Abstract
Upon cultivation with interferon-γ (IFN-γ) and granulocyte/macrophage-colony stimulating factor (GM-CSF) polymorphonuclear neutrophils (PMN) acquire characteristics of dendritic cells, including expression of major histocompatibility complex (MHC) class II antigens, of the co-stimulatory antigens CD80, CD86 and of CD83, the latter considered to be specific for dendritic cells. Dendritic-like PMN were also able to present to T cells antigens in a MHC class II-restricted manner. To assess whether dendritic-like PMN are also generated in vivo, cells of patients with acute bacterial infections and of patients with chronic inflammatory diseases (primary vasculitis) were tested. During acute infection up to 80% of PMN acquired CD83, but remained negative for MHC class II, CD80 or CD86. PMN of patients with primary vasculitis expressed MHC class II antigens, CD80 and CD86, but not CD83, indicating that up-regulation of MHC class II and of CD83 are not necessarily linked to each other. Indeed, parallel studies with PMN of healthy donors showed that while IFN-γ and granulocyte/macrophage colony stimulating factor (GM-CSF) induced both, MHC class II and CD83, tumour necrosis factor (TNF)-α selectively induced de novo synthesis of CD83. The function of CD83 on PMN is still elusive. A participation in the MHC class II-restricted antigen presentation could be ruled out, consistent with the segregation of MHC class II and CD83 expression. Regardless, however, of its function, CD83 expression could serve as a marker to differentiate between acute and chronic inflammation.
Keywords: bacterial infection, CD83, MHC class II, PMN, TNF-α, vasculitis
Introduction
The image of polymorphonuclear neutrophils (PMN) has changed considerably during the recent years. Traditionally considered to be the first-line defence against bacterial infection it became increasingly clear that PMN also participate in chronic inflammation disease and regulation of the immune response when appropriately activated. Hallmarks of PMN activation in vivo are changes in surface-receptor expression, for example loss of CD16, up-regulation of CD64, CD14 [1–3] and the prolongation of their lifespan, as it is seen in patients with severe trauma or burns, sepsis, bacterial infections [4–6], or chronic inflammatory diseases [7].
Prolongation of lifespan is also achieved by cultivating PMN, for example with cytokines such as interferon-gamma (IFN-γ), granulocyte/macrophage colony stimulating factor (GM-CSF) or interleukin (IL) -3 [8–11] or, as shown more recently, with low doses of tumour necrosis factor alpha (TNF-α) [12]. Prolongation of lifespan depends on de novo protein synthesis and is linked to numerous phenotypical and functional changes [13–17], a process we refer to as transdifferentiation [18,19].
We focused on the transdifferentiation of PMN to dendritic-like cells. Following culture with IFN-γ or IFN-γ plus GM-CSF PMN de novo synthesized major histocompatibility complex (MHC) class II antigens, the co-stimulatory receptors CD80 and CD86 [17–19] and acquired the ability to present antigen in a MHC class II-restricted manner. Consistent with the transdifferentiation to antigen-presenting cells we and others have found more recently that PMN also produced CD83, a glycoprotein of 45 kDa, thought to be specific for dendritic cells [18,20].
In this study we tested whether CD83-positive PMN also occur in vivo. Because PMN of patients with Wegener's granulomatosis express MHC class II antigens, CD80 and CD86, antigens typical for antigen-presenting cells [7,19], they were the most obvious choice. We also included patients with severe systemic bacterial infections, and for comparison healthy donors as well. Unexpectedly, CD83 expression was found only during acute bacterial infection, but not in patients with Wegener's disease. Studies with cultivated PMN confirmed that MHC class II expression was not linked to that of CD83 and indicated that CD83 was not required for antigen presentation.
Patients, materials and methods
Patients and donors
After approval by the Ethikkommission (Ethics Committee) of the University of Heidelberg and after having obtained informed consent, patients with bacterial infections and patients with primary, ANCA-associated vasculitis (Wegener's granulomatosis) attending the renal unit of the Heidelberg University Hospital were included in a prospective study. Acute bacterial infections were diagnosed by X-ray, positive blood cultures, high levels of C-reactive peptide (CRP), high leucocyte counts, high erythrocyte sedimentation rate (ESR) or by measuring procalcitonin levels> 1 ng/ml. Twenty-six patients were included in the study. The patients presented with pneumonia (n = 8); peritonitis (n = 4); appendicitis (n = 2); pancreatitis (n = 1); cholangitis/–cystitis (n = 2); diverticulitis (n = 1); infection of soft tissue (n = 4) or infected implants (n = 5). Wegener's granulomatosis was diagnosed according to the definition of the Chapel Hill conference [21] and to the ACR criteria [22] as described elsewhere [7]. Disease activity was determined by the Birmingham Vasculitis Activity Score (BVAS) [23]. Patients with active disease (BVAS 2 or higher) were included in the study (n = 7), as were patients with inactive disease (n = 12). Also included in the study were 22 healthy donors. Blood was taken by venous puncture using 7·5 ml heparin-coated tubes (Sarstedt, Nümbrecht, Germany) and was analysed within 2 h.
