Abstract
An increased number of apoptotic bodies have been detected in glomeruli of non-nephritic kidneys of C1q-deficient mice. In these mice an in vivo impaired uptake of apoptotic cells by peritoneal macrophages was also demonstrated. Here we investigated whether C1q plays a role in the in vitro clearance of apoptotic cells by glomerular mesangial cells. Phagocytosis was assessed using a novel flow cytometric assay that was validated by immunofluorescence studies. The uptake of apoptotic cells by mesangial cells, measured as percentage of mesangial cells ingesting apoptotic cells, was ∼25%, 10% and 10% for a T cell lymphoma line (RMA), thymocytes and neutrophils, respectively. The uptake reached a plateau phase after 3 h, was specific for apoptotic cells and was mediated by serum but not by complement components C1q or C3. The phagocytosis of apoptotic cells was significantly inhibited by Arg-Gly-Asp-Ser (RGDS), a peptide capable of blocking the interaction of thrombospondin with CD36 or the vitronectin receptor. Pretreatment of the mesangial cells with dexamethasone (200 nm) but not with LPS increased the uptake markedly. These findings indicate that murine mesangial cells are capable of taking up syngeneic apoptotic cells, although much less efficiently than professional phagocytic cells. They also show that serum proteins other than complement components mediate the removal of apoptotic cells by murine mesangial cells in vitro.
Keywords: apoptosis, complement, mesangial cell, mice
Introduction
Programmed cell death, or apoptosis, is an essential physiological process for the maintenance of normal tissue homeostasis and for the safe clearance of unwanted cells during resolution of inflammation. During the process of apoptosis cells undergo typical structural changes including membrane blebbing and condensation/fragmentation of the nucleus, which allow their specific recognition by professional phagocytic cells. Although several receptors by which professional phagocytes recognize and ingest apoptotic cells have been identified [1], the mechanisms that control the phagocytic capacity for the clearance of apoptotic cells by semiprofessional phagocytes are less well understood.
There is now growing evidence that the phagocyte type rather than the lineage of apoptotic cell determines which recognition mechanism is employed. Some receptors expressed on specific macrophage populations mediate professional, highly efficient phagocytosis of apoptotic cells. These include the vitronectin receptor (VnR) αvβ3 [2], CD36 [3], phosphatidylserine receptor [4], CD14 [5], the ATP-binding cassette transporter ABC1 [6], lectin-like receptors [7] and scavenger receptors [8,9]. In addition, a role for complement and the members of the collectin family has recently been reported [10–13]. Macrophages are clearly the key phagocytes responsible for the clearance of apoptotic cells without the elicitation of proinflammatory secretory responses. However, other cells with the potential for phagocytosis such as endothelial cells, fibroblasts, vascular smooth muscle cells, hepatocytes and glomerular mesangial cells may provide an important contribution to the local resolution of an inflammatory insult. There is evidence that these semiprofessional phagocytes may deploy different mechanisms [14–17]. In particular cultured human glomerular mesangial cells have been shown to utilize a novel CD36-independent mechanism involving the αvβ3 VnR receptor together with thrombospondin (TSP) [18].
Inherited deficiencies of the early components of the classical pathway of complement, particularly C1q and C4, strongly predispose to the development of systemic lupus erythematosus (SLE) [19]. Systemic lupus erythematosus is a multisystem autoimmune disease characterized by the production of a large array of autoantibodies. Interaction of these autoantibodies with their cognate antigens leads to widespread inflammatory injury, especially in the kidneys and skin. Autoantibodies in sera from SLE patients recognize antigens expressed on the surface of apoptotic cells, implying that apoptotic cells may be the source of autoantigens in SLE [20]. In addition injection of apoptotic cells into mouse strains not normally susceptible to the development of SLE was shown to induce an autoantibody response [21]. C1q has been shown to bind in an antibody-independent manner to the surface blebs of apoptotic cells via its globular head [22,23]. This finding, together with the observations that C1q-deficient mice developed a lupus-like disease with proliferative glomerulonephritis and had an increased number of glomerular apoptotic bodies in the non-nephritic kidneys [24], led to the hypothesis that C1q may be implicated in the clearance of apoptotic cells and that deficiency of this activity may predispose to the development of SLE. The demonstration of an in vivo impaired phagocytic uptake of apoptotic cells by inflammatory macrophages in the C1q-deficient mice has provided further support for this hypothesis. In this context it is relevant to note that monocyte-derived macrophages from C1q-deficient humans cultured in autologous serum also exhibited impaired phagocytosis of apoptotic cells in vitro and that this defect was rectifiable with purified human C1q [12].
In this study we used cultured murine mesangial cells to characterize the recognition mechanisms employed by these cells in the phagocytosis of syngeneic apoptotic cells. We investigated whether complement components, particularly C1q or C3, were involved in the clearance of apoptotic cells by these nonprofessional phagocytes in vitro.
Materials and methods
Mice
C1q-deficient mice were generated as described previously [24] and were studied on the pure inbred 129/Sv genetic background or back-crossed onto C57BL/6 for 10 generations. C3-deficient mice [25] were kindly provided by Prof M. C. Carroll (Boston, USA). All of the animals were kept in a specific pathogen-free environment. Wild-type C57BL/6 and 129/Sv mice were purchased from Harlan Olac, UK. Serum was obtained from all of these mice by terminal bleeding under general anaesthesia. All animal work was conducted in accordance with institutional guidelines.
