have contributed in part to protection in the nose. IgA anti-RSV antibody response in serum was highest in the RSV immunization group than in the F·rVV intradarmal immunization group. Although the role of serum IgA antibody is not clear, the low IgA antibody titres in BAL and in nasal wash in the F·rVV intradermal immunization group did not indicate the exudation of IgA from serum to respiratory mucosa, nor the contribution of serum IgA antibody to protection in the nose.
The long-term effects of these immunization on protection were not clarified here; however, exacerbation of lung inflammation was observed after RSV challenge on the day 35 (date not shown) even with the reduced viral replication.
Lung inflammation was observed in all F·rVV-immunized mice after challenge with RSV, although this pathology was milder in the mucosal immunization group than in the intradermal immunized group. The mechanism promoting this inflammation is unknown. Serum IgG antibody may participate in the formation of immune complexes resulting in lung injury. In addition, mice primed with F·rVV induce strong RSV-specific cytotoxic T cell response and these T cells recognize RSV antigen on respiratory epithelial cells and may cause lung pathology [29–31]. However, in mice immunized intranasally with live RSV, lung inflammation was very mild after RSV challenge. Here, neither serum IgG antibody nor cytotoxic T cell activity appeared to have a role in the induction of lung inflammation.
Intranasal administration of IgG possessing high RSV neutralizing activity dramatically reduced RSV replication and when combined with IgG administration via the intraperitoneal route, resulted in protection of both the lung and nose without aggravated lung pathology. These findings suggest that, for RSV eradication from the nose, local administration of neutralizing IgG antibody is effective in mediating protection at the mucosal level.
A promising RSV vaccine candidate should induce effective immunity, by suppressing virus replication and reducing lung inflammation [32]. For complete protection of the respiratory tract, the presence of local neutralizing antibody is necessary, but cytotoxic T cell activity is not. The mechanism of aggravated lung pathology induced by immunization with F·rVV still remains uncertain. Antiviral immunity appears to both protect against infection and to contribute to lung pathology.
Once-combined nasal/parenteral administration of a neutralizing antibody against RSV could eradicate virus from the respiratory tract without aggravated lung pathology. In this study, intranasal immunization with F glycoprotein transfected to vaccinia virus protects against RSV replication in the respiratory tract, but contributes to lung pathology. Local neutralizing antibody is indispensable for protection in the nose. Local admistration of even a low dose of anti-RSV antibody with high neutralizing activity combined with parenteral administration, may control viral replication very effectively without unwanted inflammatory reaction in patients with serious RSV infection.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan.
REFERENCES
- 1.Kim HW, Arrobio JO, Brandt CD, et al. Epidemiology of respiratory syncytial virus infection in Washington, D.C. 1. Importance of the virus in different respiratory tract disease syndromes and temporal distribution of infection. Am J Epidemiol. 1973;89:422–34. doi: 10.1093/oxfordjournals.aje.a121550. [DOI] [PubMed] [Google Scholar]
- 2.Belsh RB, Van Voris LP, Mufson MA. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982;145:311–9. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- 3.Norryby E, Akerlind B, Mufson MA. The immunobiology of respiratory syncytial virus vaccine: prospects for a vaccine. Adv Exp Med Biol. 1990;257:147–53. doi: 10.1007/978-1-4684-5712-4_14. [DOI] [PubMed] [Google Scholar]
- 4.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evalution of a respiratory syncytial virus vaccine and a trivarent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–63. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 5.McIntosh K, Masters HB, Orr I, Chao RK, Barkin RM. The immunologic response to infection with respiratory syncytial virus in infants. J Infect Dis. 1978;138:24–32. doi: 10.1093/infdis/138.1.24. [DOI] [PubMed] [Google Scholar]
- 6.Mclntosh K, Fishaut JM. Immunopathologic mechanism in lower respiratory tract disease of infants due to respiratory syncytial virus. Prog Med Virol. 1980;26:94–118. [PubMed] [Google Scholar]
- 7.Prince GA, Jenson AB, Hemming VG, et al. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactivated virus. J Virol. 1986;57:721–8. doi: 10.1128/jvi.57.3.721-728.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piedra PA. Respiratory syncytial virus vaccines: recent developments. Pediatr Infect Dis J. 2000;19:805–8. doi: 10.1097/00006454-200008000-00035. [DOI] [PubMed] [Google Scholar]
- 9.Openshaw PJ, Clarke SL, Record FM. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 10.Pecbles RJ, Sheller JR, Jr, Collins RD, Jarzecka K, Mitchell DB, Graham BS. Respiratory syncytial virus (RSV)-induced airway hyperresponsiveness in allergically sensitized mice is inhibited by live RSV and exacerbated by formalin-inactivated RSV. J Infect Dis. 2000;182:671–7. doi: 10.1086/315783. [DOI] [PubMed] [Google Scholar]
