Abstract
Metastatic clear cell renal cell cancer has traditionally been treated with cytokines (interferon or interleukin-2). Improved understanding of biology has engendered novel targeted therapeutic agents that have altered the natural history of this disease. The vascular endothelial growth factor and its related receptor and the mTOR signal transduction pathway have particularly been exploited. Sunitinib malate, sorafenib tosylate, temsirolimus, and bevacizumab have improved clinical outcomes in randomized trials. Other multitargeted tyrosine kinase inhibitors (lapatinib, axitinib, pazopanib) and antiangiogenic agents (VEGF Trap, lenalidomide) have also demonstrated activity in early studies. Combinations of these agents are being evaluated. The future of the therapy of renal cancer appears promising owing to the efficacy of these novel agents.
In the USA, 38,890 new cases and 12,840 deaths were predicted to occur from renal cell cancer (RCC) in 2006 (1). Although surgery is a potential cure for patients with early stage disease, many patients experience recurrence after surgery or have metastatic disease at the time of diagnosis. Few treatment options have been available for these patients. RCC, a highly vascular disease, is resistant to chemotherapy, with no single agent showing significant antitumor activity (2, 3). Observations of late relapses after nephrectomy, prolonged stabilization of disease in the absence of therapy, and a small number of spontaneous remissions led to trials exploring immunotherapy. Interferon alfa (IFN α) and interleukin-2 (IL-2) have been widely utilized as treatment for metastatic RCC. However, the majority of patients do not benefit from IFN α or IL-2, and the few responses seen are not durable, with only about 10% of patients remaining progression free at 3 years (4, 5).
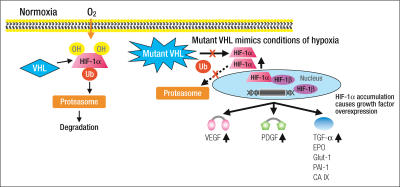
Angiogenesis, the process of new blood vessel formation, is known to play a critical role in the growth of tumors and is believed to be an important target for the development of novel anticancer therapies (6). Recent work in understanding the von Hippel-Lindau (VHL) gene has demonstrated a role for angiogenesis in the pathophysiology of RCC. Clear cell RCC is characterized by its frequent loss of the VHL tumor suppressor gene. VHL normally encodes a protein (p-VHL) that targets hypoxia-inducible factor (HIF) for proteolysis (Figure) (7–12). As a result of VHL inactivation, a defective p-VHL is produced, and HIF is up-regulated (13). Activated HIF then translocates into the nucleus and leads to the transcription of several genes that play a central role in tumorigenesis. These genes include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor-α, and basic fibroblast growth factor (14–20). Angiogenesis is stimulated, in part, by VEGF binding its receptor (VEGFR), and this pathway has been heavily exploited for the therapy of RCC (21). Tumor angiogenesis is also stimulated by growth factors through the phosphatidylinositol-3 kinase PI3K-AKT-mTOR signal transduction pathway (22, 23). Agents targeting this pathway can also be expected to have antitumor activity.
Figure.
Consequences of mutation or inactivation of the von Hippel Lindau (VHL) gene. VHL normally encodes a protein (p-VHL) that targets hypoxia-inducible factor (HIF) for proteolysis. As a result of VHL inactivation, a defective p-VHL is produced and HIF is up-regulated, translocates to the nucleus, and results in the transcription of several genes involved in angiogenesis and tumor growth. These genes include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor (TGF)-α, basic fibroblast growth factor (bFGF), carbonic anhydrase IX (CA IX) or G250, erythropoietin (EPO), and others. OH indicates hydroxyl group; Ub, ubiquitin; Glut-1, glucose transporter 1; PAI-1, plasminogen activator inhibitor 1. Adapted from Kim WY. J Clin Oncol 2004;22:4991–5004.
Based upon an improved understanding of the molecular biology of clear cell RCC, progress has been made in the development and use of targeted agents in patients with metastatic disease (Table 1). VEGF, its related receptor VEGFR, and the mTOR signal transduction pathway have particularly been exploited. Sunitinib malate (24, 30, 31), sorafenib tosylate (25, 26), and temsirolimus (29) have improved clinical outcomes in randomized trials, resulting in the first new drug approvals for treatment of advanced RCC in almost 2 decades. Large multi-institutional trials evaluating the use of these agents as adjuncts to surgery in patients with locally advanced RCC are ongoing. This review provides an update on these novel agents as therapy for metastatic clear cell RCC.