Mesangial cells
Primary mesangial cell cultures were established from glomeruli isolated, as reported previously [26]. Briefly, 10–12-week-old mice were killed by cervical dislocation and 10 ml of a magnetized iron oxide suspension (1% Fe3O4 in isotonic solution) was injected into the left ventricle of the heart. Kidneys were removed and kept on ice in PBS. The kidneys were minced by mechanical sieving and the glomeruli were isolated from the remaining renal tissue by placing the kidney extract on a Dynal magnet. The resulting preparation contained>95% glomeruli as judged by light microscopy. The recovered glomeruli were digested with collagenase (380 U/ml) (Sigma-Aldrich, Dorset, UK) for 30 min at 37°C and plated on fibronectin-coated 25 cm2 culture flasks. The cells were resuspended in DMEM containing glutamax-I (Gibco BRL, Life Technologies Laboratories, Paisley, UK) supplemented with 15% heat-inactivated fetal calf serum (FCS) (Gibco BRL), 100 U/ml penicillin, 100 µg/ml streptomycin, 1/100 insulin/selenium/transferrin growth supplement (Sigma-Aldrich, Dorset, UK) and 20 mm Hepes. Approximately 3 × 103 glomeruli were plated in each flask and cultured in humidified air with 5% CO2. After glomerular cells reached confluence (approximately 2 weeks), they were passaged regularly using standard methods of serial culture/trypsinization (5× trypsin) (Gibco BRL). Phenotypically stable cells between passages 10 and 20 were used for all in vitro studies. Mesangial cells were identified by their typical stellate morphology when subconfluent, while upon becoming confluent they adopted the well-recognized elongated conformation. To exclude contamination with other cell types, immunofluorescence studies were carried out using the following antibodies: a rabbit antimyosin (Sigma-Aldrich), a mouse monoclonal antipancytokeratin (Sigma-Aldrich) and a rat antimouse CD11b (M1-70) (Pharmingen-Becton Dickinson, San Diego, CA, USA). The primary antibodies were followed by the appropriate FITC-conjugated secondary antibodies.
Apoptotic cells
Three different populations of murine apoptotic cells were used in the phagocytic assays: RMA cells, an H-2b T cell lymphoma line, kindly provided by Prof E. Simpson (London, UK), thymocytes and neutrophils. All three types of cells were labelled with 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) (Molecular Probes, Leiden, the Netherlands) according to the manufacturer's protocol (5 µm per 107 cells) before induction of apoptosis or necrosis. Apoptosis was induced in the RMA cells by mitomycin C (50 µg/ml for 60 min at 37°C) (Sigma-Aldrich) followed by overnight culture in RPMI-1640 with 0·1% FCS. This resulted in a population of cells that was ∼80% apoptotic and>95% viable. Mouse thymocytes were obtained by mechanical dissociation of thymi from 3–5-week-old mice and were induced to undergo apoptosis by 3 h culture in RPMI-1640/0·4% BSA in the presence of 1 µm dexameth asone (Sigma-Aldrich). This resulted in a population of cells that was ∼55% apoptotic and>95% viable. Mouse neutrophils were obtained from peritoneal exudate cells of mice injected with 1 ml sterile 4% thioglycollate 12h prior to collection. Red blood cells were removed by hypotonic lysis, peritoneal cells were centrifuged at 700 r.p.m. at 24°C for 15 min, resuspended at a concentration of 106 cells/ml in sterile 0·25%BSA/HBSS and cultured for 4 h. This resulted in a population of cells that was ∼45% apoptotic and>95% viable. Apoptosis was confirmed by annexin V binding, propidium iodide staining (assessed by flow cytometry) and morphological changes including nuclear fragmentation and condensation, loss of cell volume and membrane blebbing (assessed on cytospin preparations). Cells were considered viable when they excluded propidium iodide and trypan blue. Necrotic cells were obtained by exposing the cells (107/ml in DMEM) to 3–5 cycles of rapid freezing/thawing until cell membrane integrity was lost. Staining with propidium iodide and trypan blue confirmed necrosis.
In vitro phagocytosis assays
Phagocytosis of apoptotic cells was assessed by flow cytometry and immunofluorescence. In all experiments cycling mesangial cells were used between passages 10–20. Mesangial cells (5 × 104/ml) were grown in DMEM supplemented with 15% FCS overnight on a fibronectin-coated 24-well plate. For immunofluorescence studies only, the mesangial cells were labelled with PKH26 according to the manufacturer's protocol (Sigma-Aldrich). The cells were washed three times with PBS before adding ∼1 × 106 CFSE-labelled apoptotic cells in DMEM with or without 20% mouse serum. The two cell types were co-cultured in 5% CO2 at 37°C for different time-points, as stated for each experiment, up to 24 h. The interaction was stopped by the addition of cold PBS and the non-adherent cells were removed by three washes with PBS. The mesangial cell monolayer was trypsinized (10X trypsin), and the cells were collected for the FACS analysis and/or the cytospin preparations. The effect of various soluble inhibitors was investigated by adding the reagent to the medium during the co-culture period. The reagents and the final concentrations used were the following: the tetrapeptide Arg-Gly-Asp-Ser (RGDS) (2 mm), the control peptide Arg-Gly-Glu-Ser (RGES) (2 mm), the water-soluble phosphatidylserine receptor inhibitor phospho-l-serine (1 mm) (Sigma-Aldrich) and RMV-7 (10 µg/ml), a monoclonal antibody antimouse αv of the VnR, kindly provided by Prof B. Imhof (Geneva, Switzerland). In some experiments, as stated in the text, the mesangial cells were activated by adding to the standard medium 200 nm of dexamethasone (Sigma-Aldrich) or different concentrations of LPS (from 0·1 µg/ml to 10 µg/ml) (Escherichia coli serotype 0111:B4) (Sigma-Aldrich) 24 h before the incubation with apoptotic cells.