Media and reagents
AIM-V was obtained from Gibco, Eggenstein (Germany). Recombinant human (rh)GM-CSF, rhTNF-α, rhIL-1, rhIL-2, rhIL-4, rhIL-5, rhIL-6 rhIL-8 and rhIL-10 were purchased from Sigma (St Louis, MO, USA); rhIFN-γ from Boehringer Mannheim (Mannheim, Germany); and NAP 2 from R&D Systems (Wiesbaden, Germany).
Antibodies
For cytofluorometry fluorescein isothiocyanate (FITC) and phycoerythrin (PE)-labelled murine MoAbs were used. CD66b-FITC and CD83 PE (clone HB15A) was obtained from IOTest (Marseilles, France). MHC class II, CD80 and CD86 were obtained from Immunotec (Marseilles, France), anti-CD83 PE (clone HB15E) was purchased from Research Diagnostics, Flanders, NJ, USA and isotypic mouse IgG1 FITC and IgG2a PE were used in the same final concentration (Immunotech, Marseilles, France).
Cytofluorometry
To avoid alteration of antigen expression, PMN from patients or donors were analysed in whole blood. Anti-CD66b-FITC was used as a PMN marker for PMN. The respective PE-labelled antibody were titrated before use. The final concentrations varied between 1 and 20 µg/ml. Erythrocytes were lysed using fluorescence-activated cell sorter (FACS) lysing solution as recommended by the supplier (Becton Dickinson, Heidelberg, Germany). Cells were analysed by FACSCalibur® and CellQuest® software (Becton Dickinson). Results are expressed as percentage of positive cells in the respective gate or quadrant as well as mean fluorescence. Isotype controls were used in all experiments.
Differences between the groups: (1) patients with bacterial infections; (2) patients with Wegener's granulomatosis and active disease; (3) patients with Wegener's granulomatosis and inactive disease; and (4) healthy donors in antigen expression of CD83 were calculated using a non-parametric t-test.
When analysing isolated PMN 106 cells were used; 100 µl of whole blood was incubated for 20 min with the respective antibodies in the presence of 1% BSA to reduce non-specific binding.
Purification of PMN
PMN were separated from heparinized blood of healthy donors by PolymorphPrep® (Nycomed, Oslo, Norway). Remaining red cells were lysed by two hypotonic/hypertonic lysis steps with 0·2%/1·6% saline. The purity of the isolation was measured by FACScan with a MoAb against CD66b as a specific marker for PMN. Usually up to 98–99% cells were positive for CD66b. In addition cells were Giemsa-stained and 300 cells were counted. Normally 0·5–1·5% of cells were identified as not being PMN. These cells were mainly CD3-positive cells; CD83- or CD14-positive cells were not detectable. Viability of PMN was determined by exclusion of propidium iodide. For the antigen-presentation test, PMN were purified further by adsorption to CD15 as described in [19], using the devices of the supplier (MiltenyBiotech, Bergisch-Gladbach, Germany).
Cultivation of PMN
Purified PMN (1 × 106/ml) were cultivated in AIM V with 2·5% autologous serum (heat inactivated at 56°C for 30 min, normal human serum (NHS)). Cytokine concentrations for PMN cul-ture were: GM-CSF 50 U/ml, IFN-γ 100 U/ml, IL-1 2 ng/ml, IL-2 30 U/ml, IL-4 3 ng/ml, IL-5 5 ng/ml, IL-6 1 ng/ml, IL-8 10 ng/ml, IL-10 1 ng/ml, NAP 2 0·5 µg/ml and TNF-α 5 ng/ml. Cells were incubated for 2 days at 37°C with 5% CO2 in air. Viability of PMN was determined by propidium iodide staining. Only experiments with survival greater than 85% were utilized.
