Abstract
High radiation exposure among male radiologists has been reported to result in a significantly higher proportion of female offspring. This study examined whether work-related radiation exposure was associated with a higher propensity for female offspring among male interventional cardiologists. On behalf of the interventional committee of the Society for Cardiovascular Angiography and Interventions, an Internet-based questionnaire was sent to the society's 2063 members. The 402 male respondents had a total of 518 biological offspring; 48.6% of them were female. Among the 172 high-volume male diagnostic operators (those who performed >300 cases annually), there were 218 biological offspring, of whom 46.8% were female. Among the 59 high-volume male interventional operators, there were 70 biological offspring, of whom 45.7% were female. P values were nonsignificant for all three groups. In conclusion, work-related radiation exposure of male invasive and interventional cardiologists was not associated with a statistically significant preponderance of female offspring.
A reduced offspring sex ratio, defined as the ratio of male to female offspring or the ratio of male offspring to total offspring, has been hypothesized to be a potential marker of reproductive hazard in paternal subjects (1, 2). Exposure to varying forms of radiation and toxic substances, including but not limited to electromagnetic radiation (3–6), environmental pollutants known as dioxins (7–9), tobacco (10, 11), metal fumes (12), and alcohol and lead (13), has been implicated in reducing the sex ratio. A significantly higher proportion of female offspring has also been observed among male radiologists and male surgeons exposed to ionizing radiation (14, 15). Changes in the paternal hormonal profile and Y chromosomal damage have been put forward as potential mechanisms for this change in sex ratio (16, 17). However, data have been inconsistent, and other studies have shown that ionizing radiation in nonmedical fields may not affect the sex ratio (18–22).
With the advent of more intricate, technically difficult procedures, it is now thought that cardiologists performing fluoroscopic-guided procedures are exposed to the highest levels of ionizing radiation among those using x-ray technology (23–25). Diagnostic or interventional collar-level exposure can range from 0.04 to 0.16 mSv per procedure, and waist-level exposure beneath a 0.5-mm lead apron can range from 0.01 to 0.02 mSv per case (26–31). Due to their complexity, interventional procedures expose operators to higher levels of radiation than diagnostic angiography (32, 33). The aim of this study is to determine if the occupational radiation exposure of male invasive and interventional cardiologists is associated with a higher proportion of female offspring.
METHODS
On behalf of the interventional committee of the Society for Cardiovascular Angiography and Interventions, an Internet-based questionnaire was sent to the society's members. The survey asked respondents about their age, gender, number of years performing angiographic procedures, average annual number of procedures, and number and gender of biological offspring. Participants were also asked to specify the number of diagnostic and interventional procedures performed. High-volume operators were defined as those averaging >300 cases annually. For this study, offspring sex ratio was defined as the ratio of male to female offspring.
Statistical analysis
Data were presented as mean ± standard deviation. Differences in offspring gender ratio were examined using a chi-square test. Statistical significance was assumed with P < 0.05. It was determined that at least 375 offspring were required to detect a 20% absolute difference (odds ratio, 1.2) in the proportion of female vs male offspring given a one-tailed test (alpha, 0.025; power, 80%).
RESULTS
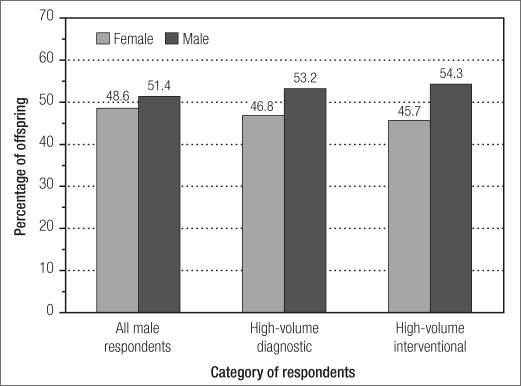
Questionnaires were sent to 2063 members of the Society for Cardiovascular Angiography and Interventions, with 423 responses (21%). Of the respondents, 402 (95%) were male. The study group's demographics are shown in the Table. There were 518 total biological offspring, of whom 252 (48.6%) were female and 266 (51.4%) were male (Figure). The calculated sex ratio was 1.06. Among the 172 high-volume male diagnostic operators, there were 218 biological offspring, with 102 (46.8%) female offspring. Among the 59 high-volume male interventional operators, there were 70 biological offspring, with 32 (45.7%) female offspring. The calculated sex ratios for high-volume diagnostic and interventional operators were 1.14 and 1.19, respectively. P values were nonsignificant for all three groups.