Flow cytometric analysis
FACS analysis of phagocytic uptake was performed on unlabelled mesangial cells co-cultured with CFSE-labelled apoptotic cells on a FACSCalibur (Becton-Dickinson, Mountain View, CA, USA). Annexin V binding and propidium iodide stainings (Pharmingen-Becton Dickinson) were carried out according to the manufacturer's instructions.
Statistics
Results are expressed as mean ± s.e.m. Statistics were calculated using GraphPad PrismTM (version 2·0; GraphPad Software). Statistical significance (defined as P < 0·05) was evaluated by using Student's t-test.
Results
Characterization of apoptotic cell clearance by mouse mesangial cells in vitro
In order to establish whether murine mesangial cells were capable of phagocytosing apoptotic cells as described for human and rat cells [18,27,28], mesangial cells from two different strains of mice, 129/Sv and C57BL/6, were isolated. Pre-confluent mesangial cells, between passages 10–20, were initially phenotyped by immunofluorescence using various surface markers as previously reported. The cells stained uniformly for myosin but were negative for the epithelial cell marker cytokeratin and the mouse macrophage marker CD11b (data not shown). In all experiments the mesangial cells were cultured on fibronectin-coated 24-well plates and incubated with apoptotic cells with or without 20% mouse serum. Initially mitomycin-treated RMA cells were used as source of apoptotic cells and the uptake was assessed in parallel by two methods, immunofluorescence and flow cytometry (Fig. 1). In the immunofluorescence study, the CFSE-labelled ingested RMA cells appeared as a granular pattern within the mesangial cell cytoplasm, while the uningested RMA cells were identified as bright green condensed cells (Fig. 1a). The PKH26-stained mesangial cells analysed with the FITC filter appeared as dull green cells (Fig. 1b). In contrast, in the FACS analysis uningested apoptotic RMA cells were identified by the combination of their green fluorescence (FL1) due to the CFSE labelling and their relatively smaller size (FSC) compared with the mesangial cell (Fig. 1c,e: R1). Mesangial cells that had ingested apoptotic labelled cells (Fig. 1e: R3) could be distinguished from normal mesangial cells, depicted in Fig. 1d,e: R2, by their fluorescence staining. Because the results obtained with these two methods showed a correlation of 85% ± 10 (n = 5) we decided to use the flow cytometric assay for all subsequent experiments. This latter method allowed us to analyse the uptake in a greater number of cells compared to the cytospin preparations.
Fig. 1.
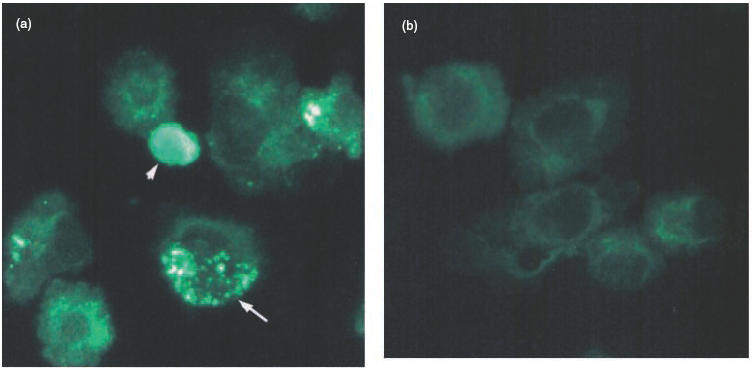
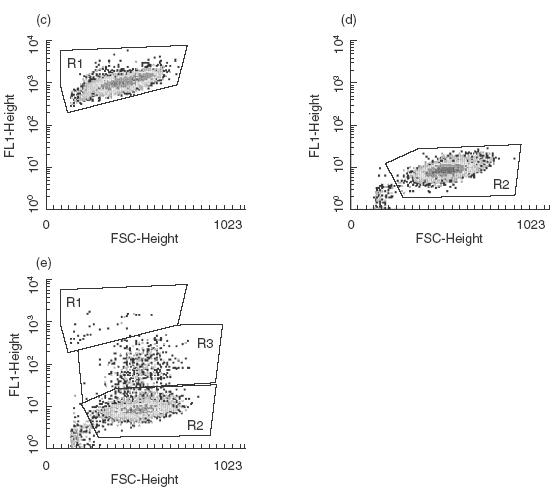
Phagocytosis of apoptotic RMA cells by murine mesangial cells in vitro. (a) Representative photomicrographs of cytospin preparations of PKH26-labelled mesangial cells incubated with CFSE-labelled apoptotic RMA cells for 3 h in the presence of mouse serum. Note that the ingested apoptotic RMA cells gave a green granular pattern within the mesangial cells (example arrowed), while the uningested RMA cells retained their condensed morphology and appeared merely to adhere to the mesangial cells (arrowhead). (b) PKH26-stained mesangial cells alone. (c,d) FACS profiles of CFSE-labelled apoptotic RMA and unlabelled mesangial cells, respectively. (e) FACS profile of unlabelled mesangial cells incubated with CFSE-labelled apoptotic RMA cells for three hours in presence of mouse serum. Three populations of cells can be defined according to their size and fluorescence: uningested apoptotic RMA cells (R1), ingested apoptotic RMA cells (R3) and mesangial cells alone (R2).
