Abstract
Staphyllococcus aureus-induced infections often result in high mortality and permanent joint destruction, despite treatment with antibiotics. IL-10 is typically regarded as an anti-inflammatory cytokine because it promotes a T helper cell type 2 response, and subsequently down-regulates cell mediated immune functions. To investigate the role of IL-10 in S. aureus-induced arthritis and sepsis, Balb/c mice, intact or defective with respect to IL-10 gene were intravenously inoculated with bacteria. IL-10−/− mice develop a more frequent and destructive arthritis compared to their congeneic controls. The mechanisms regulating such outcome may be due not only to the anti-inflammatory properties of IL-10 but also, directly or indirectly, to antibacterial features of this molecule. Indeed, inoculation of staphylococci to IL-10−/− mice resulted in higher bacterial load in blood and kidneys compared to congeneic controls. Altogether our data indicate that IL-10 is essential for efficient elimination of bacteria and thereby for protection against septic arthritis.
Introduction
Systemic Staphylococcus aureus infections with manifestations such as septic arthritis are often very severe conditions, accompanied by high mortality and rapid destruction of the joints. The bacteria trigger an exaggerated immune response in the host which causes most of the sequels seen. In our murine model of septic arthritis we have the ability to study these immunological mechanisms in detail. Mice are inoculated intravenously with a superantigen-producing S. aureus strain, LS-1 [1,2], which homes to the joints and gives rise to arthritis and other manifestations of septicaemia. A series of studies using this model suggest that S. aureus arthritis is a T cell-dependent and superantigen-mediated disease [3,4]. We have earlier shown that the T cell response is skewed towards a Th1 response where the joint destruction caused by the bacterium is dependent partly on cytokines such as IL-12 [5], TNF/LT-α[6] and IFN-γ[7]. The importance of Th2 cytokines in this infection is not understood completely, but it is known that the presence of IL-4 functions as promotor for staphylococcal growth [8,9].
IL-10 was discovered around 10 years ago as a factor produced by mouse T helper type 2 cells which inhibits Th1 cytokine synthesis [10]. IL-10 is produced by many cell types including monocytes/macrophages, T and B cells and keratinocytes [11]. This cytokine acts primarily as an anti-inflammatory molecule inhibiting the synthesis of proinflammatory cytokines (e.g. TNF, IL-1, IL-8) by macrophages/monocytes [12] and neutrophils [13]. It has been shown in vivo to ameliorate aseptic collagen II-induced arthritis [14].
The aim of this study has been to investigate the role of IL-10 in S. aureus-induced arthritis by using IL-10 deficient Balb/c mice and their congeneic controls. Our results demonstrate that the lack of IL-10 causes impaired clearance of bacteria leading to a more destructive course of arthritis.
Materials and methods
Mice
Embryonic stem cells (129/Ola) were disrupted for the IL-10 gene [15] and injected into blastocysts of C57BL/6 mice. These IL-10−/− mice were bred onto Balb/c background and back-crossed for more than 12 generations [16]. Mice of both sexes, 6–12 weeks of age, were used. As controls, age- and sex-matched healthy Balb/c mice were obtained from B&K Universal (Sollentuna, Sweden). Up to 10 mice were kept in each cage and they were maintained in the animal facility of the Department of Rheumatology, Göteborg University, under standard conditions of light and temperature, and fed standard laboratory chow and water ad libitum.
Bacteria and inoculation
S. aureus LS-1 was isolated originally from a swollen joint of a spontaneously arthritic NZB/W mouse [1]. One of the characteristics of this staphylococcal strain is that it produces large amounts of TSST-1 [2], an exotoxin with superantigenic properties. The bacteria were cultured on blood agar for 24 h and then reincubated on blood agar for another 24 h. They were kept frozen at −20°C in phosphate buffered saline (PBS) [0·13 m sodium chloride, 10 mm sodium phosphate (pH 7·4)] containing 5% bovine serum albumin and 10% dimethyl sulfoxide (C2H6OS) until use. Prior to use, the bacteria solution was thawed, washed in PBS and diluted in PBS to achieve the desired concentration of bacteria. Mice were inoculated with 200 µl of bacteria solution in one of the tail veins. Viable counts were used to ascertain the number of bacteria injected.