Table.
Demographic data for the 402 male respondents
| Variable | Result |
| Age (years) | 48 ± 8.8 |
| Years as practicing invasive cardiologist | |
| 0–4 | 18% |
| 5–10 | 20% |
| 11–16 | 25% |
| 17–20 | 14% |
| >21 | 23% |
Figure.
Percentage of male and female offspring among respondents.
DISCUSSION
In our study, the work-related radiation exposure of male invasive and interventional cardiologists was not associated with a statistically significant preponderance of female offspring. The sex ratio of all biological offspring of paternal subjects in this study, 1.06, is remarkably close to the observed sex ratio of 1.046 to 1.059 within the US population over the last 60 years (34). Among high-volume male interventional operators, whose radiation exposure is higher, a reduction in the offspring sex ratio was also not observed.
The current literature is conflicting regarding the effect of ionizing radiation exposure on offspring sex ratio. In a questionnaire study of 586 male radiologists, Hama et al reported that radiation exposure among male radiologists was associated with a significantly higher proportion of female offspring: 51.5% vs 48.5% in the control group, with a relative risk of 1.13 (95% confidence interval [CI], 1.00–1.27). The same study found that high levels of radiation exposure, defined as one or more incidents of annual radiation exposure >10 mSv, among male radiologists were associated with an even higher proportion of female offspring (66%; P = 0.002; relative risk, 2.01) (14). Zadeh and Briggs have demonstrated a nonsignificant increase in the female proportion (53%, P = 0.13) in the sex ratio among male orthopaedic surgeons exposed to ionizing radiation. However, their obstetric and gynecological counterparts who served as controls because of their limited exposure to ionizing radiation also had a significant increase in female offspring (52%, P = 0.05 and P = 0.01 if both groups were combined) (15).
Outside the field of medicine, Irgens et al demonstrated that men in the smelter industry and wire-producing industry who were exposed to extremely low-frequency electromagnetic fields demonstrated a reduced sex ratio (3). Milham also confirmed a reduced sex ratio among the 139 offspring of carbon setters in Olympia, Washington, who were exposed to electromagnetic radiation; there was a 62% female preponderance (P = 0.0026) (4). Limited data have also shown reduced sex ratios in maternal subjects in the smelter and wire-producing industries (3) and among female physiotherapists (6) exposed to electromagnetic radiation.
In contrast, when the sex ratio of over 39,500 offspring of male nuclear industry workers in the UK was studied, 19,156 (48.5%) live births were female, corresponding to a sex ratio equal to that of the general population (odds ratio, 1.00; 95% CI, 0.98–1.02) (18). Likewise, in the analysis of 413 offspring of 259 surveyed US naval submarine officers, 46.25% of the biological offspring were female. These results were not significantly different from the sex ratio of the US general population at the time (P = 0.29). Nonetheless, there was a nonsignificant trend towards lower sex ratios as time lived within the submarine community increased for these paternal subjects (19). Lastly, in a study of male nuclear installation workers in northern England, the observed offspring sex ratio was 1.094 (95% CI, 1.060–1.128) vs 1.055 (95% CI, 1.046–1.063) in the control group. Furthermore, in the same study, among fathers with high radiation exposure, defined as >10 mSv of external radiation in the 90 days prior to conception, an even higher proportion of male offspring was observed (sex ratio, 1.396; 95% CI, 1.127–1.729) (20).
The mechanisms that underlie these changes in offspring sex ratio due to environmental exposures are not clearly understood; however, several theories have been proposed. The hormonal hypothesis purports that certain exposures can affect paternal levels of testosterone and gonadotropins—hormones that are directly involved in human sex determination (16). In contrast to human somatic cells, human spermatozoa have been shown to have a dose-dependent increase in structural chromosomal aberrations with chronic x-ray exposure (35). Therefore, radiation-induced damage to the sex-determining gene SRY on the Y chromosome or selective injury of Y-chromosome–bearing spermatocytes may also increase the proportion of female offspring (17). Lastly, ionizing radiation has also been shown to affect fertility in general by reducing sperm counts and altering sperm structure (36).