Table 1.
Reported randomized trials of targeted agents in renal cell cancer
| Trial (ref) | Eligibility | N | Experimental arm | Control arm | Median PFS | Median OS |
| Motzer et al (24) | First-line, good and intermediate risk | 750 | Sunitinib | IFN α | 11 vs5 mo∗ | NA |
| Escudier et al (25) | Second-line, good and intermediate risk | 905 | Sorafenib | Placebo | 24 vs12 wks∗ | 19.3 vs 15.9 mo∗ |
| Escudier et al (26) | First-line, good risk | 189 (II) | Sorafenib | IFN α | NA | NA |
| Yang et al(27) | Second-line, IL-2 refractory | 116 (II) | Bevacizumab (high-dose)† | Placebo | 4.8 vs 2.5 mo∗ | NA |
| Bukowski et al (28) | First-line | 104 (II) | Bevacizumab + erlotinib | Bevacizumab | 9.9 vs 8.5 mo | NA |
| Hudes et al (29) | First-line, poor risk | 626 | Temsirolimus‡ | IFN α | 3.7 vs 1.9 mo∗ | 10.9 vs 7.3 mo∗ |
∗Statistically significant.
†Study contained a low-dose bevacizumab arm that did not extend PFS.
‡Study contained another arm of temsirolimus + IFN that did not extend survival.
Ref indicates reference; N, number of patients; PFS, progression-free survival; OS, overall survival; IFN, interferon; mo, months; NA, not available; II, randomized phase II trial; EGFR, epidermal growth factor receptor.
SUNITINIB
Sunitinib malate (Sutent; Pfizer Inc., New York, NY) is a highly potent, selective inhibitor of multiple receptor tyrosine kinases, including VEGFR-1, VEGFR-2, VEGFR-3, PDGFRα, PDGFR-β, c-kit, and Flt-3. Preclinical data suggest that sunitinib has antitumor activity that may result from both inhibition of angiogenesis and direct antiproliferative effects (32, 33). The recommended dose was defined in phase I trials as 50 mg orally once daily for 4 weeks, followed by 2 weeks off, in repeated 6-week cycles (34, 35). Dose-limiting toxicities included fatigue, gastrointestinal toxicity, cytopenias, and skin toxicity, which were reversible upon discontinuation of treatment.
Two phase II trials have been reported in patients with metastatic RCC progressing after prior therapy with IFN α or IL-2 (30, 31). A pooled analysis of prognostic features for response and progression-free survival (PFS) based on investigator assessment was performed on 168 evaluable patients. Patients' median age was 57 years, they had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1, and nearly all had undergone nephrectomy. Overall, 71 patients (42%) responded; 40 (24%) had stable disease of 3 or more months' duration; and 57 (34%) had stable disease for <3 months, had progressive disease, or were not evaluable. The median PFS for all 168 patients was 8.2 months and for responding patients, 14.8 months. A longer PFS was observed in patients with favorable ECOG performance status and normal serum hemoglobin levels; the multivariate analysis demonstrated low hemoglobin to be an independent predictor of a shorter PFS. Sunitinib was tolerable, and severe toxicities (grade 3–4) seldom occurred. Fatigue was a common treatment-related adverse event (approximately 10% reported grade 3 fatigue), and hypothyroidism has been recently recognized as a probable cause of fatigue in some patients. Approximately 5% of patients experienced a reversible decline in left ventricular ejection fraction without development of clinical congestive heart failure.