Immunoprecipitation of CD83
PMN membranes were prepared by two repeated freeze- and thawing cycles followed by centrifugation for 30 min at 10 000 g. The membrane proteins were extracted using 1% CHAPS in phosphate-buffered saline containing 0·01 m EDTA, 1 mm PMSF. To 500 µl extract obtained from 106 PMN 50 µl of packed protein A sepharose beads (Pharmacia) was given. After 60 min at room temperature with constant rotation, the beads were removed by centrifugation. Twenty µl of anti-CD83 was added, and after 30 min at room temperature 50 µl protein A sepharose beads. After 16 h rotation at 4°C the beads were harvested, washed in PBS and boiled with 100 µl SDS-PAGE buffer. The supernatants were subjected to SDS-PAGE (7%) under reducing conditions and the gel was silver-stained.
Differentiation of monocytes in vitro
Monocytes were isolated from a buffy coat obtained from a healthy donor by centrifugation on Ficoll followed by positive selection of the cells using CD14 beads (MiltenyBiotech, Bergisch-Gladbach, Germany). The cells were then cultivated with IL-4 and TNF-α as described by Zhou and Tedder [24] to acquire CD83 and other characteristics of dendritic cells.
T cell line
A T cell line specific for tetanus toxoid (TT) was established and propagated as described previously [17].
Antigen presentation
Essentially the method described by Radsak et al. [17] was used. In brief, purified PMN (105) were placed into a 96-well plate and incubated at 37°C with 5% C02 atmosphere in either AIM V/2·5% NHS alone or combined with IFN-γ/G-CSF or additional TNF-α. Cells were washed once in RPMI and then T cells (105) and TT (0·2 µg/well) were added. After incubation for 4 days cells were pulsed by adding 1 µCi of [3H]-thymidine (Amersham, Life Science, Braunschweig, Germany) to each well and harvested 14–16 h later. [3H]-thymidine incorporation into DNA was measured with a scintillation counter (Packard, Groningen, the Netherlands) and expressed as counts per minute (cpm).
For inhibition experiments, unlabelled antibodies (2 µg/ml) to MHC class II, CD83 or IgG were added to the cells.
RNA isolation and RT-PCR
Total RNA was isolated from 1 × 107 PMN after 24 h of culture with the respective cytokines. RNA isolation was performed using a RNAeasy kit by Quiagen (Hilden, Germany) following the instructions of the manufacturer exactly. RNA was transcribed using a Gibco RT Superscript II kit (Gibco Life Technologies, Karlsruhe, Germany). To detect CD83 the following primers, yielding a 445-bp PCR product, were used: sense: 5′-GCC ATG TCG CGC GGC CTC CAG CTT-3′; antisense: 5′-GGA CAA TCT CCG CTC TGT ATT TC-3′. The primers were synthesized by ARK Scientific Biosystems (Darmstadt, Germany). PCR was performed in a Hybaid PCR express thermocycler as follows: 2 min at 95°C once; 30 min at 95°C; 30 min at 66°C; 30 min at 72°C 10 times; 30 min at 95°C; 30 min at 56°C; 45 min at 72°C 25 times and finally 7 min at 72°C. To 2 µg of RT-product 1 U of Taq-DNA polymerase (Boehringer), 5 µl 10× buffer with Mg2+, 25 mm of sense- and antisense-primer, 10 mm of dNTPs (Gibco) was given and filled with DEPC water (final volume 50 µl). The resulting PCR product was separated by agarose gel electrophorese, yielding the anticipated length of 445 bp. Sequencing confirmed identify with CD83 (Medigenomix, Planegg Martinsried, Germany). For quantitative PCR a primer for β-actin, kindly provided by Dr Giese (Institute for Immunology, Heidelberg, Germany), was used with essentially the same conditions as above, yielding a 450-bp PCR product. The RT products were separated by a 1·5% agarose gel, stained with SybrGreen (Molecular Probes, Leiden, Netherlands) and analysed by FLA2000 (Fuji, Japan) using Image Gauge V3·0 (Fuji) as software. We used a Boehringer DNA Marker VI (range 154–2176 bp) at a concentration of 30 µg/ml.
Determination of TNF-α in plasma
Patients’ plasma was stored at − 20°C; for determination of TNF-α commercially available ELISA (Quantikine, R&D Systems, Wiesbaden, Germany) was used. Of the plasma 100 µl were used and for comparison plasma of six healthy donors.
Results
Expression of CD83 on PMN in vivo
As determined by cytofluorometry, PMN of healthy donors expressed little or no CD83 (range 0–8·7%). In 17 of 26 patients with bacterial infections CD83 expression was found, ranging from 5·5% to 80%, whereas patients with Wegener's granulomatosis did not express CD83 (range 0–3·3%) (an example is shown in Fig. 1a; data of all patients are summarized in Fig. 1b). From one patient, PMN were isolated and CD83 was immunoprecipated by anti-CD83. A band in the area of 45 kDa was found, in a position identical to proteins precipitated from in vitro activated PMN (see below) or from in vitro differentiated monocytes (Fig. 2). By RT-PCR using RNA from the highly purified PMN yielded a 445-bp product, identical in sequence to the monocyte-derived protein (Fig. 2).