Limitations exist in this and other attempts to calculate a sex ratio based on an occupational hazard such as ionizing radiation. For numerous reasons, including varying operator technique, use of safety measures, and fluoroscopic and cineangiography technology, radiation exposure to primary operators in the cardiac catheterization laboratory is not uniform. Other confounding variables include age of paternal subjects at the time of conception and other undocumented paternal and maternal exposures. Lastly, the use of average number of annual cases as a surrogate for preconceptional radiation exposure is inexact. Existing studies suggest that the mean collar-level exposure per case for cardiologists performing both angiography and interventional procedures is 0.10 mSv per procedure (26–31). Assuming 300 cases per year, this would translate to an annual exposure of 30 mSv. Current recommendations for dose limits by the International Commission on Radiological Protection and the National Council on Radiation Protection mandate that whole-body exposure should not average >20 mSv per year and not exceed an annual limit of 50 mSv per year (37, 38). However, collar-level measurements overestimate the under-apron exposure, which is closer to the true “effective dose” of whole-body radiation received by fluoroscopic operators. One author has estimated that the mean annual effective dose for physicians performing angiography and intervention to be closer to 2.5 ± 2.6 mSv, with a maximum of 16.2 mSv (39). Interventional cardiologists, therefore, may not be exposed to the levels of ionizing radiation needed to affect their proportion of female offspring.
Further studies with more exact measurements of radiation exposure and uniform methods of estimating occupational risk to physicians are needed before making a definitive conclusion concerning the ability of ionizing radiation to affect the sex ratio of paternal subjects. While this study did not demonstrate a skewed offspring sex ratio among interventional cardiologists, ionizing radiation is still known to be a biological hazard. Cardiologists performing diagnostic angiography and interventional procedures should continue to practice strict safety measures and adhere to the safety guidelines recommended by their institutions and governing bodies.
References
- 1.Davis DL, Gottlieb MB, Stampnitzky JR. Reduced ratio of male to female births in several industrial countries: a sentinel health indicator? JAMA. 1998;279(13):1018–1023. doi: 10.1001/jama.279.13.1018. [DOI] [PubMed] [Google Scholar]
- 2.James WH. Was the widespread decline in sex ratios at birth caused by reproductive hazards? Hum Reprod. 1998;13(4):1083–1084. doi: 10.1093/humrep/13.4.1083. [DOI] [PubMed] [Google Scholar]
- 3.Irgens A, Kruger K, Skorve AH, Irgens LM. Male proportion in offspring of parents exposed to strong static and extremely low-frequency electromagnetic fields in Norway. Am J Ind Med. 1997;32(5):557–561. doi: 10.1002/(sici)1097-0274(199711)32:5<557::aid-ajim19>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Milham S., Jr Unusual sex ratio of births to carbon setter fathers. Am J Ind Med. 1993;23(5):829–831. doi: 10.1002/ajim.4700230516. [DOI] [PubMed] [Google Scholar]
- 5.Saadat M. Offspring sex ratio in men exposed to electromagnetic fields. J Epidemiol Community Health. 2005;59(4):339. doi: 10.1136/jech.2004.025304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen AI, Olsen J, Svane O. Gender-specific reproductive outcome and exposure to high-frequency electromagnetic radiation among physiotherapists. Scand J Work Environ Health. 1991;17(5):324–329. doi: 10.5271/sjweh.1695. [DOI] [PubMed] [Google Scholar]