Bacteriological examination
Bacterial growth in blood was examined 24 h after i.v. bacterial inoculation by plating 50–100 µl of heparinized blood, taken from the tail vein, on 5% horse blood agar dishes. The bacterial load in kidneys was examined at time of sacrifice. The kidneys were aseptically removed, ground and diluted with 10 ml of sterile PBS. Appropriate dilutions were made, and 100 µl samples of tissue suspension were plated on agar dishes containing 5% horse blood. Samples for bacteriological examination of joints were obtained using sterilized cotton sticks, after dissection of talocrural and radiocarpal joints, and transferred to 5% horse blood agar dishes. After incubation for 24–48 h colonies were counted, and the results were expressed as the number of colony-forming units (CFU) per ml blood or per whole organ.
Clinical evalution of arthritis
All mice were followed-up individually. Joints were inspected at regular intervals. Arthritis was defined as visible joint erythema and/or swelling of at least one joint. To evaluate the intensity of arthritis, a clinical scoring (arthritic index) was carried out using a system where macroscopic inspection yielded a score of 0–3 points for each limb (0, neither swelling nor erythema; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; 3, marked swelling and erythema) [17]. The total score was calculated by adding the scores for each animal tested. The overall condition was evaluated by assessment of body weight and general appearance.
Histopathological examination
Histopathological examination of the joints was performed after routine fixation, decalcification and paraffin embedding. Tissue sections from fore- and hindpaws were cut and stained with haematoxylin–eosin. All slides were coded and evaluated by a blinded observer, and evaluated with regard to synovial hypertrophy and cartilage/subchondral bone destruction. The degree of synovitis and bone and cartilage destruction yielded a score from 0 to 3 in every joint, i.e. fingers/toes, wrists/ankles, elbows and knees.
Cytokine analysis
TNF
Τhe levels of TNF in serum and supernatants were determined using ELISA kit from R&D Systems (Minneapolis, MN, USA).
Interferon gamma assay
Levels of IFN-γ were measured by ELISA using 2 µg/ml of purified rat antimouse IFN-γ MoAb (PharMingen, San Diego, CA, USA) in sodium bicarbonate pH 9.6 for coating. All samples were serially diluted in Tris-NaCl and incubated in wells. Biotinylated rat antimouse IFN-γ MoAb (2 µg/ml) (PharMingen) were added to measure the level of IFN-γ bound to solid phase. This procedure was followed by stepwise addition of streptavidin alkaline phosphatase (Dako, Glostrup, Denmark). The enzyme substrate was then added, and the absorbance was measured in a SpectraMax plus photometer (Molecular Devices) at 405 nm. The samples were tested in twofold dilutions and compared with recombinant mouse IFN-γ standard (Genzyme, Cambridge, MO, USA).
Intracellular killing activity of intraperitoneal macrophages
The intracellular killing activity of intraperitoneal macrophages was tested by the modification of a previously described method [18,19]. Macrophages were recovered by injecting 3 ml of ice-cold medium (Iscove's medium, 10% FCS, 1% gentamycin) into the peritoneal cavity of sacrificed mice and aspirated after 1 min of massage. The cells were adjusted to the approximate concentration of 2 × 106/ml medium (Iscove's medium, 10% FCS, 1% gentamycin), seeded in 200-µl volumes into 24-wells plates (Nunc) and incubated at room temperature 90 min. Afterwards, 500 µl medium was added to each well, and the cells were incubated for 4 h at 37°C. The medium was then removed and 500 µl of medium free from antibiotics was added to the cells. After incubation overnight at 37°C the cells were washed once with Iscove's medium, and 500 µl of S. aureus LS-1 suspension at a concentration of 8 × 106 bacteria/ml were added for 50 min. Then the cells were washed three times with PBS to remove extracellular bacteria which were not ingested. The bacterial content was analysed immediately (time-point 0) and 26 h after bacterial incubation. To avoid the extracellular replication of bacteria Iscove's medium supplemented with 5% FCS and minimal inhibitory concentration of gentamycin for S. aureus LS-1 strain (4 µg/ml) was added. The macrophages were lysed with distilled water for 20 min, and the lysate, diluted 1 : 1, 1 : 10, 1 : 100 and 1 : 1000 was cultured on 5% horse blood agar plates. The plates were incubated for 24 h and the number of bacterial colonies counted.