We initially established the kinetic of the uptake by wild-type mesangial cells in the presence of normal mouse serum. The clearance of apoptotic RMA cells by the mesangial cells isolated from both strains of mice, 129/Sv and C57BL/6, was similar and reached a plateau phase after three hours of co-culture (Fig. 2). Guided by these results, we selected the 3-h time-point for most of the subsequent experiments. Comparison of the phagocytic uptake of an equivalent number of mitomycin-treated RMA cells with viable and necrotic cells confirmed that the mesangial cells preferentially ingested apoptotic cells [percentage of mesangial cells ingesting mitomyicin-treated apoptotic RMA cells = 24·8% ± 1·6 (n = 4) compared with 5·9% ± 1 (n = 4) and 10·5% ± 1·5 (n = 4) with untreated and necrotic cells (mean ± s.e.m.), P = 0·013 and P = 0·0086 for apoptotic versus alive and necrotic cells, respectively].
Fig. 2.
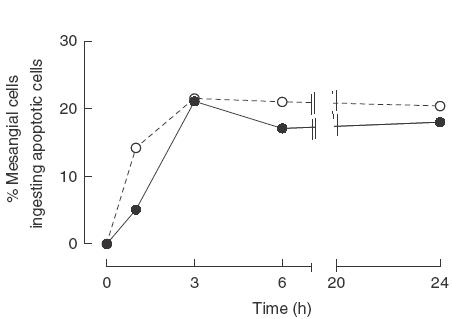
Kinetic of apoptotic RMA cell clearance. Graph showing the percentage of mesangial cells from 129/sv and C57BL/6 mice phagocytosing apoptotic RMA cells at different time-points. The kinetic of the uptake was similar with phagocytosis reaching a plateau phase after 3 h of co-culture. Data shown are representative of three independent experiments. •, C57BL/6; ▴, 129/Sv.
The kinetic of the clearance observed with the mitomycin-induced apoptotic RMA cells was common to the other apoptotic cell types that we studied. Dexamethasone-induced apoptotic thymocytes and starvation-induced apoptotic neutrophils were ingested in a similar way, suggesting that the lineage of the cells or the method used for inducing apoptosis was not determining the recognition mechanisms employed by the mesangial cells (Fig. 3). Although for each cell type analysed the phagocytic uptake peaked after 3 h, the uptake of apoptotic RMA cells was approximately twofold higher when compared with the uptake of apoptotic thymocytes and neutrophils, probably a reflection of the higher percentage of apoptosis present in the RMA preparation. Moreover, in keeping with previous findings, phagocytosis was observed only when the cells were kept at 37°C but not at 4°C (data not shown). Guided by these data, we selected to use the RMA cell line as source of apoptotic cells for all of the subsequent experiments. However apoptotic thymocytes and neutrophils were also used to validate the findings obtained with the RMA cells.
Fig. 3.
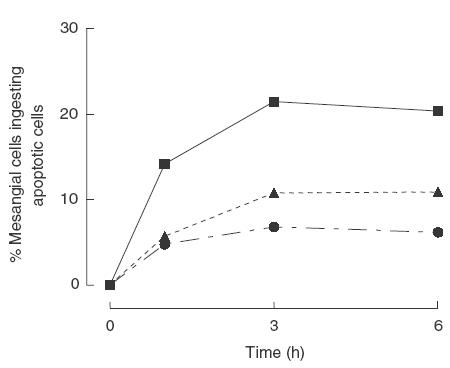
Phagocytic uptake of murine apoptotic cells of different origin. Graph showing the percentage of mesangial cells phagocytosing apoptotic RMA cells (∼80% apoptotic) or neutrophils (∼55% apoptotic) or thymocytes (∼45% apoptotic) at different time-points. Plateau was reached after 3 h of co-culture irrespective of the origin of the cells. The higher uptake observed using the RMA cells paralleled the higher percentage of apoptosis present in the RMA cell preparations. Data shown are representative of three independent experiments., ▪, Mitomycin-induced apoptotic RMA; ▴, starvation-induced apoptotic neutrophils; •, dexamethazone-induced apoptotic thymocytes.
Our study was carried out with mesangial cells that had been passaged at least 10 times to ensure homogeneity and loss of contaminating cells from the primary culture, but no more than 20 times. We confirmed that the cells remained homogeneous during the entire period in culture by repeated immunofluorescence staining for myosin (uniformly positive) and cytokeratin (negative). However, in both mouse strains we noticed that the percentage of uptake was passage-dependent. In the same cell line the phagocytic uptake ranged between 15% (passages 10–15) and 45% (passages 25–30). Despite the absence of noticeable changes in the immunofluorescence staining, subtle changes in the morphology were observed. While early passage cultures displayed morphology of elongated spindle-shaped cells, later cultures (over 20 passages) became less elongated. For this reason all comparisons between different mesangial cell lines were carried out with cells at similar passages (between passages 10 and 20).
