Abstract
An experiment was conducted on Catharanthus roseus to study the effect of seed treatments with native diazotrophs on its seedling growth and antioxidant enzyme activities. The treatments had significant influence on various seedling parameters. There is no significant influence on dry matter production with the diazotrophs, Azospirillum and Azotobacter. However, the vital seedling parameters such as germination percentage and vigour index were improved. Azotobacter treatment influenced maximum of 50% germination, whereas Azospirillum and Azotobacter were on par with C. roseus with respect to their vigour index. There was significant difference in the population of total diazotrophs. Azospirillum and Azotobacter between rhizosphere and non-rhizosphere soils of C. roseus had the same trend and were observed at various locations of the study. The activities of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POX) and catalase (CAT) were increased to a significant extent due to the treatment with diazotrophs.
Keywords: Rhizosphere, Non-rhizosphere, Azospirillum, Azotobacter, Antioxidant enzyme, Catharanthus roseus
INTRODUCTION
The strong and rapidly stimulating effect of fungal elicitor on plant secondary metabolism in medicinal plants attracts considerable attention (Zhao et al., 2005). The reasons responsible for the diverse stimulating effects of fungal elicitors are complicated and could be related to the interactions between fungal elicitors and plant cells, elicitor signal transduction, and plant defense responses (Nurnberger et al., 1994; Somssich and Hahlbrock, 1998). Certain secondary metabolite pathways in plants are induced by infection with microorganisms (Verpoorte et al., 2002). Diazotrophic rhizobiocoenosis is an important biological process that plays a major role in satisfying the nutritional requirements of plants. Studies on the diazotrophic population in the rhizosphere region and testing the suitability of the isolated diazotroph as seed inoculant will be highly useful in improving the productivity of commercially important medicinal plants. Diazotrophs secrete plant growth hormones such as auxins, gibberellins and cytokinins (Deka et al., 1992).
Plants are equipped with oxygen radical detoxifying enzymes such as superoxide dismutase (SOD), peroxidase (POX), catalase (CAT) and antioxidant molecules like ascorbic acid and reduced glutathione in order to survive under stress conditions (Prochazkova et al., 2001). Generation of reactive oxygen species (ROS) such as superoxide, H2O2 and hydroxyl molecules causes rapid cell damage by triggering off a chain reaction (Imlay, 2003). Plants under stress produce some defense mechanisms to protect themselves from the harmful effect of oxidative stress. ROS scavenging is one among the common defense responses against abiotic stresses (Vranova et al., 2002). ROS scavenging depends on the detoxification mechanism provided by an integrated system of non-enzymatically reduced molecules like ascorbate and glutathione and enzymatic antioxidants (Prochazkova et al., 2001).
Catharanthus roseus (L.) G. Don. (Madagascar periwinkle) is a perennial tropical plant belonging to the family Apocynaceae that produces more than 100 monoterpenoid indole alkaloids (MIAs) including two commercially important cytotoxic dimeric alkaloids used in cancer chemotherapy (Magnotta et al., 2006). Roots of this plant are the main source of an anti-hypertension alkaloid ajmalicine (Jaleel et al., 2006). It is also a popular ornamental plant. There are commonly two varieties in this plant based on the flower colour viz., pink flowered rosea and white flowered alba (Jaleel et al., 2007). All parts of the plant are rich in alkaloids, with maximum concentrations found in the root bark, particularly during flowering. An infusion of the leaves is used to treat menorrhagia. The juice of the leaves is applied externally to relieve wasp stings. All parts of the plant have hypoglycaemic properties and are used to treat diabetes (Kar et al., 2003). The major practical problem in the cultivation of C. roseus is the poor germination percentage at field level. To the best of our knowledge, no information on the germination, seedling vigour and antioxidant potentials of C. roseus under diazotrophs treatment is available. The purpose of this study was to provide additional information on the germination and seedling vigour and enzymatic (SOD, POX and CAT) antioxidant constituents of C. roseus under seed priming with native diazotrophs such as Azospirillum and Azotobacter.
MATERIALS AND METHODS
Seed collection and diazotrophs isolation
The seeds of Catharanthus roseus (L.) G. Don. were collected from Jaya Priya Laboratories, Rajapalayam, Tamilnadu, India. The rhizosphere and non-rhizosphere soil samples of C. roseus were collected from three locations viz., horticultural farm of Annamalai University, microbiological potculture yard of Annamalai University and Horticultural Research Station, Pondicherry, Tamil Nadu, India with the samples being denoted as AHF, AMG and HRS. The population of the total diazotrophs was estimated as suggested by Watanabe and Barraquio (1979). Diazotrophic Azospirillum are isolated from the surface sterilized roots of C. roseus and the free-living diazotrophic Azotobacter was isolated from the rhizosphere soil samples of C. roseus.