Analysis of the phagocytic activity of leucocytes
Freshly obtained heparinized whole blood was vortexed and aliqouted on the bottom of a 5-ml tube. Precooled flourescein isothiocyanate (FITC)-conjugated bacteria were added (1 × 109/ml) and incubated for 20 min at 37°C. Ice-cold quenching solution was then added to remove cell surface-bound FITC. The erythrocytes were lysed and leucocyte membranes solubilized to permit detection of intracellular FITC-labelled bacterial deposits (Pharma, Heidelberg, Germany). Measurements were performed with FACScan (Becton Dickinson, San Jose, CA, USA).
In vitro stimulation of spleen mononuclear cells
Spleen mononuclear cells were incubated at 2 × 106/ml in Iscove's complete medium with either formalin-killed S. aureus strain LS-1 (2 × 107/ml), 1·25 µg/ml of concavalin A (Con A; ICN Biochemicals, Cleveland, OH, USA) or 10 µg/ml of purified TSST-1 (Toxin Technology, Sarasota, FL, USA). Supernatants from cell cultures incubated for 24 h were used for determination of cytokine levels.
Experimental protocol
Three in vivo experiments have been performed and data has been pooled when appropriate.
Experiment no. 1
Female mice were used, IL-10−/− (n = 9) and controls (n = 13). The bacterial dose given was 3 × 107 CFU/ml and 200 µl was injected i.v. Blood was taken for culture and cytokine analysis 24 h after bacterial inoculation. The mice were sacrificed at day 11 and kidneys removed for bacterial culture, blood was obtained for culture and cytokine analysis, and joints for histopathological examination.
Experiment no. 2
Both male and female mice were used, IL-10−/− (n = 13) and controls (n = 13). The bacterial dose given was 2 × 107 CFU/ml and 200 µl was injected i.v. Half of the mice were sacrificed at day 4 and half at day 10. Kidneys and paws were removed for bacterial culture and blood for cytokine analysis at both occassions.
Experiment no. 3
Both male and female mice were used, IL-10−/− (n = 10) and controls (n = 11). The bacterial dose given was 2 × 107 CFU/ml and 200 µl was injected i.v. Blood samples were taken from two different mice at every occasion, before bacterial inoculation and at days 1, 3 and 11 for analysing peripheral leucocyte expression of Annexin V and propiodium iodide. Blood was taken for cultures at 24 h and 3 days. Mice were sacrificed at day 11, kidneys and paws were removed for culture and blood for cytokine analysis.
Statistical analysis
The mortality rate and frequency of arthritis were analysed using Fisher's exact test. All the remaining parameters were analysed by the Mann–Whitney U-test. All the data are expressed as mean ± s.e.m. or median and IQR unless indicated otherwise.
Results
IL-10-deficient mice exhibit more frequent and severe arthritis
The development of arthritis showed higher severity in the IL-10−/− mice with significant values at day 6 + 7 compared to congeneic controls (P = 0·02, Fig. 1a). These clinical findings were verified by the histopathological examination which showed clearly that the IL-10 knock-out mice displayed a significantly higher level of both cartilage- (P = 0·01) and bone- (P = 0·02) destruction, Fig. 1b. The frequency of arthritis was increased in the IL-10−/− animals but did not reach the level of significance (Table 1). Mortality in the IL-10 deficient group was 5/32 (16%) and in the control group 3/37 (16%).