Uptake of apoptotic cells was serum but not complement dependent
Recently the early components of the classical pathway of the complement system, in particular C1q, have been shown to be involved in the clearance of apoptotic cells by macrophages in vivo [12] and in vitro [10,13]. The uptake assays were carried out with sera deficient in C1q or C3 to determine whether the complement system also played an important role in the apoptotic cell clearance by non-professional phagocytic cells, such as the mesangial cells. As shown in Fig. 4, at 3 h time-point a marked reduction in the uptake of apoptotic cells was observed in the absence of serum (5·4 ± 2·6 and 7·1 ± 2·5 for wild-type and C1q-deficient mesangial cells, respectively, P < 0·0001 versus control standardized to 100%). However, the absence of C1q or C3 in the serum did not inhibit the phagocytosis by mesangial cells. The same findings were obtained irrespective of the source of apoptotic cells and the time-point analysed (data not shown). Mesangial cells are known to be capable of producing complement components [29]. Therefore, to avoid the confounding effect of an endogenous production of C1q, the experiments were performed with mesangial cells isolated from C1q-deficient mice on the same genetic background as the wild-type cells (129/Sv or C57BL/6). In the presence of wild-type serum the C1q-deficient mesangial cells showed no defects in the phagocytic uptake compared with wild-type cells, suggesting that these cells had no intrinsic phagocytic defects. In addition serum lacking C1q or C3 (Fig. 4) or different ratios of apoptotic cells to mesangial cells (from 50 : 1 to 5 : 1) had no detectable effects on the role played by complement in the uptake (data not shown).
Fig. 4.
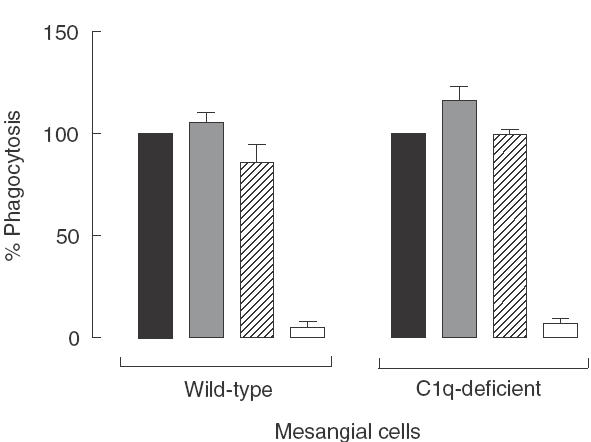
Phagocytosis of apoptotic RMA cells by mesangial cells from wild-type (n = 4) and C1q-deficient mice (n = 4). Graph showing the percentage of phagocytosis after 3 h of co-culture in the presence of 20% serum from wild-type animals or C1q- or C3-deficient mice and in the absence of serum. The results are expressed as a mean percentage of the phagocytic signal in the well containing wild-type serum for each experiment ± s.e.m. The control well was standardized to 100%. No inhibition in phagocytosis was detected using complement-deficient sera, while the absence of serum reduced the uptake to about 5–7% of the control. No difference of phagocytic uptake was observed between mesangial cells isolated from C1q-deficient or wild-type mice. ▪, Wild-type serum;  , C1q-deficient serum;
, C1q-deficient serum;  , C3-deficient serum; □, no serum.
, C3-deficient serum; □, no serum.
Recognition mechanisms employed by the murine mesangial cells
Because complement did not appear to mediate the phagocytosis of apoptotic cells by mesangial cells in our experimental systems, we went on to ask whether the murine mesangial cells employed similar recognition mechanisms to those described in human cells. In keeping with previous observations [18], the RGDS tetrapeptide, which is able to block the CD36/αvβ3 VnR-dependent recognition pathway, but not the control peptide RGES, at 2 mm exerted the strongest inhibitory effect upon the phagocytosis of apoptotic RMA cells [75·4% ± 4·5 inhibition (n = 4); P < 0·0001 versus control value standardized to 100%]. Moreover, the phosphatidylserine receptor inhibitor phospho-l-serine (1 mm) and the RMV-7 antibody (10 µg/ml) caused a partial reduction in the uptake [19·3% ± 5 and 21·2% ± 6·8 inhibition (n = 4), respectively; P < 0·05 versus control value standardized to 100%] (Fig. 5).
Fig. 5.

The effect of RGDS and RGES tetrapeptides, phospho-l-serine and RMV-7 upon mesangial cell phagocytosis of apoptotic RMA cells at the 3-h time-point. The higher inhibition of phagocytosis was seen following the addition of 2 mm RGDS in the mesangial cell/apoptotic RMA interaction medium while the control tetrapeptide RGES had no effect. The results are expressed as a mean percentage of the phagocytic signal in the well containing wild-type serum for each experiment ± s.e.m. The control well was standardized to 100%. Data shown are the result of four independent experiments. *P < 0·05; **P < 0·0001.