To further evaluate the efficacy of sunitinib in previously untreated patients with metastatic RCC, a large multinational phase III trial was conducted (24). A total of 750 patients with good- and intermediate-risk clear cell RCC were randomized to receive either sunitinib or IFN α. To qualify for the study, patients were required to have good performance status and measurable disease. The primary endpoint was PFS, with secondary endpoints of patient-related outcomes, overall survival, response rate, and safety. Stratification factors were lactate dehydrogenase levels, performance status, and nephrectomy status. Patients were randomized 1:1 to receive oral sunitinib 50 mg/day for 4 weeks followed by a 2-week off period (6-week cycles) or IFN α 9 MU (million units) subcutaneously 3 times weekly (3 MU per dose the first week and 6 MU per dose the second week). The treatment arms were well balanced; patients had a median age of 60 years, and 90% had undergone prior nephrectomy. Approximately 35% had good-risk disease, 58% had intermediate-risk disease, and 7% had poor-risk disease.
The median PFS was 11 months for the sunitinib arm and 5 months for the IFN α arm (hazard ratio [HR] = 0.415, P < 0.000001) (Table 1). The objective response rate assessed by independent review was 31% vs 6% (P < 0.000001). There was only one complete response, and this occurred in the sunitinib arm. Severe adverse events (grade 3–4 toxicities) were acceptable, with neutropenia (12%), thrombocytopenia (8%), hyperamylasemia (5%), diarrhea (5%), hand-foot syndrome (5%), and hypertension (8%) being noteworthy in the sunitinib arm and fatigue more common with IFN α (12% vs 7%). Adverse events leading to withdrawal from the study occurred in 8% of patients on sunitinib and 13% on IFN α. Although survival endpoints are still premature, the HR was 0.65 (P < 0.02) in favor of sunitinib. Based upon the highly statistically significant improvement in PFS and its tolerability, sunitinib should be considered the new standard of care for the first-line treatment of good- and intermediate-risk metastatic clear cell RCC. Studies combining sunitinib with IFN α, bevacizumab, and temsirolimus are ongoing.
SORAFENIB
Sorafenib tosylate (Nexavar; Bayer Pharmaceuticals, West Haven, CT, and Onyx Pharmaceuticals, Richmond, CA) is an oral, bi-aryl urea molecule that was designed as a c-Raf and b-Raf kinase inhibitor but was also found to inhibit several receptor tyrosine kinases, including VEGFR-1, VEGFR-2, VEGFR-3, PDGFR-β, Flt3, and c-kit (36–40). A phase II randomized discontinuation trial evaluated the effects of sorafenib in patients with a variety of cancers, including 202 patients with RCC (41). Patients received oral sorafenib 400 mg twice daily during the initial run-in period. After 12 weeks, patients with changes in bidimensional tumor measurements that were <25% were randomized to sorafenib or placebo for an additional 12 weeks; patients with 25% tumor shrinkage continued open-label sorafenib; patients with 25% tumor growth discontinued treatment. The primary endpoint was PFS at 24 weeks after the initiation of sorafenib. Seventy-three patients had tumor shrinkage of 25%. Sixty-five patients with stable disease at 12 weeks were randomly assigned to sorafenib or placebo. At 24 weeks, 50% of the sorafenib-treated patients were progression free vs 18% of the placebo-treated patients (P = 0.0077). Median PFS from randomization was significantly longer with sorafenib than placebo (24 vs 6 weeks; P = 0.0087). Median overall PFS was 29 weeks for the entire study population.
These encouraging results led to a phase III placebo-controlled randomized trial, known as TARGET (Treatment Approaches in RCC Global Evaluation Trial) (25). Nine hundred and five patients who had failed one prior systemic therapy in the last 8 months and had measurable disease, clear cell histology, a good or intermediate prognosis, and an ECOG performance status of 0 to 1 were entered in this largest randomized trial of advanced RCC conducted to date (Table 1). Almost all patients had undergone nephrectomy. The primary aim of the trial was to assess overall survival, and the secondary endpoint was PFS. In a preliminary report, tumor control (stable disease or partial response) with sorafenib was achieved in 80% of patients, although only 2% attained a partial response. Sorafenib significantly prolonged median PFS compared with placebo (24 vs 12 weeks, P < 0.000001), and median survival improvement was preliminarily reported (19.3 vs 15.9 months, HR = 0.77, P = 0.015). Benefit was evident across all subsets evaluated.