Fig. 1.
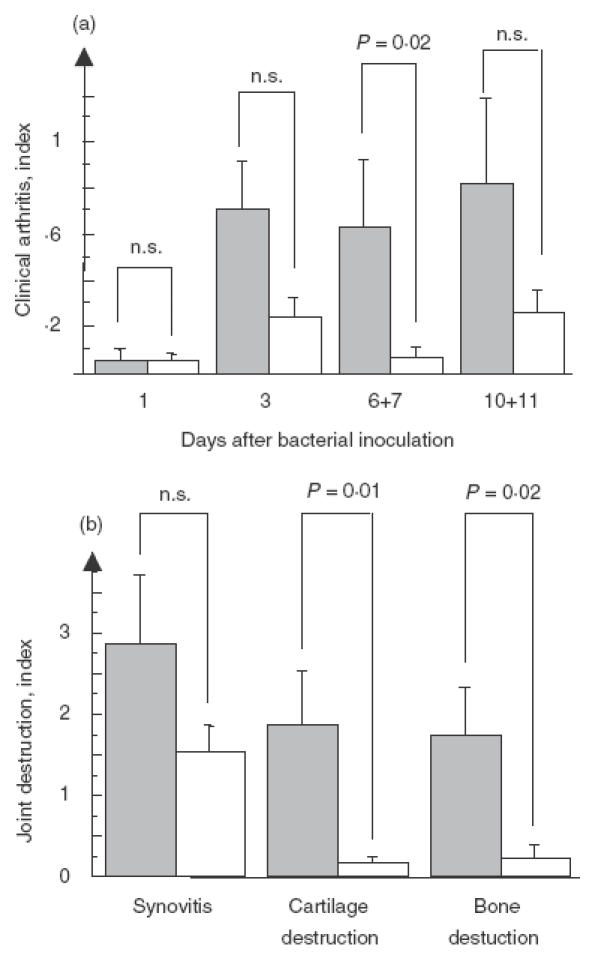
Pooled data from the three pooled experiments show the severity of arthritis in IL-10−/− (n = 32) and their congeneic controls (n = 37) (a). Severityof histopathological changes in IL-10−/− (n = 8) and control mice (n = 13) in synovia, cartilage and bone at day 11 after bacterial inoculation (b).  , IL-10−/−; □ controls.
, IL-10−/−; □ controls.
Table 1.
Frequency of arthritis from three pooled experiments, %
| Mouse type | Day 3 | Day 7 | Day 10 + 11 |
|---|---|---|---|
| IL-10−/− | 29 | 32 | 40 |
| IL-10+/+ | 19 | 7 | 22 |
IL-10-deficient mice exhibit an increased bacterial burden after infection with S. aureus
Number of circulating staphylococci was significantly higher in the knock-out animals at 24 h after bacterial inoculation (P = 0·04) as shown in Fig. 2a, but not detectable in either of the groups after 3 days. Median level of bacteria in kidneys from IL-10−/− mice at day 4 was 12·5 × 105 CFU/kidney in comparison with controls where staphylococi were not detectable (P = 0·009). At day 10 + 11 the tendency was the same, without reaching the level of significance (Fig. 2b). There were no detectable staphylococci in the joints in neither IL-10−/− mice nor in the control group either at day 4 or at day 10 + 11.
Fig. 2.