Effects of mesangial cell activation on apoptotic cell clearance
Inflammatory mediators have been shown to be able to modulate the ability of mesangial cells to respond to injury. In addition, glucocorticoids have been reported to increase the phagocytosis of apoptotic bodies by human glomerular mesangial cells in a concentration-dependent manner [30]. Therefore we sought to assess the effects of LPS or dexamethasone on the phagocytic uptake by murine mesangial cells. A 24-h pretreatment with different amounts of LPS ranging from 0·1 µg/ml to 10 µg/ml had no detectable effects on the mesangial cell phagocytosis of apoptotic cells assessed at the 3-h time-point (data not shown). Similar results were observed if LPS was added to the culture medium only during the phagocytic assays. In contrast, pretreatment of the mesangial cells with 200 nm of dexamethasone for 24 h induced an increase in the percentage of phagocytosis (253·8% ± 66·6 versus control standardized to 100%) (Fig. 6). The failure of dexamethasone pretreatment to potentiate the phagocytosis of viable or necrotic RMA cells confirmed that the observed augmentation in the phagocytic uptake was specific for the apoptotic cells (data not shown). A similar dexamethasone-induced increase in the mesangial cell uptake was observed using apoptotic neutrophils or thymocytes. This effect, irrespective of the lineage of the apoptotic cells used, was serum-dependent but complement-independent (data not shown). Furthermore, in keeping with previous observations in nontreated cells, RGDS (and not RGES) markedly inhibited the uptake by dexamethasone pretreated mesangial cells to about 70% (Fig. 6). However, differently to the findings with non-activated mesangial cells, the monoclonal antibody to αv (RMV-7) and the phosphatidylserine receptor inhibitor phospho-l-serine (1 mm) had no effect on the phagocytic uptake (Fig. 6).
Fig. 6.
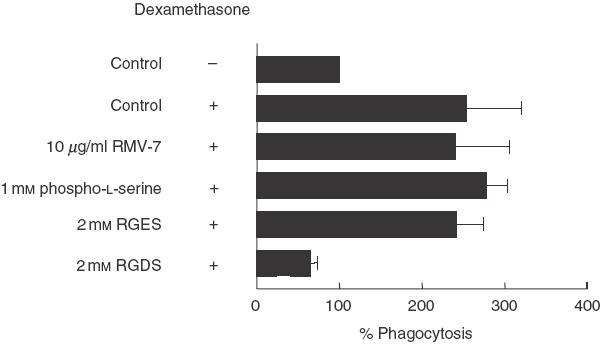
The effect of RGDS and RGES tetrapeptides, phospho-l-serine and RMV-7 upon dexamethasone-treated mesangial cell phagocytosis of apoptotic RMA cells at the 3-h time-point. Inhibition of phagocytosis was seen only following the inclusion of 2 mm RGDS in the mesangial cell/apoptotic RMA interaction medium. Note that addition of dexamethasone to wild-type serum induced a significant increase in the phagocytic uptake to about 250% of the control. The results are expressed as a mean percentage of the phagocytic signal in the well containing wild-type serum for each experiment ± s.e.m. The control well was standardized to 100%. Data shown are the result of three independent experiments.
Discussion
In this paper we have used a novel uptake assay to demonstrate for the first time that cultured mouse glomerular mesangial cells employ serum-mediated mechanisms to specifically recognize and engulf syngeneic apoptotic cells. However, our findings indicated also that components of the complement system, particularly C1q and C3, were not essential for apoptotic cell clearance under the conditions employed in these in vitro assays.
The phagocytic recognition of cells dying by apoptosis is essential for the safe resolution of inflammatory insults to the kidney. Although recent data suggested that a failure in the rapid clearance of apoptotic leucocytes may lead to persistent inflammation resulting in scarring and renal failure [31], the cells implicated in this process and the mechanisms that govern glomerular inflammation resolution are still poorly characterized. Apoptotic cells are recognized, ingested and degraded in a non-phlogistic pathway mainly by macrophages, the professional phagocytic cells [32]. However, there is now compelling evidence from in vivo and in vitro studies that myofibroblast-like mesangial cells from human and rat can contribute to the phagocytic clearance of both apoptotic leucocytes [18,27,28] and apoptotic resident glomerular cells [33]. In this paper we have provided further support to these observations demonstrating that murine mesangial cells also have similar capacity to recognize and ingest syngeneic apoptotic cells. In addition using three different syngeneic apoptotic cell lines we have confirmed the observations present in the literature that uptake/recognition mechanisms controlling the engulfment of apoptotic cells by semiprofessional phagocytes are determined by the type of phagocytic cells and not by the nature of the apoptotic cells. To assess the phagocytic uptake we initially established a novel flow cytometric assay that allowed us to analyse more efficiently the uptake in a greater number of cells compared to the cytospin preparations. Using this method we found that, although the murine mesangial cells, as shown previously with cells obtained from other species [18,27,28], could take up apoptotic cells, they were not efficient. The highest percentage of mesangial cells ingesting apoptotic cells that we observed after 3 h of co-culture was ∼25%. However, the mesangial cells did preferentially ingest apoptotic cells irrespective of the lineage of the apoptotic cells used. Notably the apoptotic RMA cells were ingested by a higher proportion of mesangial cells when compared with apoptotic thymocytes or neutrophils. The explanation for this difference is unlikely to be the nature of the cells as the RMA cells and thymocytes belong to the same lineage. On the other hand, the percentage of apoptosis present in the RMA cell preparations was higher (∼80% in the RMA cell preparation versus ∼55% and 45% in apoptotic thymocytes or neutrophils, respectively) and this may have resulted in an increased uptake by the mesangial cells.
Mesangial cells are generally considered to be nonprofessional phagocytes; however, we observed a progressive increase in the uptake with the number of passages in culture. Although the lack of staining with the mouse macrophage marker CD11b ruled out the possibility of a contamination by bone marrow-derived monocyte macrophages, it is possible that the different capacity to ingest apoptotic cells reflected a change in the properties of the mesangial cell consequent upon culture. In agreement with this explanation, despite the failure to detect variations in the immunofluorescence staining, a subtle change of morphology was observed in the late passages. While early passage cultures displayed morphology of elongated spindle-shaped cells, later cultures (>20) became less elongated. Therefore our observations would indicate that cultured cells may acquire new phenotypic characteristic and properties and may no longer be representative of the cell type of origin.