Crossover from the placebo to the sorafenib arm has been allowed owing to the magnitude of effect on PFS. The patients who crossed over to sorafenib also demonstrated a 30% improvement in survival (42). With the placebo arm censored at the time of crossover, median survival was 19.3 months for sorafenib vs 14.3 months for placebo (HR = 0.74, P = 0.01). Adverse effects were manageable with grade 3 to 4 hand-foot syndrome, fatigue, and hypertension observed in 5%, 2%, and 1% of patients, respectively. A randomized phase II trial of sorafenib vs IFN α in previously untreated patients has been conducted, and results are awaited (26). Further investigation of sorafenib combined with a variety of other agents is being pursued.
TEMSIROLIMUS
Temsirolimus (Torisel; Wyeth Pharmaceuticals, Philadelphia, PA), an intravenously administered mTOR inhibitor, regulates nutritional needs, cell growth, and angiogenesis by down-regulating or up-regulating a variety of proteins, including HIF-1 (43). A randomized trial was designed for the first-line therapy of RCC patients with ≥3 of 6 poor-risk features (4 from the Memorial Sloan-Kettering Cancer Center criteria excluding nephrectomy and including >1 metastatic site and time from diagnosis to therapy <1 year). The primary endpoint was survival (29). Other notable eligibility criteria were Karnofsky Performance Status ≥60, measurable disease, and acceptable lipid levels (fasting cholesterol ≤350 mg/dL, triglycerides ≤400 mg/dL). The trial randomized 626 patients in a 1:1:1 ratio to three arms: IFN α up to 18 MU subcutaneously 3 times weekly, temsirolimus 25 mg/week intravenously, or a combination of temsirolimus 15 mg/week plus IFN α 6 MU 3 times weekly. The arms were well balanced, and approximately 70% were poor risk, 80% had predominantly clear cell histology, and nephrectomy had been performed in 67% of patients.
The median survival of patients was superior in the temsirolimus-alone arm compared with IFN α (10.9 vs 7.3 months, HR = 0.73, P < 0.007), while the combination arm was not superior to IFN α (8.4 months, HR = 0.95, P = 0.69). The median PFS was statistically superior for both temsirolimus arms (3.7 months) compared with IFN α (1.9 months) (Table 1). Objective responses occurred in 7%, 9%, and 11% of patients in the IFN α, temsirolimus, and combination arms, respectively.
Any toxicity of grade 3 or higher was seen in 69% of patients on temsirolimus vs 85% to 87% of patients on the IFN-containing arms (P < 0.001). Grade 3 toxicity was primarily asthenia, which occurred less frequently in the temsirolimus arm (12%) than in the IFN α arms (approximately 30%). Rash was more common in the temsirolimus arm (37% vs 5% on IFN α and 16% on the combination). Adverse events leading to withdrawal from the study occurred in 7%, 14%, and 22% of patients on temsirolimus, IFN α, and the combination arms, respectively. The investigators concluded that temsirolimus should be considered a standard in poor-risk clear cell RCC, and a potential benefit in a broader population may exist. Combination with VEGF targeted therapy is being evaluated.
BEVACIZUMAB
Bevacizumab (Avastin; Genentech, San Francisco, CA) is a recombinant monoclonal antibody that binds and neutralizes circulating VEGF-A and has demonstrated activity against metastatic RCC in several clinical trials. In a phase II randomized trial, 116 patients with metastatic RCC refractory to IL-2 were randomized to receive either bevacizumab at two different dose levels (10 mg/kg or 3 mg/kg every 2 weeks) or placebo (27). Bevacizumab 10 mg/kg yielded a 10% response rate (with several patients having prolonged periods of stability or minor responses) and led to a PFS of 4.8 months vs 2.5 months with placebo (Table 1). No difference in PFS was seen with low-dose bevacizumab. Hypertension was observed in 36% of patients in the high-dose bevacizumab arm, 3% in the lower-dose arm, and 5% in the placebo arm. Proteinuria (without renal dysfunction) and epistaxis were also significantly higher in the high-dose bevacizumab group.