Germination and seedling vigour
To find out the effect of these isolated diazotrophic Azospirillum and Azotobacter on the germination and vigour index of C. roseus, 100 seeds were taken in a sterile petriplate and treated with 10 ml broth culture of (with an initial population 107 cells/ml) Azospirillum and Azotobacter as separate treatments. The seeds were treated for 30 min and then shade dried. Then, these inoculated seeds were tested for the germination rate using paper towel method (ISTA, 1976). The germination percentage was calculated from 8 days after sowing (DAS) to 12 DAS. The morphological parameters like shoot length and root length were measured on 20 DAS. The vigour index (VI) of the seedlings was estimated as suggested by Abdul-Baki and Anderson (1973):
| VI=RL+SL×GP, |
where RL is root length (cm), SL is shoot length (cm) and GP is germination percentage.
Antioxidant enzyme extractions and assays
1. Superoxide dismutase (SOD, EC 1.15.1.1)
The activity of SOD was assayed as described by Beauchamp and Fridovich (1971). The reaction mixture contained 1.17×10−6 mol/L riboflavin, 0.1 mol/L methionine, 2×10−5 mol/L KCN and 5.6×10−5 mol/L nitroblue tetrazolium (NBT) salt dissolved in 3 ml of 0.05 mol/L sodium phosphate buffer (pH 7.8). Three millilitres of the reaction medium was added to 1 ml of enzyme extract. The mixtures were illuminated in glass test tubes by two sets of Philips 40 W fluorescent tubes in a single row. Illumination was started to initiate the reaction at 30 °C for 1 h. Identical solutions that were kept under dark served as blanks. The absorbance was read at 560 nm in the spectrophotometer against the blank. SOD activity is expressed in U/(mg protein). One U is defined as the change in 0.1 absorbance per hour per mg protein.
2. Peroxidase (POX, EC 1.11.1.7)
POX was assayed by the method of Kumar and Khan (1982). Assay mixture of POX contained 2 ml of 0.1 mol/L phosphate buffer (pH 6.8), 1 ml of 0.01 mol/L pyrogallol, 1 ml of 0.005 mol/L H2O2 and 0.5 ml of enzyme extract. The solution was incubated for 5 min at 25 °C after which the reaction was terminated by adding 1 ml of 2.5 mol/L H2SO4. The amount of purpurogallin formed was determined by measuring the absorbance at 420 nm against a blank prepared by adding the extract after the addition of 2.5 mol/L H2SO4 at zero time. The activity was expressed in U/(mg protein). One U is defined as the change in the absorbance per 0.1 min per mg protein.
3. Catalase (CAT, EC 1.11.1.6)
The activity of CAT was measured according to the method of Chandlee and Scandalios (1984) with small modification. The assay mixture contained 2.6 ml of 50 mmol/L potassium phosphate buffer (pH 7.0), 0.4 ml of 15 mmol/L H2O2 and 0.04 ml of enzyme extract. The decomposition of H2O2 was followed by the decline in absorbance at 240 nm. The enzyme activity was expressed in U/(mg protein). One U is defined as 1 mmol/L of H2O2 reduction per min per mg protein. The enzyme protein was estimated by the method of Bradford (1976) for all the enzymes.
Statistical analysis
Statistical analysis was performed using one way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT). The values are mean±SD for six samples in each group. P values ≤0.05 were considered as significant.
RESULTS AND DISCUSSION
Significant differences have been observed in germination rate, root length, shoot length, dry matter production and vigour index between untreated seeds and treated seeds of C. roseus. The maximum germination percentage (70%) was recorded in Azotobacter treatment followed by Azospirillum (66%). The native isolates of Azotobacter and Azospirillum significantly increased the germination rate in C. roseus which was 70% as against 35% recorded by untreated control (Table 1). Treatments with these diazotrophs resulted in significantly higher dry matter production than control. Similar trend has been observed for the increase in vigour index of C. roseus for the Azospirillum and Azotobacter seed treatment. There is no significant difference in the dry matter production between Azospirillum and Azotobacter treatments in C. roseus.
Table 1.