There is an increasing body of evidence in vitro and in vivo indicating that the early components of the classical pathway of the complement system and in particular C1q are involved in the clearance of apoptotic cells by professional phagocytes and this may prevent the development of an autoimmune disease [12]. In addition, the histological finding in the C1q-deficient mice of an increased number of glomerular apoptotic bodies in the non-nephritic kidneys [24] suggested a potential role for C1q in the clearance of apoptotic cells by non-professional phagocytes such as the mesangial cells. We tested this hypothesis in vitro by comparing the mesangial cell phagocytosis in the presence of mouse serum with or without C1q or C3. At all time-points analysed the complement proteins (C1q and C3) did not appear to contribute to the uptake. Notably, the apoptotic cells were prepared in the absence of serum to avoid the possibility of exogenous complement components binding to the apoptotic cells during the incubation in vitro. In addition, there was no detectable difference in the phagocytic uptake between normal and C1q- or C3-deficient serum irrespective of the nature of apoptotic cells or of the ratio of apoptotic cells to phagocytic cells used. Because mesangial cells have been shown to be capable of producing complement proteins [29], as in previous studies with macrophages [12], we avoided the possibility of C1q being present on cell surface due to local synthesis by using mesangial cells obtained from C1q-deficient animals. Notably the C1q-deficient mesangial cells showed similar phagocytic capacity to the wild-type cells ruling out the possibility of an intrinsic phagocytic defect in these cells. Therefore our findings in vitro indicate that the complement system is not involved in the clearance of apoptotic cells by these non-professional phagocytic cells. Consistent with these data is the recent observation that C1q does not appear to play a critical role in the recognition and removal of sunburn cells by keratinocytes in the skin [34]. Nevertheless, several lines of evidence support a role for C1q in the clearance of apoptotic cells by macrophages [10,12,13]. Therefore our data demonstrate that the contribution of the complement system to the uptake appears to vary according to the nature of the phagocytic cells; this is in agreement with the data in the literature indicating the existence of specific recognition mechanisms employed by different cells [1,35].
Despite the lack of a complement-mediated effect, the phagocytic uptake by the mesangial cells required the presence of serum. Thus we investigated whether the pathways operating in the murine mesangial cells were akin to those described for human cells. Consistent with a previous study [18] we found that, under our culture conditions, mesangial cell phagocytosis of apoptotic cells involved the TSP/αvβ3 VnR-mediated mechanism. However, in contrast to the observations with human cells [18,27], our study showed that the phosphatidylserine pathway may also contribute to the uptake. Notably, in our studies the maximum inhibition of phagocytosis obtained with RGDS was to about 75% of control suggesting that multiple mechanisms might operate in determining the phagocytic capacity of the mesangial cells.
The efficient removal of apoptotic cells is thought to play an important role in promoting the resolution of an inflammatory insult and local inflammatory stimuli may modulate this clearance. In a previous study glucocorticoids have been shown to have potentiating effects on the apoptotic cell clearance by human mesangial cells [30]. In agreement with this study we found that pretreatment with dexamethasone had a specific promoting effect on the uptake of apoptotic cells (approximately twofold increase). However, the addition of LPS, known to have multiple activation effects on mesangial cells in vitro, at different concentrations did not modulate the phagocytic capacity of the cells. Furthermore, in keeping with the observations obtained with resting mesangial cells, complement did not contribute to the uptake under either condition. In addition, in dexamethasone-treated mesangial cells only RGDS exerted an inhibitor effect (∼70%), while phospho-l-serine and the antiαv antibody had no effects. These findings, taken together, would indicate that the recognition mechanisms operating in mesangial cells might contribute differently to the phagocytic uptake under inflammatory conditions. Further studies will be required to elucidate these mechanisms in vivo.
In conclusion, our findings suggest that C1q does not play a critical role in the removal of apoptotic cells by murine mesangial cells in vitro, unlike its role in the clearance of apoptotic cells by the macrophages.
Acknowledgments
This work was supported by Wellcome Trust grant 054838. JCH was a recipient of a fellowship from the National Institute of Health, Spain (BEFI 99/9212). We thank all of the staff in the animal facility for their technical assistance.