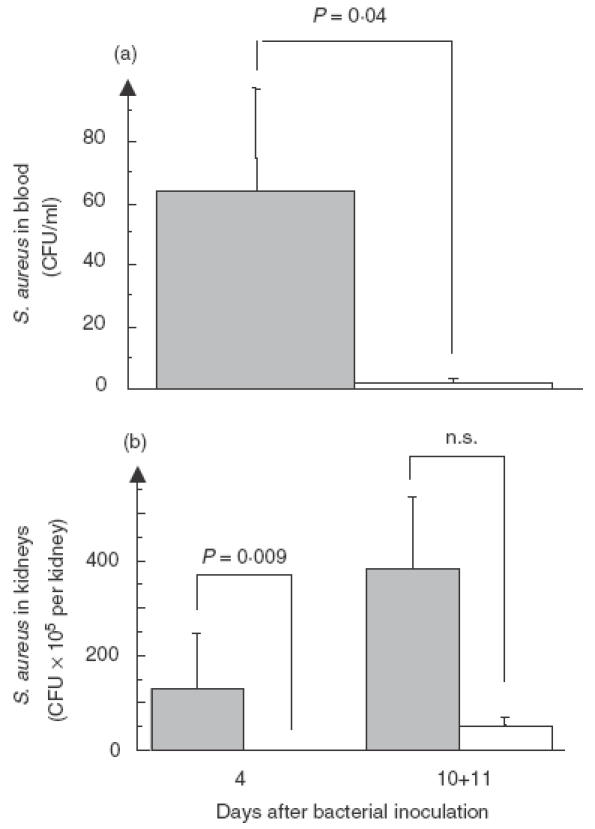
Bacterial counts in blood of IL-10−/− (n = 11) and control mice (n = 11) 24 h after bacterial inoculation (a). At day 3 no staphylococci were detectable in the blood (data not shown). Bacterial counts in kidneys of IL-10−/− (n = 6) and control mice (n = 6) at day 4 after inoculation. At day 10 + 11 kidneys from the IL-10−/− mice (n = 12) contain more bacteria than the controls (n = 17) but do not reach the level of significance (b).  , IL-10−/−,□, controls.
, IL-10−/−,□, controls.
IL-10-deficient mice exhibit an altered cytokine pattern in response to S. aureus infection
At day 1 there was no difference in the in vivo levels of TNF between the IL-10−/− and congeneic mice. Surprisingly, the serum TNF levels in the IL-10 deficient animals decreased during the infection compared to controls, and at day 10 were significantly (P = 0·008) lower compared to controls, Fig. 3a. The circulating IFN-γ levels were increased in the IL-10−/− mice, although the difference did not reach statistical significance, Fig. 3b.
Fig. 3.

Proinflammatory cytokine response following inoculation of S. aureus in IL-10−/− mice. There was no statistical difference between the serum levels of TNF early (IL-10−/−n = 19, IL-10+/+, n = 21) during the infection. At day 10 + 11 the TNF levels in the IL-10−/− mice (n = 11) were significantly decreased compared to controls (n = 16) (a). Differences regarding serum levels of IFN-γ were not found at any of the time-points studied (IL-10−/−,n = 8, IL-10+/+, n = 12) (b).  , IL-10−/−,□, controls.
, IL-10−/−,□, controls.
In vitro stimulation of spleen mononuclear cells from IL-10 deficient mice showed significantly increased (P = 0·03) levels of IFN-γ in the supernatant in response to ConA (Fig. 4a). A similar relationship (P = 0·03) was obtained concerning TNF levels in the supernatant but now in response to staphylococcal cell wall components (Fig. 4b).
Fig. 4.

Cytokine production in response to in vitro stimulation of mononuclear spleen cells. (a) Significantly increased IFN-γ level (P = 0·03) in supernatants from the IL-10−/− mice (n = 5) compared to controls (n = 5) upon stimulation with ConA. TNF production is also significantly higher (P = 0·03) in the IL-10-deficient animals in response to cell wall components (b).  , IL-10−/−□, controls.
, IL-10−/−□, controls.
IL-10-deficient mice display intact phagocytosis
There was no significant difference between the ability of PMNC and monocytes to phagocytose FITC-labelled staphylococci between IL-10−/− and congeneic controls when analysed by FACS. Addition of rIL-10 significantly impaired the phagocytosis by macrophages when added to the knock-out animals (P = 0·03) and in granulocytes when added to controls (P = 0·009) (Fig. 5). Intracellular killing of staphylococci by peritoneal macrophages did not differ between the IL-10−/− mice (n = 3) and controls (n = 3) checked at time-point 0 at 26 h after bacterial stimulation.