Fig. 1.
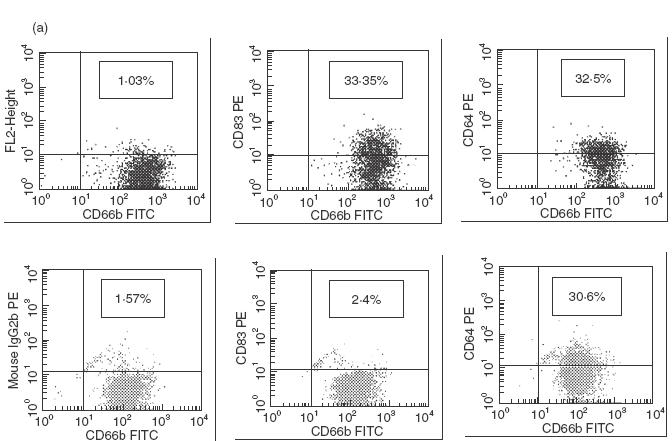
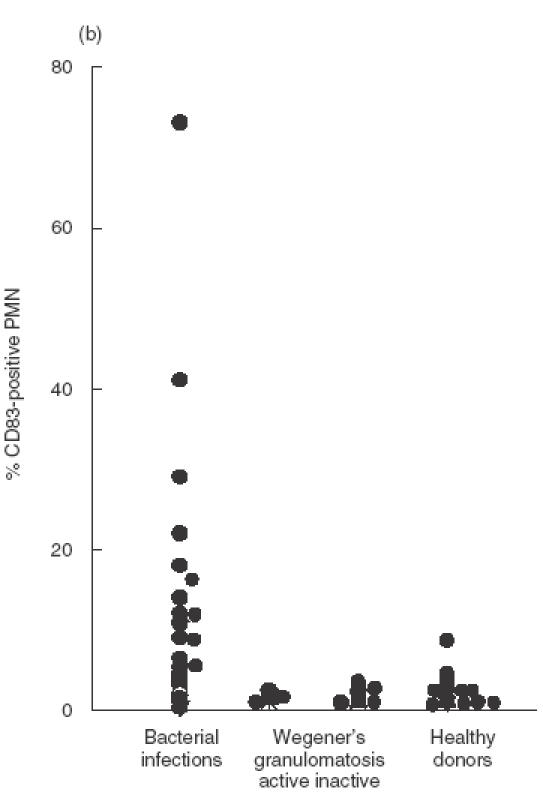
Expression of CD83 on PMN of patients with acute bacterial infection. (a) PMN in whole blood were examined by flow cytofluorometry; cells were labelled with CD66b-FITC as a specific marker for PMN and CD83 PE (clone HB15A) or the isotypic-negative control, respectively. The upper panel shows PMN of a patient with infection; here 33·4% of PMN are double-positive for CD66b/CD83 (right upper quadrant). The right panel shows CD64 expression as a ‘positive control’ for PMN activation. The lower panel shows cells of a patient with active Wegener's granulomatosis. (b) Expression of CD83 on PMN of patients with either bacterial infections, or Wegener's granulomatosis, or of healthy donors. Data are shown for patients with acute bacterial infections (n = 26), patients with Wegener's granulomatosis with active disease (BVAS > 2) (n = 7) or inactive disease (n = 12), and healthy donors (n = 22) and is expressed as percentage of CD83-positive PMN. Each dot represents one patient or donor, respectively, The values of the group ‘bacterial infection’ differed from all the others (largest P-value: 0·05).
Fig. 2.