REFERENCES
- 1.Fadok VA, Bratton DL, Henson PM. Phagocyte receptors for apoptotic cells: recognition, uptake and consequences. J Clin Invest. 2001;108:957–62. doi: 10.1172/JCI14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savill J, Dransfield I, Hogg N, et al. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–3. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 3.Ren Y, Silverstein RL, Allen J, et al. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J Exp Med. 1995;181:1857–62. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadok VA, Bratton DL, Rose DM, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405:85–90. doi: 10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- 5.Devitt A, Moffatt OD, Raykundalia C, et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–9. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- 6.Luciani MF, Chimini G. The ATP binding cassette transporter ABC1, is required for the engulfment of corpses generated by apoptotic cell death. Embo J. 1996;15:226–35. [PMC free article] [PubMed] [Google Scholar]
- 7.Duvall E, Wyllie AH, Morris RG. Macrophage recognition of cells undergoing programmed cell death (apoptosis) Immunology. 1985;56:351–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Platt N, Suzuki H, Kurihara Y, et al. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci USA. 1996;93:12456–60. doi: 10.1073/pnas.93.22.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukasawa M, Adachi H, Hirota K, et al. SRB1, a class B scavenger receptor, recognizes both negatively charged liposomes and apoptotic cells. Exp Cell Res. 1996;222:246–50. doi: 10.1006/excr.1996.0030. [DOI] [PubMed] [Google Scholar]
- 10.Mevorach D, Mascarenhas JO, Gershov D, et al. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313–20. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schagat TL, Wofford JA, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol. 2001;166:2727–33. doi: 10.4049/jimmunol.166.4.2727. [DOI] [PubMed] [Google Scholar]
- 12.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogden CA, deCathelineau A, Hoffmann PR, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and cd91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med. 2001;194:781–96. doi: 10.1084/jem.194.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dini L, Autuori F, Lentini A, et al. The clearance of apoptotic cells in the liver is mediated by the asialoglycoprotein receptor. FEBS Lett. 1992;296:174–8. doi: 10.1016/0014-5793(92)80373-o. [DOI] [PubMed] [Google Scholar]
- 15.Dini L, Lentini A, Diez GD, et al. Phagocytosis of apoptotic bodies by liver endothelial cells. J Cell Sci. 1995;108:967–73. doi: 10.1242/jcs.108.3.967. [DOI] [PubMed] [Google Scholar]
- 16.Bennett MR, Gibson DF, Schwartz SM, et al. Binding and phagocytosis of apoptotic vascular smooth muscle cells is mediated in part by exposure of phosphatidylserine. Circ Res. 1995;77:1136–42. doi: 10.1161/01.res.77.6.1136. [DOI] [PubMed] [Google Scholar]
- 17.Hall SE, Savill JS, Henson PM, et al. Apoptotic neutrophils are phagocytosed by fibroblasts with participation of the fibroblast vitronectin receptor and involvement of a mannose/fucose-specific lectin. J Immunol. 1994;153:3218–27. [PubMed] [Google Scholar]
- 18.Hughes J, Liu Y, Van Damme J, et al. Human glomerular mesangial cell phagocytosis of apoptotic neutrophils: mediation by a novel CD36-independent vitronectin receptor/thrombospondin recognition mechanism that is uncoupled from chemokine secretion. J Immunol. 1997;158:4389–97. [PubMed] [Google Scholar]
- 19.Pickering MC, Botto M, Taylor PR, et al. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 20.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mevorach D, Zhou JL, Song X, et al. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J Exp Med. 1998;188:387–92. doi: 10.1084/jem.188.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korb LC, Ahearn JM. C1q binds directly and specifically to surface blebs of apoptotic human keratinocytes: complement deficiency and systemic lupus erythematosus revisited. J Immunol. 1997;158:4525–8. [PubMed] [Google Scholar]
- 23.Navratil JS, Watkins SC, Wisnieski JJ, et al. The globular heads of C1q specifically recognize surface blebs of apoptotic vascular endothelial cells. J Immunol. 2001;166:3231–9. doi: 10.4049/jimmunol.166.5.3231. [DOI] [PubMed] [Google Scholar]
- 24.Botto M, Dell’Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 25.Wessels MR, Butko P, Ma M, et al. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA. 1995;92:11490–4. doi: 10.1073/pnas.92.25.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattell V, Cook HT, Ebrahim H, et al. Anti-GBM glomerulonephritis in mice lacking nitric oxide synthase type 2. Kidney Int. 1998;53:932–6. doi: 10.1111/j.1523-1755.1998.00892.x. [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich S, Sato T, Schmidt M, et al. Induction of mesangial interleukin-6 synthesis by apoptotic U937 cells and monocytes. Kidney Int. 1997;52:318–28. doi: 10.1038/ki.1997.337. [DOI] [PubMed] [Google Scholar]
- 28.Savill J, Smith J, Sarraf C, et al. Glomerular mesangial cells and inflammatory macrophages ingest neutrophils undergoing apoptosis. Kidney Int. 1992;42:924–36. doi: 10.1038/ki.1992.369. [DOI] [PubMed] [Google Scholar]
- 29.Zhou W, Marsh JE, Sacks SH. Intrarenal synthesis of complement. Kidney Int. 2001;59:1227–35. doi: 10.1046/j.1523-1755.2001.0590041227.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Cousin JM, Hughes J, et al. Glucocorticoids promote non-phlogistic phagocytosis of apoptotic leukocytes. J Immunol. 1999;162:3639–46. [PubMed] [Google Scholar]
- 31.Savill J, Mooney A, Hughes J. Apoptosis and renal scarring. Kidney Int Suppl. 1996;54:S14–7. [PubMed] [Google Scholar]
- 32.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784–8. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 33.Baker AJ, Mooney A, Hughes J, et al. Mesangial cell apoptosis: the major mechanism for resolution glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest. 1994;94:2105–16. doi: 10.1172/JCI117565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering MC, Fischer S, Lewis MR, et al. Ultraviolet-radiation-induced keratinocyte apoptosis in C1q-deficient mice. J Invest Dermatol. 2001;117:52–8. doi: 10.1046/j.0022-202x.2001.01381.x. [DOI] [PubMed] [Google Scholar]
- 35.Fadok VA, Savill JS, Haslett C, et al. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J Immunol. 1992;149:4029–35. [PubMed] [Google Scholar]