Fig. 5.

Addition of rIL-10 (10 ng/ml) impaires the phagocyting capacity of macrophages and granulocytes. Only significant values are shown. Data shown as median and inter quartil range. □, IL-10−/−, n = 5; □, IL-10−/− + rIL-10, n = 4; □, control, n = 5;  , control + rIL-10, n = 5.
, control + rIL-10, n = 5.
Discussion
The results of this study clearly show that the absence of IL-10 aggravates, both the frequency, and the severity of S. aureus-induced arthritis. This is in line with the findings in aseptic collagen II arthritis where Kasama et al. [20] demonstrated an acceleration of the onset and an increase in the severity of arthritis, when mice were administered neutralizing monoclonal anti-IL-10 antibodies. A series of studies [21–25] show that IL-10 ameliorates the outcome of collagen arthritis. One of the explanations of the very destructive course of arthritis seen in S. aureus-induced infections is due to the fact that the IL-10 knock-out situation leads to increased production of Th1 cytokines, which can be illustrated with the elevated levels of TNF and IFN-γ in splenocytes when stimulated with staphylococcal cell wall components and TSST-1, respectively.
An additional factor which has to be considered in staphylococcal arthritis is the bacterium itself. Because IL-10 has a deactivating capacity on macrophages and neutrophils, cells that are of utmost importance for clearing the staphylococcal infection in our mice model [26,27], one would therefore assume that lack of IL-10 would enhance the bacterial clearance. However, the IL-10 knock-out mice showed a higher bacterial burden in several body compartments including blood and kidneys, especially early during the infection. No bacteria were found in the joints in any of the groups, probably due to the relatively low dose of bacteria inoculated and relatively insensitive detection technique. The increased bacterial burden in the IL-10 deficient mice infected with S. aureus LS-1 was not entirely unexpected. Indeed, Sasaki et al. [28] showed that mice infected with S. aureus 834 and treated with IL-10 neutralizing antibodies had a higher bacterial load in kidneys. Our in vitro results suggest that recombinant IL-10 impairs the phagocytic capacity of macrophages and granulocytes, due possibly to higher concentration of rIL-10 compared to the endogenous production of IL-10.
In vitro stimulation of splenocytes from IL-10−/− animals showed significantly higher IFN-γ levels, although no differences were found when measured in vivo. The significance of IFN-γ in S. aureus arthritis is multi-faceted. We have earlier shown that IFN-γ plays a critical role in the clearance of bacteria in the early state of the infection, but is of less importance later. Administration of rIFN-γ enhances the systemic clearance of staphylococci, probably by increasing the phagocytic capacity of neutrophils, but worsens the arthritis [7,29]. It is also well known that the susceptibility to collagen-induced arthritis (CIA) is increased in IFN-γ receptor knock-out mice [30,31]. In contrast, IFN-γ given systemically in the CIA model ameliorated the disease [32].
Arai et al. [33] demonstrated that IL-10 plays an essential role in protection of Salmonella-infected macrophages from apoptosis caused by exessive production of TNF. One potential explanation for the impaired clearance of staphylococci in the IL-10 knock-out mice is enhanced apoptosis of phagocytosing cells, i.e. macrophages. We have addressed this question by analysing peripheral blood leucocytes with respect to their expression of Annexin V and propidium iodide. However, we could not detect any changes of frequency of apoptotic cells between IL-10−/− and controls (results not shown). Futhermore, the intracellular killing capacity of macrophages showed no difference compared to the control mice, as evaluated in vitro. Finally, the in vivo migration capacity of neutrophils [34] was not affected by IL-10 deficiency (data not shown).