Subsequently, a multicenter 63-patient phase II study combined bevacizumab 10 mg/kg intravenously every 2 weeks with an oral EGF receptor tyrosine kinase inhibitor, erlotinib 150 mg orally daily (44). Eligibility criteria for this study included metastatic RCC (at least 75% clear cell component) and no more than one prior treatment regimen. Twenty-five percent of 59 assessable patients had objective responses (one complete response, 14 partial responses). The median PFS was 11 months, and the median survival was >20 months. Despite these encouraging results, a recently reported randomized phase II 104-patient study suggests that the addition of erlotinib to bevacizumab does not improve outcomes (PFS approximately 9 months, response rate approximately 14%) compared with bevacizumab alone (28).
Two randomized phase III trials (CALGB-90206 and Roche B017705) of bevacizumab with or without IFN α in previously untreated patients with metastatic RCC have completed accrual, and the results are anxiously awaited. Combination studies of bevacizumab with sunitinib, sorafenib, mTOR inhibitors (temsirolimus and everolimus), and IL-2 (high or low dose) are in progress.
CONCLUSION
The availability of rationally designed, targeted therapy for metastatic RCC has significantly impacted the natural history of this disease. Sunitinib, sorafenib, temsirolimus, and bevacizumab have demonstrated significant improvements in response rates, PFS, and overall survival (temsirolimus) with manageable side effects. A variety of other novel targeted agents are currently under development for the treatment of metastatic RCC, including mTOR inhibitors (everolimus [RAD001]), VEGF ligand inhibitors (VEGF Trap), and tyrosine kinase inhibitors (axitinib and pazopanib) (Table 2).
Table 2.
Targeted agents in development for renal cell cancer
| Agent | Mechanism of action | Manufacturer | Status |
| Everolimus | mTOR inhibitor | Novartis | Phase III |
| Lenalidomide | Imid angiogenesis inhibitor | Celgene | Phase II |
| Pazopanib | VEGFR, PDGFR, and c-kit tyrosine kinase inhibitor | GSK | Phase III |
| Axitinib | VEGFR and PDGFR tyrosine kinase inhibitor | Pfizer | Phase II |
| Lapatinib | EGFR and erb-2 tyrosine kinase inhibitor | GSK | Phase II |
| Vatalanib | VEGFR, PDGFR, and c-kit tyrosine kinase inhibitor | Novartis | Phase II |
| VEGF Trap | VEGF ligand inhibitor | Various | Phase II |
VEGFR indicates vascular endothelial growth factor receptor; PDGFR, platelet-derived growth factor receptor; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor.
Given the availability of multiple treatment options, several questions emerge as to how to integrate these new therapies into the management of metastatic RCC: Is there an optimal sequence or combination of therapies? Is there a current role for cytokine therapy in RCC? What is the level of activity of these agents in non–clear cell RCC? What is the future role of cytoreductive nephrectomy? Most importantly, can cure rates be improved with use of these agents earlier in the disease (neoadjuvant or adjuvant therapy)? Ongoing and planned clinical trials hope to provide insight into many of these questions.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56(2):106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Yagoda A, Abi-Rached B, Petrylak D. Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Semin Oncol. 1995;22(1):42–60. [PubMed] [Google Scholar]
- 3.Motzer RJ, Vogelzang NJ. Chemotherapy for renal cell carcinoma. In: Raghaven D, Scher HI, Leibel SA, Lang P, editors. Principles and Practice of Genitourinary Oncology. Philadelphia: Lippincott-Raven; 1997. pp. 885–896. [Google Scholar]
- 4.McDermott DF, Atkins MB. Application of IL-2 and other cytokines in renal cancer. Expert Opin Biol Ther. 2004;4(4):455–468. doi: 10.1517/14712598.4.4.455. [DOI] [PubMed] [Google Scholar]
- 5.Hutson TE, Quinn DI. Cytokine therapy: a standard of care for metastatic renal cell carcinoma? Clin Genitourin Cancer. 2005;4(3):181–186. doi: 10.3816/CGC.2005.n.030. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 7.Schraml P, Struckmann K, Hatz F, Sonnet S, Kully C, Gasser T, Sauter G, Mihatsch MJ, Moch H. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol. 2002;196(2):186–193. doi: 10.1002/path.1034. [DOI] [PubMed] [Google Scholar]