- 7.Mocarelli P, Gerthoux PM, Ferrari E, Patterson DG, Jr, Kieszak SM, Brambilla P, Vincoli N, Signorini S, Tramacere P, Carreri V, Sampson EJ, Turner WE, Needham LL. Paternal concentrations of dioxin and sex ratio of offspring. Lancet. 2000;355(9218):1858–1863. doi: 10.1016/S0140-6736(00)02290-X. [DOI] [PubMed] [Google Scholar]
- 8.Ryan JJ, Amirova Z, Carrier G. Sex ratios of children of Russian pesticide producers exposed to dioxin. Environ Health Perspect. 2002;110(11):A699–A701. doi: 10.1289/ehp.021100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.del Rio Gomez I, Marshall T, Tsai P, Shao YS, Guo YL. Number of boys born to men exposed to polychlorinated byphenyls. Lancet. 2002;360(9327):143–144. doi: 10.1016/s0140-6736(02)09386-8. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M, Fukuda K, Shimizu T, Andersen CY, Byskov AG. Parental periconceptional smoking and male: female ratio of newborn infants. Lancet. 2002;359(9315):1407–1408. doi: 10.1016/S0140-6736(02)08362-9. [DOI] [PubMed] [Google Scholar]
- 11.Retherford RD. Tobacco smoking and sex ratios in the United States. Soc Biol. 1974;21(1):28–38. doi: 10.1080/19485565.1974.9988087. [DOI] [PubMed] [Google Scholar]
- 12.Figa-Talamanca I, Petrelli G. Reduction in male births among workers exposed to metal fumes. Int J Epidemiol. 2000;29(2):381. doi: 10.1093/ije/29.2.381. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson H, Parker L. Do alcohol and lead change the sex ratio? J Theor Biol. 1994;169(3):313–315. doi: 10.1006/jtbi.1994.1152. [DOI] [PubMed] [Google Scholar]
- 14.Hama Y, Uematsu M, Sakurai Y, Kusano S. Sex ratio in the offspring of male radiologists. Acad Radiol. 2001;8(5):421–424. doi: 10.1016/S1076-6332(03)80550-0. [DOI] [PubMed] [Google Scholar]
- 15.Zadeh HG, Briggs TW. Ionising radiation: are orthopaedic surgeons' offspring at risk? Ann R Coll Surg Engl. 1997;79(3):214–220. [PMC free article] [PubMed] [Google Scholar]
- 16.James WH. The data sources which may help strengthen the epidemiological evidence for the hormonal hypothesis of sex determination in man. Hum Reprod. 2001;16(6):1081–1085. doi: 10.1093/humrep/16.6.1081. [DOI] [PubMed] [Google Scholar]
- 17.Joffe M. Infertility and environmental pollutants. Br Med Bull. 2003;68:47–70. doi: 10.1093/bmb/ldg025. [DOI] [PubMed] [Google Scholar]
- 18.Maconochie N, Roman E, Doyle P, Davies G, Smith PG, Beral V. Sex ratio of nuclear industry employees' children. Lancet. 2001;357(9268):1589–1591. doi: 10.1016/S0140-6736(00)04748-6. [DOI] [PubMed] [Google Scholar]
- 19.Volk B. Evaluating the sex ratio in the offspring of U.S. Navy submariners. Mil Med. 2004;169(11):890–893. doi: 10.7205/milmed.169.11.890. [DOI] [PubMed] [Google Scholar]
- 20.Dickinson HO, Parker L, Binks K, Wakeford R, Smith J. The sex ratio of children in relation to paternal preconceptional radiation dose: a study in Cumbria, northern England. J Epidemiol Community Health. 1996;50(6):645–652. doi: 10.1136/jech.50.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winther JF, Boice JD, Jr, Thomsen BL, Schull WJ, Stovall M, Olsen JH. Sex ratio among offspring of childhood cancer survivors treated with radiotherapy. Br J Cancer. 2003;88(3):382–387. doi: 10.1038/sj.bjc.6600748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guberan E, Campana A, Faval P, Guberan M, Sweetnam PM, Tuyn JW, Usel M. Gender ratio of offspring and exposure to shortwave radiation among female physiotherapists. Scand J Work Environ Health. 1994;20(5):345–348. doi: 10.5271/sjweh.1387. [DOI] [PubMed] [Google Scholar]