We conclude that absence of IL-10 significantly increases the severity of S. aureus-induced arthritis. This phenomenon is probably due to increased production of Th1 cytokines such as TNF and a heavier bacterial burden. Futher studies are required to assess if addition of rIL-10 to mice infected with S. aureus might, in combination with antibiotics, would be a novel approach to treatment of this severe infection.
Acknowledgments
We thank Donna Rennick and Elisabeth Süri-Payer for kindly providing the IL-10-deficient mice, and Lena Svensson, Ing-Marie Jonsson, Margareta Verdrengh and Zai-Qing Liu for excellent technical assistance. This work was supported from the Göteborg Medical Society, the Swedish Association against Rheumatism, the King Gustaf V Foundation, the Swedish Medical Research Council, Inflammation network, Infection and Vaccinology network, the Nanna Swartz Foundation, AME Wolff Foundation and the University of Göteborg.
REFERENCES
- 1.Bremell T, Lange S, Yacoub A, et al. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–23. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–85. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdelnour A, Bremell T, Holmdahl R, et al. Role of T lymphocytes in experimental Staphylococcus aureus arthritis. Scand J Immunol. 1994;39:403–8. doi: 10.1111/j.1365-3083.1994.tb03392.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdelnour A, Bremell T, Tarkowski A. Toxic shock syndrome toxin 1 contributes to the arthritogenicity of Staphylococcus aureus. J Infect Dis. 1994;170:94–9. doi: 10.1093/infdis/170.1.94. [DOI] [PubMed] [Google Scholar]
- 5.Hultgren OH, Stenson M, Tarkowski A. Role of IL-12 in Staphylococcus aureus-triggered arthritis and sepsis. Arthritis Res. 2001;3:41–7. doi: 10.1186/ar138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hultgren O, Eugster HP, Sedgwick JD, et al. TNF/lymphotoxin-alpha double-mutant mice resist septic arthritis but display increased mortality in response to Staphylococcus aureus. J Immunol. 1998;161:5937–42. [PubMed] [Google Scholar]
- 7.Zhao YX, Tarkowski A. Impact of interferon-gamma receptor deficiency on experimental Staphylococcus aureus septicemia and arthritis. J Immunol. 1995;155:5736–42. [PubMed] [Google Scholar]
- 8.Hultgren O, Kopf M, Tarkowski A. Staphylococcus aureus-induced septic arthritis and septic death is decreased in IL-4-deficient mice: role of IL-4 as promoter for bacterial growth. J Immunol. 1998;160:5082–7. [PubMed] [Google Scholar]
- 9.Hultgren O, Kopf M, Tarkowski A. Outcome of Staphylococcus aureus-triggered sepsis and arthritis in IL-4-deficient mice depends on the genetic background of the host. Eur J Immunol. 1999;29:2400–5. doi: 10.1002/(SICI)1521-4141(199908)29:08<2400::AID-IMMU2400>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 10.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson AW. The cytokine handbook. London: Academic Press; 1998. [Google Scholar]
- 12.de Waal Malefyt R, Abrams J, Bennett B, et al. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes. an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–20. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassatella MA, Meda L, Bonora S, et al. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J Exp Med. 1993;178:2207–11. doi: 10.1084/jem.178.6.2207. California 94304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyata M, Sasajima T, Sato H, et al. Suppression of collagen induced arthritis in mice utilizing plasmid DNA encoding interleukin 10. J Rheumatol. 2000;27:1601–5. [PubMed] [Google Scholar]