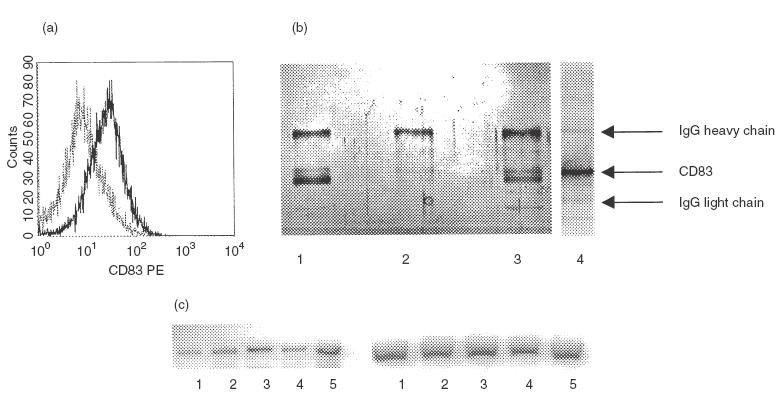
Identification of CD83 on PMN of a patient with acute bacterial infection: of PMN derived from a patient with staphylococcus-induced soft tissue infection, expressing CD83 (cytofluorometry using anti-CD83 clone clone HB15E, panel (a), membranes were prepared and CD83 was precipitated using anti-CD83 (clone HB15A). (b) Silver-stained gel; a band with an apparent molecular weight of 45 kDa (lane 4) is seen; for comparison, immunoprecipitates from in vitro differentiated monocytes are shown (lane 1), freshly isolated PMN (lane 2) or PMN stimulated with IFN-γ+ GM-CSF for 48 h (lane 3). (c) CD83-specific RT-PCR products with the expected size of 445 bp derived from RNA from: PMN cultivated for 8 h in AIM V/NHS (lane 1); IFN-γ (lane 2); TNF-α (lane 3), freshly isolated PMN (lane 4) and patient's PMN (lane 5). The right panel shows the respective amplification products of β-actin.
As described previously, the PMN of the patients with active Wegener's granulomatosis expressed MHC class II antigens (range: 6·8–24%); less than 2·5% MHC class II-positive PMN were found in patients with inactive disease, and less than 2% in patients with bacterial infections or in healthy donors.
Induction of CD83 and MHC class II in vitro: effect of various cytokines
CD83 and MHC class II are de novo synthesized in PMN when cultured with IFN-γ, GM-CSF or a combination thereof [17,18]. Because of the discrepancy of in vivo data numerous cytokines, including IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, NAP-2 and TNF-α, were tested for their capacity to induce CD83 and/or MHC class II expression, respectively. Under our culture conditions (Aim V containing 2·5% autologous heat-inactivated serum) in most of the experiments the majority of PMN survived for up to 48 h as measured by propidium iodide. When testing the effect of the cytokines, only experiments with a survival rate greater 85% were considered. None of these cytokines affected survival considerably or induced MHC class II expression. Only TNF-α, in concentrations ranging from 2 to 20 ng, was able to induce CD83 synthesis. TNF-α was more potent than IFN-γ and GM-CSF and also enhanced the effect of IFN-γ and GM-CSF (examples are shown in Figs 2c and 3; data of different experiments are summarized in Table 1).
Fig. 3.

Expression of CD83 on isolated PMN: highly purified PMN were cultivated for 48 h under different conditions. From left to right: AIM V containing 2·5% NHS (extreme left), 100 U/ml IFN-γ, 100 U/ml IFN-γ/ 50 U/ml GM-CSF or (extreme right) 2 ng/ml TNF-α.
Table 1.
Induction of CD83 expression on PMN
| % CD83-positive PMN* | |||
|---|---|---|---|
| PMN cultivated for 48 h with | Range | Mean ± s.d. | Individual donors |
| AIM/NHS 2·5% | 2·2–8·0 | 5·4 ± 2·1 | 6 |
| Plus | |||
| IL-1 (2 ng/ml)** | 4·3–13·4 | 10·8 ± 4·4 | 4 |
| IL-2 (30 U/ml) | 2·5–17·7 | 10·3 ± 7·6 | 3 |
| IL-4 (3 ng/ml) | 1·4–16·6 | 7·5 ± 6·4 | 4 |
| IL-5 (5 ng/ml) | 2·05–7·0 | 3·8 ± 2·8 | 3 |
| IL-6 (1 ng/ml) | 1·7; 5·9 | 2 | |
| IL-8 (10 ng(ml) | 3·0–13·4 | 8·9 ± 5·3 | 4 |
| IL-10 (1 ng/ml) | 1·7; 2·0 | 2 | |
| NAP-2 (0·5 µg/ml) | 0·9–6·2 | 2·7 ± 1·7 | 7 |
| IFN-γ (100 U/ml) | 2·0–34·6 | 9·8 ± 11·2 | 7 |
| GM-CSF (50 U/ml) | 2·0–41·6 | 20·7 ± 19·9 | 3 |
| TNF-α (5 ng(ml) | 25–65·8 | 30·3 ± 18·6 | 6 |
| IFN-γ+ GM-CSF + TNF-α | 22·4–74·2 | 46·9 ± 19·3 | 8 |
Only experiments with survival >85% are included
cytokine doses were used which stimulated other functions of PMN.