- 8.Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER. Inactivation of the von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosomes Cancer. 1998;22(3):200–209. doi: 10.1002/(sici)1098-2264(199807)22:3<200::aid-gcc5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994;91(21):9700–9704. doi: 10.1073/pnas.91.21.9700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kibel A, Iliopoulos O, DeCaprio JA, Kaelin WG., Jr Binding of the von Hippel-Lindau tumor suppressor protein to Elongin B and C. Science. 1995;269(5229):1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 11.Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1α and HIF-2α under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19(48):5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 12.Gallou C, Joly D, Mejean A, Staroz F, Martin N, Tarlet G, Orfanelli MT, Bouvier R, Droz D, Chretien Y, Marechal JM, Richard S, Junien C, Beroud C. Mutations of the VHL gene in sporadic renal cell carcinoma: definition of a risk factor for VHL patients to develop an RCC. Hum Mutat. 1999;13(6):464–475. doi: 10.1002/(SICI)1098-1004(1999)13:6<464::AID-HUMU6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 13.Gnarra JR, Zhou S, Merrill MJ, Wagner JR, Krumm A, Papavassiliou E, Oldfield EH, Klausner RD, Linehan WM. Post-transcriptional regulation of vascular endothelial growth factor mRNA by the product of the VHL tumor suppressor gene. Proc Natl Acad Sci U S A. 1996;93(20):10589–10594. doi: 10.1073/pnas.93.20.10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23(5):1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 15.Kourembanas S, Hannan RL, Faller DV. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Paulsen N, Brychzy A, Fournier MC, Klausner RD, Gnarra JR, Pause A, Lee S. Role of transforming growth factor-alpha in von Hippel-Lindau (VHL)–/– clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci U S A. 2001;98(4):1387–1392. doi: 10.1073/pnas.031587498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwabara K, Ogawa S, Matsumoto M, Koga S, Clauss M, Pinsky DJ, Lyn P, Leavy J, Witte L, Joseph-Silverstein J, et al. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci U S A. 1995;92(10):4606–4610. doi: 10.1073/pnas.92.10.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grabmaier KA, de Weijert MC, Verhaegh GW, Schalken JA, Oosterwijk E. Strict regulation of CAIX(G250/MN) by HIF-1α in clear cell renal cell carcinoma. Oncogene. 2004;23(33):5624–5631. doi: 10.1038/sj.onc.1207764. [DOI] [PubMed] [Google Scholar]
- 19.Lee YS, Vortmeyer AO, Lubensky IA, Vogel TW, Ikejiri B, Ferlicot S, Benoit G, Giraud S, Oldfield EH, Linehan WM, Teh BT, Richard S, Zhuang Z. Coexpression of erythropoietin and erythropoietin receptor in von Hippel-Lindau disease-associated renal cysts and renal cell carcinoma. Clin Cancer Res. 2005;11(3):1059–1064. [PubMed] [Google Scholar]
- 20.Giordano FJ, Johnson RS. Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev. 2001;11(1):35–40. doi: 10.1016/s0959-437x(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 21.Goodsell DS. The molecular perspective: VEGF and angiogenesis. Stem Cells. 2003;21(1):118–119. doi: 10.1634/stemcells.21-1-118. [DOI] [PubMed] [Google Scholar]
- 22.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22(20):7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mita MM, Mita A, Rowinsky EK. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003;2(4 Suppl 1):S169–S177. [PubMed] [Google Scholar]
- 24.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 25.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Szczylik C, Demkow T, Staehler M, Rolland F, Negrier S, Hutson TE, Scheuring UJ, Schwartz B, Bukowski RM. Randomized phase II trial of the multi-kinase inhibitor sorafenib versus interferon (IFN) in treatment-naïve patients with metastatic renal cell carcinoma (mRCC). 2006 ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24(18S):4501. [Google Scholar]
- 27.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349(5):427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bukowski RM, Kabbinavar F, Figlin RA, Flaherty K, Srinivas S, Vaishampayan U, Drabkin H, Dutcher J, Scappaticci F, McDermott D. Bevacizumab with or without erlotinib in metastatic renal cell carcinoma (RCC). 2006 ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24(18S):4523. [Google Scholar]