- 15.Kuhn R, Lohler J, Rennick D, et al. Interleukin-10-deficient mice develop chronic enterocolitis [see comments] Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 16.Berg DJ, Davidson N, Kuhn R, et al. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4 (+) TH1-like responses. J Clin Invest. 1996;98:1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelnour A, Arvidson S, Bremell T, et al. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993;61:3879–85. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiedermann U, Tarkowski A, Bremell T, et al. Vitamin A deficiency predisposes to Staphylococcus aureus infection. Infect Immun. 1996;64:209–14. doi: 10.1128/iai.64.1.209-214.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lissner CR, Swanson RN, O'Brien AD. Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vitro. J Immunol. 1983;131:3006–13. [PubMed] [Google Scholar]
- 20.Kasama T, Strieter RM, Lukacs NW, et al. Interleukin-10 expression and chemokine regulation during the evolution of murine type II collagen-induced arthritis. J Clin Invest. 1995;95:2868–76. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Persson S, Mikulowska A, Narula S, et al. Interleukin-10 suppresses the development of collagen type II-induced arthritis and ameliorates sustained arthritis in rats. Scand J Immunol. 1996;44:607–14. doi: 10.1046/j.1365-3083.1996.d01-355.x. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka Y, Otsuka T, Hotokebuchi T, et al. Effect of IL-10 on collagen-induced arthritis in mice. Inflamm Res. 1996;45:283–8. doi: 10.1007/BF02280992. [DOI] [PubMed] [Google Scholar]
- 23.Joosten LA, Lubberts E, Durez P, et al. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997;40:249–60. doi: 10.1002/art.1780400209. [DOI] [PubMed] [Google Scholar]
- 24.Lubberts E, Joosten LA, Van Den Bersselaar L, et al. Intra-articular IL-10 gene transfer regulates the expression of collagen-induced arthritis (CIA) in the knee and ipsilateral paw. Clin Exp Immunol. 2000;120:375–83. doi: 10.1046/j.1365-2249.2000.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walmsley M, Katsikis PD, Abney E, et al. Interleukin-10 inhibition of the progression of established collagen-induced arthritis. Arthritis Rheum. 1996;39:495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 26.Verdrengh M, Tarkowski A. Role of neutrophils in experimental septicemia and septic arthritis induced by Staphylococcus aureus. Infect Immun. 1997;65:2517–21. doi: 10.1128/iai.65.7.2517-2521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verdrengh M, Tarkowski A. Role of macrophages in Staphylococcus aureus-induced arthritis and sepsis. Arthritis Rheum. 2000;43:2276–82. doi: 10.1002/1529-0131(200010)43:10<2276::AID-ANR15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki S, Nishikawa S, Miura T, et al. Interleukin-4 and interleukin-10 are involved in host resistance to Staphylococcus aureus infection through regulation of gamma interferon. Infect Immun. 2000;68:2424–30. doi: 10.1128/iai.68.5.2424-2430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao YX, Nilsson IM, Tarkowski A. The dual role of interferon-gamma in experimental Staphylococcus aureus septicaemia versus arthritis. Immunology. 1998;93:80–5. doi: 10.1046/j.1365-2567.1998.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manoury-Schwartz B, Chiocchia G, Bessis N, et al. High susceptibility to collagen-induced arthritis in mice lacking IFN-gamma receptors. J Immunol. 1997;158:5501–6. [PubMed] [Google Scholar]
- 31.Vermeire K, Heremans H, Vandeputte M, et al. Accelerated collagen-induced arthritis in IFN-gamma receptor-deficient mice. J Immunol. 1997;158:5507–13. [PubMed] [Google Scholar]
- 32.Nakajima H, Takamori H, Hiyama Y, et al. The effect of treatment with interferon-gamma on type II collagen-induced arthritis. Clin Exp Immunol. 1990;81:441–5. doi: 10.1111/j.1365-2249.1990.tb05353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arai T, Hiromatsu K, Nishimura H, et al. Endogenous interleukin 10 prevents apoptosis in macrophages during Salmonella infection. Biochem Biophys Res Commun. 1995;213:600–7. doi: 10.1006/bbrc.1995.2174. [DOI] [PubMed] [Google Scholar]
- 34.Josefsson E, Carlsten H, Tarkowski A. Neutrophil mediated inflammatory response in murine lupus. Autoimmunity. 1993;14:251–7. doi: 10.3109/08916939309077373. [DOI] [PubMed] [Google Scholar]