Presence of TNF-α in the plasma of patients with bacterial infections
In 13 of 21 patients with bacterial infections TNF-α was found in the peripheral blood (range 5–750 pg/ml), but not in plasma of the healthy donors. The TNF-α levels did not correlate linearly with CD83 expression (Fig. 4a); however, CD83 expression coincided with plasma levels of TNF-α of 10 pg/ml or more (Fig. 4b). In patients with Wegener's granulomatosis there was no evidence for TNF-α induction, neither during active disease nor during remission (data not shown).
Fig. 4.
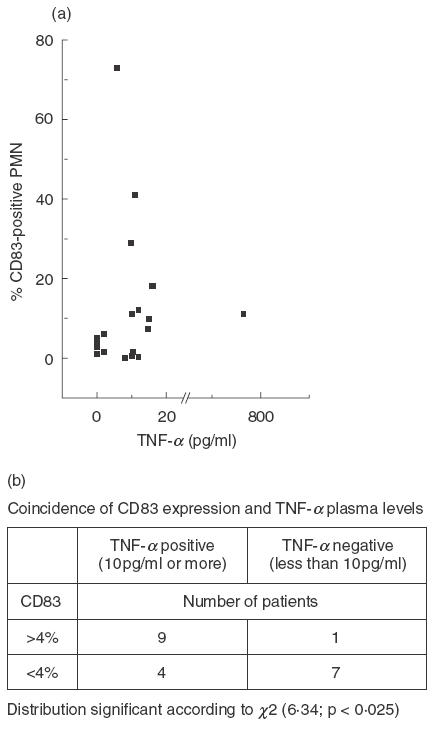
Coincidence of expression of CD83 on PMN and presence of TNF-α in the plasma: of 25 patients, CD83 expression on PMN was determined by cytofluorometry; in parallel TNF-α levels in the plasma were determined. The scattergram (a) shows that there is no linear correlation; TNF-α levels higher than 10 pg/ml, however, were found more frequently in patients with PMN expressing CD83 (b).
MHC class II restricted antigen presentation
The data so far suggest that induction of MHC class II expression and of CD83 on PMN are not linked to each other. To test whether there is a functional relationship between the two, the participation in antigen presentation of CD83 was tested. Highly purified PMN were cultivated in the presence of IFN-γ and GM-CSF with or without additional TNF-α for 24 h. Then tetanus toxoid (TT) was added and proliferation of a tetanus specific T cell line was measured after 4 days. PMN prestimulated with IFN-γ and GM-CSF induced T cell proliferation. Athough up-regulating CD83 expression, TNF-α also had no additional effect on T cell proliferation under limiting conditions, i.e. with limited number of antigen-presenting PMN (Fig. 5a). Moreover, antibodies to CD83 did not affect T cell proliferation, whereas – as expected – antibodies to MHC class II or to CD86 were inhibitory (Fig. 5b).
Fig. 5.

Induction of T cell proliferation by PMN: (a) Of a healthy donor TT-specific T cell clones was established. PMN were induced to acquire MHC class II and CD83 by culturing the cells with either IFN-γ (100 U/ml) and GM-CSF (50 U/ml) or additionally TNF-α (2 ng/ml) to further up-regulate CD83 for 24 h. After 5 days of co-culture of T cells with PMN and TT proliferation of T cells was measured by incorporation of [3H]-thymidine. (b) Antibodies (2 µg/ml) to MHC class II or CD83 were added during PMN/T cell/TT co-culture, as was mouse IgG as a negative control. Proliferation was measured after 5 days.
Discussion
CD83 is a 45-kDa protein belonging to the immunoglobulin family. Its constitutive expression is restricted to dendritic cells and thymic epithelial cells [25,26]. Expression of CD83, however, can be induced in monocytes [24,27,28], PMN precursors [29] and even in mature PMN, as has been shown recently [18,20]. In PMN up-regulation of CD83 was induced by cultivating the cells with IFN-γ and GM-CSF and depended entirely on de novo protein synthesis. Because concomitantly with CD83 MHC class II antigens, CD80 and CD86 were also up-regulated, we concluded that PMN can acquire characteristics of dendritic cells [18]. While this is still true, the present study shows that CD83 and MHC class II expression can occur independently of each other.
TNF-α, which preferentially up-regulates CD83 synthesis, is a well-known activator of PMN, and best studied as a ‘priming’ factor [30]. However, more recently it has been shown that low doses of TNF-α prolong the lifespan of PMN [12]. Its effect on PMN is consistent with that on monocytes: here TNF-α is required for the differentiation of peripheral monocytes to CD83-positive dendritic-like cells [24].