- 23.Rehani MM, Ortiz-Lopez P. Radiation effects in fluoroscopically guided cardiac interventions—keeping them under control. Int J Cardiol. 2006;109(2):147–151. doi: 10.1016/j.ijcard.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Andreassi MG. The biological effects of diagnostic cardiac imaging on chronically exposed physicians: the importance of being non-ionizing. Cardiovasc Ultrasound. 2004;2:25. doi: 10.1186/1476-7120-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vano E. Radiation exposure to cardiologists: how it could be reduced. Heart. 2003;89(10):1123–1124. doi: 10.1136/heart.89.10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zorzetto M, Bernardi G, Morocutti G, Fontanelli A. Radiation exposure to patients and operators during diagnostic catheterization and coronary angioplasty. Cathet Cardiovasc Diagn. 1997;40(4):348–351. doi: 10.1002/(sici)1097-0304(199704)40:4<348::aid-ccd4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 27.Delichas M, Psarrakos K, Molyvda-Athanassopoulou E, Giannoglou G, Sioundas A, Hatziioannou K, Papanastassiou E. Radiation exposure to cardiologists performing interventional cardiology procedures. Eur J Radiol. 2003;48(3):268–273. doi: 10.1016/s0720-048x(03)00007-x. [DOI] [PubMed] [Google Scholar]
- 28.Renaud L. A 5-y follow-up of the radiation exposure to in-room personnel during cardiac catheterization. Health Phys. 1992;62(1):10–15. doi: 10.1097/00004032-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Finci L, Meier B, Steffenino G, Roy P, Rutishauser W. Radiation exposure during diagnostic catheterization and single- and double-vessel percutaneous transluminal coronary angioplasty. Am J Cardiol. 1987;60(16):1401–1403. doi: 10.1016/0002-9149(87)90630-8. [DOI] [PubMed] [Google Scholar]
- 30.Vano E, Gonzalez L, Guibelalde E, Fernandez JM, Ten JI. Radiation exposure to medical staff in interventional and cardiac radiology. Br J Radiol. 1998;71(849):954–960. doi: 10.1259/bjr.71.849.10195011. [DOI] [PubMed] [Google Scholar]
- 31.Limacher MC, Douglas PS, Germano G, Laskey WK, Lindsay BD, McKetty MH, Moore ME, Park JK, Prigent FM, Walsh MN, American College of Cardiology ACC expert consensus document. Radiation safety in the practice of cardiology. J Am Coll Cardiol. 1998;31(4):892–913. doi: 10.1016/s0735-1097(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 32.Pitney MR, Allan RM, Giles RW, McLean D, McCredie M, Randell T, Walsh WF. Modifying fluoroscopic views reduces operator radiation exposure during coronary angioplasty. J Am Coll Cardiol. 1994;24(7):1660–1663. doi: 10.1016/0735-1097(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 33.Dash H, Leaman DM. Operator radiation exposure during percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1984;4(4):725–728. doi: 10.1016/s0735-1097(84)80398-8. [DOI] [PubMed] [Google Scholar]
- 34.Mathews TJ, Hamilton BE. Trend analysis of the sex ratio at birth in the United States. Natl Vital Stat Rep. 2005;53(20):1–17. [PubMed] [Google Scholar]
- 35.Kamiguchi Y, Tateno H. Radiation- and chemical-induced structural chromosome aberrations in human spermatozoa. Mutat Res. 2002;504(1–2):183–191. doi: 10.1016/s0027-5107(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 36.Subcommittee on Reproductive and Neurodevelopmental Toxicology. Committee on Biologic Markers. Board on Environmental Studies and Toxicology. Commission on Life Sciences. National Research Council . Biological Markers in Reproductive Technology. Washington, DC: National Academy Press; 1989. [Google Scholar]
- 37.Sinclair WK. Radiation protection recommendations on dose limits: the role of the NCRP and the ICRP and future developments. Int J Radiat Oncol Biol Phys. 1995;31(2):387–392. doi: 10.1016/0360-3016(94)00275-p. [DOI] [PubMed] [Google Scholar]
- 38.International Commission on Radiological Protection . 1990 Recommendations of the International Commission on Radiological Protection (Publication 60, Annals of the ICRP) Oxford, UK: Pergamon Press; 1991. [PubMed] [Google Scholar]
- 39.Al-Shakhrah IA, Abu-Khaled YS. Estimation of effective radiation dose for physicians and staff members in contrast angiocardiography. Heart Lung. 2000;29(6):417–423. doi: 10.1067/mhl.2000.109696. [DOI] [PubMed] [Google Scholar]