- 11.Boelen A, Andeweg A, Kwakkel J, et al. Both immunization with a formalin-inactivated respiratory syncytial virus (RSV) vaccine and a mock antigen vaccine induce severe lung pathology and a Th2 cytokine profile in RSV-challenged mice. Vaccine. 2000;19:982–91. doi: 10.1016/s0264-410x(00)00213-9. [DOI] [PubMed] [Google Scholar]
- 12.Van Schaik SM, Welliver RC, Kimpen JK. Novel pathways in the pathognesis of respiratory syncytial virus disease. Padiatr Pulmonol. 2000;30:131–8. doi: 10.1002/1099-0496(200008)30:2<131::aid-ppul8>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 13.Siber GR, Leszczynski J, Pena-Cruz V, et al. Protective activity of a human respiratory syncytial virus immune globulin prepared from donors screened by microneutralization assay. J Infect Dis. 1992;165:456–63. doi: 10.1093/infdis/165.3.456. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez PJ. Immunoprophylaxis of respiratory syncytial virus disease. Pediatr Infect Dis J. 2000;19:791–801. doi: 10.1097/00006454-200008000-00033. [DOI] [PubMed] [Google Scholar]
- 15.Murphy BR, Collins PL, Lawrence L, Zubak J, Chanock RM, Prince GA. Immunosuppression of syncytial virus (RSV) pre-existing serum antibodies: partial prvention by topical infection of the respiratory tract with vaccina virus-RSV recombinants. J General Virol. 1989;70:2185–90. doi: 10.1099/0022-1317-70-8-2185. [DOI] [PubMed] [Google Scholar]
- 16.Ueba O. Respiratory syncytial virus. 1. Concentration and purification of the infectious virus. Acta Med Okayama. 1978;32:265–72. [PubMed] [Google Scholar]
- 17.Olmsted RA, Elango N, Prince GA, et al. Expression of the F glycoprotein of respiratory syncytial virus by a recombinant vaccina virus: comparison of the individual contributions of the F and G glycoproteins to host immunity. Proc Natl Acad Sci USA. 1986;83:7462–6. doi: 10.1073/pnas.83.19.7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbas CF, Crowe JEJ, Jr, Cabana D, et al. Human monoclonal Fab fragments derived from a combinational library bind to respiratory syncytial virus F glycoprotein and neutralize infectivity. Proc Natl Acad Sci USA. 1992;89:10164–8. doi: 10.1073/pnas.89.21.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsutsumi H, Flanagan T, Ogra PL. Munoclonal antibodies to the large glycoproteins of respiratory syncytial virus: posible evidence for several functional antigenic sites. J Gen Virol. 1987;68:2161–9. doi: 10.1099/0022-1317-68-8-2161. [DOI] [PubMed] [Google Scholar]
- 20.Coates HV, Alling DW, Chanock RM. An antigenic analysis of respiratory syncytial virus isolates by plaque reduction neutralization test. Am J Epidemiol. 1965;83:299–313. doi: 10.1093/oxfordjournals.aje.a120586. [DOI] [PubMed] [Google Scholar]
- 21.Liew FY, Russell SM, Appleyard G, Brand CM, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotocxic T cell reactivity. Eur J Immunol. 1984;14:350–6. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 22.Mills J, Vankirk JE, Wright PF, Chanock RM. Experimental respiratory syncytial virus infection of adults. J Immunol. 1971;107:123–30. [PubMed] [Google Scholar]
- 23.McDermott MR, Bienenstock J. Evidence for a common mucosal immunologic system. 1. Migration of B immunoblasts into intestinal, respiratory, and genital tissues. J Immunol. 1979;122:1892–8. [PubMed] [Google Scholar]
- 24.Carrington PW, Roux ME, McWilliams M, Phillips-Quagliata JM, Lamm ME. Organ and isotype distribution of plasma cells producting specific antibody after oral immunization: evidence for a generalized secretory immune system. J Immunol. 1979;123:1705–8. [PubMed] [Google Scholar]
- 25.Russell MW, Mestecky J. Induction of the mucosal immune response. Rev Infect Dis. 1988;10:s440–s446. doi: 10.1093/cid/10.supplement_2.s440. [DOI] [PubMed] [Google Scholar]
- 26.Kanesaki T, Murphy BR, Collins PL, Ogra PL. Effectiveness of enteric immunization in the development of secretory immunoglobulin A response and the outcome of infection with respiratory syncytial virus. J Virol. 1991;65:657–63. doi: 10.1128/jvi.65.2.657-663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIntosh K, McQuillin J, Gardner PS. Cell-free and cell-bound antibody in nasal seretions from infants with respiratory syncytial virus infection. Infect Immune. 1979;23:276–81. doi: 10.1128/iai.23.2.276-281.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumagai T, Wong DT, Ogra PL. Development of cell-mediated cytotoxic activity in the respiratory tract after experimental infection with respiratory syncytial virus. Clin Exp Immunol. 1985;61:351–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Cannon MJ, Openshaw PJ, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–8. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497–502. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- 31.Graham BS, Henderson GS, Tang Y, Lu X, Neuzil KM, Colley DG. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–40. [PubMed] [Google Scholar]
- 32.Vujovic O, Mills J. Preventive and therapeatic strategies for respiratory syncytial virus infection. Curr Op Pharmcol. 2001;1:497–503. doi: 10.1016/s1471-4892(01)00087-x. [DOI] [PubMed] [Google Scholar]