The data lead to the concept that depending on the cytokine pattern, PMN transdifferentiate to different phenotypes: with IFN-γ and GM-CSF PMN acquire the characteristics of dendritic cells, including expression of CD83, MHC class II, CD80 and CD86; following exposure to TNF-α only CD83 is up-regulated. The coincidence of TNF-α and CD83 expression in patients with acute bacterial infection favours that concept. The fact that neither TNF-α nor CD83 are found in all patients with bacterial infections does not argue against that concept, because both molecules are expressed only transiently, and the patients differed with regard to onset, severity and duration of the infection.
The functional consequences of CD83 up-regulation are not yet known, particularly since the function of CD83 is still elusive. A role in antigen presentation was presumed due to its association with antigen-presenting cells. More recently, a regulatory function for T cells has been described [31,32]. With regard to PMN, our data argue against a role of CD83 in antigen presentation, that because (1) antibodies to CD83 (at least clones Hb15A and Hb15E which we tested) did not inhibit the MHC class II-restricted T cell activation; (2) high expression of CD83 did not enhance T cell activation; and (3) CD83 is also up-regulated in the absence of MHC class II.
More recently, sialic acid was identified as a ligand for CD83, putting this receptor – at least functionally – into the group of Siglecs, surface receptors involved in pattern- and self-recognition, respectively [33]. It might be speculated that the function of CD83 lies in the recognition of possible pathogens and that it is involved in uptake and intracellular processing of those pathogens, rather than in the presentation of those antigens to T cells. This assumption is in line with the coincidence of bacterial infection and CD83 expression.
Regardless, however, of the possible function, expression of CD83 on PMN is an indicator for de novo protein synthesis. It therefore serves as a marker for PMN transdifferentiation and of extended lifespan and allows differentiation between acute and chronic inflammation.
REFERENCES
- 1.White-Owen C, Alexander JM, Babcock GF. Reduced expression of neutrophil CD11b and CD16 after server traumatic injury. J Surg Res. 1992;52:22–6. doi: 10.1016/0022-4804(92)90273-3. [DOI] [PubMed] [Google Scholar]
- 2.Müller-Kobold AC, Tulleken JE, Zijlstra JG, et al. Leukocyte activation in sepsis; correlation with disease state and mortality. Intensive Care Med. 2000;26:883–92. doi: 10.1007/s001340051277. [DOI] [PubMed] [Google Scholar]
- 3.Wagner C, Deppisch R, Denefleh B, et al. Expression patterns of the lipopolysaccharide receptor CD14, and the Fc receptors CD16 and CD64 on polymorphonuclear neutrophils (PMN): data from patients with severe bacterial infection and LPS-exposed cells. Shock. 2002 doi: 10.1097/00024382-200301000-00002. in press. [DOI] [PubMed] [Google Scholar]
- 4.Keel M, Ungethüm U, Steckholzer U, et al. Interleukin 10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90:3356–63. [PubMed] [Google Scholar]
- 5.Chitnis D, Dickerson C, Munster AM, et al. Inhibition of apoptosis in polymorphonuclear neutrophils from burn patients. J Leukoc Biol. 1996;59:835–9. doi: 10.1002/jlb.59.6.835. [DOI] [PubMed] [Google Scholar]
- 6.Wagner C, Pioch M, Meyer C, et al. Differentiation of polymorphonuclear neutrophils (PMN) in patients with systemic infections and chronic inflammatory diseases: evidence of prolonged life-span and de novo synthesis of fibronectin. J Mol Med. 2000;78:337–45. doi: 10.1007/s001090000107. [DOI] [PubMed] [Google Scholar]
- 7.Hänsch GM, Radsak M, Wagner C, et al. Expression of major histocompatibility class II antigens on polymorphonuclear neutrophils in patients with Wegener's granulomatosis. Kidney Int. 1999;55:1181–8. doi: 10.1046/j.1523-1755.1999.00446.x. [DOI] [PubMed] [Google Scholar]
- 8.William R, Watson G, Rotstein OD, et al. The IL-1β-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1β. J Immunol. 1998;161:957–62. [PubMed] [Google Scholar]
- 9.Klebanoff SJ, Olszowski S, Van Voorhis WC, et al. Effects of γ-interferon on human neutrophils: protection from deterioration on storage. Blood. 1992;80:225–34. [PubMed] [Google Scholar]
- 10.Lee A, Whyte MKB, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–8. [PubMed] [Google Scholar]
- 11.Colotta F, Re F, Polentarutti N, et al. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–1992. [PubMed] [Google Scholar]