- 29.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, O'toole T, Park Y, Moore L. A phase 3, randomized, 3-arm study of temsirolimus (TEMSR) or interferon-alpha (IFN) or the combination of TEMSR + IFN in the treatment of first-line, poor-risk patients with advanced renal cell carcinoma (adv RCC). 2006 ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24(18S):LBA4. [Google Scholar]
- 30.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 31.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, Redman BG, Margolin KA, Merchan JR, Wilding G, Ginsberg MS, Bacik J, Kim ST, Baum CM, Michaelson MD. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295(21):2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 32.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, Murray LJ, Carver J, Chan E, Moss KG, Haznedar JO, Sukbuntherng J, Blake RA, Sun L, Tang C, Miller T, Shirazian S, McMahon G, Cherrington JM. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. [PubMed] [Google Scholar]
- 33.Chow LQ, Eckhardt SG. Sunitinib: from rational design to clinical efficacy. J Clin Oncol. 2007;25(7):884–896. doi: 10.1200/JCO.2006.06.3602. [DOI] [PubMed] [Google Scholar]
- 34.Faivre S, Delbaldo C, Vera K, Robert C, Lozahic S, Lassau N, Bello C, Deprimo S, Brega N, Massimini G, Armand JP, Scigalla P, Raymond E. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 35.Fiedler W, Serve H, Dohner H, Schwittay M, Ottmann OG, O'Farrell AM, Bello CL, Allred R, Manning WC, Cherrington JM, Louie SG, Hong W, Brega NM, Massimini G, Scigalla P, Berdel WE, Hossfeld DK. A phase 1 study of SU11248 in the treatment of patients with refractory or resistant acute myeloid leukemia (AML) or not amenable to conventional therapy for the disease. Blood. 2005;105(3):986–993. doi: 10.1182/blood-2004-05-1846. [DOI] [PubMed] [Google Scholar]
- 36.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64(19):7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 37.Strumberg D, Richly H, Hilger RA, Schleucher N, Korfee S, Tewes M, Faghih M, Brendel E, Voliotis D, Haase CG, Schwartz B, Awada A, Voigtmann R, Scheulen ME, Seeber S. Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23(5):965–972. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 38.Moore M, Hirte HW, Siu L, Oza A, Hotte SJ, Petrenciuc O, Cihon F, Lathia C, Schwartz B. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16(10):1688–1694. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 39.Awada A, Hendlisz A, Gil T, Bartholomeus S, Mano M, de Valeriola D, Strumberg D, Brendel E, Haase CG, Schwartz B, Piccart M. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92(10):1855–1861. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clark JW, Eder JP, Ryan D, Lathia C, Lenz HJ. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11(15):5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 41.Ratain MJ, Eisen T, Stadler WM, Flaherty KT, Kaye SB, Rosner GL, Gore M, Desai AA, Patnaik A, Xiong HQ, Rowinsky E, Abbruzzese JL, Xia C, Simantov R, Schwartz B, O'Dwyer PJ. Phase II placebo-controlled randomized discontinuation trial of sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(16):2505–2512. doi: 10.1200/JCO.2005.03.6723. [DOI] [PubMed] [Google Scholar]
- 42.Eisen T, Bukowski RM, Staehler M, Szczylik C, Oudard S, Stadler WM, Schwartz B, Simantov R, Shan M, Escudier B, Sorafenib TARGETs Clinical Trial Group Randomized phase III trial of sorafenib in advanced renal cell carcinoma (RCC): impact of crossover on survival. 2006 ASCO Annual Meeting Proceedings. J Clin Oncol. 2006;24(18S):4524. [Google Scholar]
- 43.Gibbons JJ, Discafani C, Peterson R. The effect of CCI-779, a novel macrolide anti-tumor agent, on growth of human tumor cells in vitro and in nude mouse xenografts in vivo [abstract 1000] Proc Am Assoc Cancer Res. 1999;40:301. [Google Scholar]
- 44.Hainsworth JD, Sosman JA, Spigel DR, Edwards DL, Baughman C, Greco A. Treatment of metastatic renal cell carcinoma with a combination of bevacizumab and erlotinib. J Clin Oncol. 2005;23(31):7889–7896. doi: 10.1200/JCO.2005.01.8234. [DOI] [PubMed] [Google Scholar]