- 12.van den Berg J, Weyer S, Weening J, et al. Divergent effects of tumor necrosis factor alpha on apoptosis of human neutrophils. J Leukoc Biol. 2001;69:467–73. [PubMed] [Google Scholar]
- 13.Dransfield I, Buckle AM, Savill J, et al. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. J Immunol. 1994;153:1254–63. [PubMed] [Google Scholar]
- 14.Gosselin EJ, Wardwell K, Rigby WF, et al. Induction of MHC class II on human polymorphonuclear neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-gamma, and IL-3. J Immunol. 1993;151:1482–993. [PubMed] [Google Scholar]
- 15.Fanger NA, Liu C, Guyre PM, et al. Activation of human T cells by major histocompatibility complex class II expressing neutrophils: proliferation in the presence of superantigen, but not tetanus toxoid. Blood. 1997;89:4128–35. [PubMed] [Google Scholar]
- 16.Reali E, Guerrini R, Moretti S, et al. Polymorphonuclear neutrophils pulsed with synthetic peptides efficiently activate memory cytotoxic T lymphocytes. J Leukoc Biol. 1996;60:207–13. doi: 10.1002/jlb.60.2.207. [DOI] [PubMed] [Google Scholar]
- 17.Radsak M, Iking-Konert C, Stegmaier S, et al. Polymorphonuclear neutrophils (PMN) as accessory cells for T-cell activation: MHC class II restricted antigen-dependent induction of T-cell proliferation. Im-munology. 2000;101:521–30. doi: 10.1046/j.1365-2567.2000.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iking-Konert C, Csekö C, Wagner C, et al. Transdifferentiation of polymorphonuclear neutrophils: acquisition of CD83 and other functional characteristics of dendritic cells. J Mol Med. 2001;79:464–74. doi: 10.1007/s001090100237. [DOI] [PubMed] [Google Scholar]
- 19.Iking-Konert C, Vogt S, Radsak M, et al. Polymorphonuclear neutrophils in Wegener's granulomatosis acquire characteristics of antigen presenting cells. Kid Int. 2001;60:2247–62. doi: 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 20.Yamashiro S, Wang JM, Gong WH, et al. Expresion of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958–63. [PubMed] [Google Scholar]
- 21.Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitis: the proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–92. doi: 10.1002/art.1780370206. [DOI] [PubMed] [Google Scholar]
- 22.Leavitt RY, Fauci AS, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33:1101–7. doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- 23.Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham vasculitis activity score (BVAS) in systemic necrotizing vasculitis. Q J Med. 1994;87:671–8. [PubMed] [Google Scholar]
- 24.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto Y, Tu L, Miller AS, et al. CD83 expression influences CD4 (+) T-cell development in the thymus. Cell. 2002;108:755–67. doi: 10.1016/s0092-8674(02)00673-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhou LJ, Schwarting R, Smith HM, et al. A novel cell surface molecule expressed by human interdigitating reticulum cells, Langerhans cells and acticated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992;149:735–42. [PubMed] [Google Scholar]
- 27.Palucka KA, Taquet N, Sanchez-Chapuis F, et al. Dendritic cells as the terminal stage of monocyte differentiation. J Immunol. 1998;160:4587–95. [PubMed] [Google Scholar]
- 28.Pickl WF, Majdic O, Kohl P, et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14 + peripheral blood monocytes. J Immunol. 1996;157:3850–9. [PubMed] [Google Scholar]
- 29.Oehler L, Majdic O, Pickl WF, et al. Neutrophil granulocyte-committed cells can be driven to acquire dendritic cell characteristics. J Exp Med. 1998;187:1019–28. doi: 10.1084/jem.187.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berlow R, Dodson M. Biochemical mechanisms involved in the priming of neutrophils by tumor necrosis factor. J Leukoc Biol. 1988;44:345–52. doi: 10.1002/jlb.44.5.345. [DOI] [PubMed] [Google Scholar]
- 31.Lechmann M, Krooshoop DJ, Dudziak D, et al. The extracellular domain of CD83 inhibits cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J Exp Med. 2001;194:1813–21. doi: 10.1084/jem.194.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schöller N, Hayden-Ledbetter M, Dahlin A, et al. CD83 regulates the development of cellular immunity. J Immunol. 2002;168:2599–602. doi: 10.4049/jimmunol.168.6.2599. [DOI] [PubMed] [Google Scholar]
- 33.Schöller N, Hayden-Ledbetter M, Hellstrom K, et al. CD83 is a sialic acid-binding Ig-like lectin (Siglec) adhesion receptor that binds monocytes and a subset of activated T-cells. J Immunol. 2001;166:3865–72. doi: 10.4049/jimmunol.166.6.3865. [DOI] [PubMed] [Google Scholar]