Effect of Azospirillum and Azotobacter seed treatment on seedling parameters of Catharanthus roseus
| Treatment | Germination (%) | Root length (cm) | Shoot length (cm) | Dry matter production (mg/seedling) | Vigour index |
| Untreated control | 35.0 | 4.5 | 2.0 | 28.4 | 227.5 |
| Azospirillum | 66.0 | 10.6 | 6.2 | 30.6 | 1108.8 |
|
Azotobacter |
70.0 |
12.6 |
8.4 |
32.4 |
1484.0 |
| CD (P=0.05) | 3.6179 | 1.8090 | 1.5077 | 351.7499 | 4.6230 |
| SD | 1.7999 | 0.9000 | 0.7501 | 175.0000 | 2.3000 |
CD: Critical difference; SD: Standard deviation
Significant difference in the population of total diazotroph Azospirillum and Azotobacter between rhizosphere and non-rhizosphere soils of C. roseus was found. On different location of sampling of soils exhibited difference significantly. The rhizosphere soil sample of C. roseus recorded the maximum mean population of total diazotroph (72.33×103 CFU/g). The population of Azotobacter was also found to be high. It was maximum in the rhizosphere soil samples of C. roseus in all the locations such as AHF, AMG, HRS viz., 60×103, 50×103 and 44×103 CFU/g, respectively (Table 2).
Table 2.
Population of total diazotrophs Azotobacter and Azospirillum sp. on the rhizosphere and non-rhizosphere samples of Catharanthus roseus at different locations
| Soil sample | Total diazotroph (×103 CFU/g) |
Azospirillum (×103 CFU/g) |
Azotobacter (×103 CFU/g) |
|||||||||
| AHF | AMG | HRS | Mean | AHF | AMG | HRS | Mean | AHF | AMG | HRS | Mean | |
| Rhizosphere | 85 | 70 | 62 | 72.31 | 44 | 38 | 34 | 38.66 | 60 | 50 | 44 | 51.33 |
| Non-rhizosphere | 28 | 20 | 18 | 22.00 | 12 | 10 | 10 | 10.66 | 18 | 16 | 12 | 15.53 |
The seed treatment with native isolates of Azospirillum and Azotobacter increased the germination percentage, root length, shoot length and vigour index of the C. roseus. The occurrence of Azospirillum and Azotobacter in and around the root system of cereals and the beneficial effect upon inoculation have been well established. In the present study, the increased seedling parameters in C. roseus may be due to the production of growth hormones (auxins, gibberellins and cytokinins) by the heterotrophic nitrogen-fixing bacteria. It is worth noting that earlier research determined that the increase in plant growth observed on inoculation with Azotobacter was not caused by nitrogen fixation, but by bacterial production of plant hormones (Brown and Burlingham, 1968).
In the present study, isolates of Azospirillum and Azotobacter were tested for their effect on seedling parameters of C. roseus and the results clearly indicated that the native isolates significantly improved the seed germination and related seedling parameters. This confirmated earlier report by Govindarajan and Kavitha (2001) that the Azospirillum was isolated to the paddy seedlings under axenic conditions. There was another report by Lakshmanan et al.(2005) which confirmed that the medicinal plant such as Senna and Ashwagandha significantly increased germination percentage, root length, shoot length and dry weight of seedlings and more importantly the homologous isolates of Azotobacter and Azospirillum had a significant effect with host.
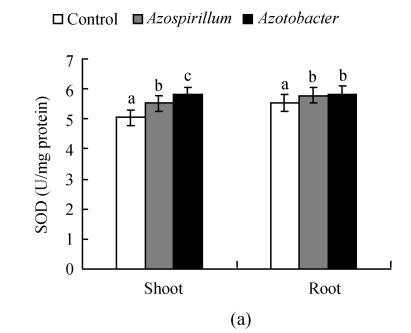
There was a significant increase in SOD, POX and CAT activities under Azotobacter and Azospirillum treatments (Fig.1). SOD activity directly modulates the amount of ROS. It catalyses the dismutation of superoxide anion radical (O2 −·) with great efficiency, resulting in the production of H2O2 and O2 (Lin and Kao, 2000). In our study, an increased level in SOD activity was found. According to Prochazkova et al.(2001), many stress situations caused an increase in the total antioxidant activity. The SOD activity showed an increase and in some, a reduction under abiotic stresses (Muthukumarasamy et al., 2000; Sairam et al., 2002). The changes in SOD activity under Azotobacter and Azospirillum treatments can be also a consequence of an altered synthesis and accumulation of less active enzymes and/or of a higher turnover of SODs (Chaparzadeh et al., 2004).
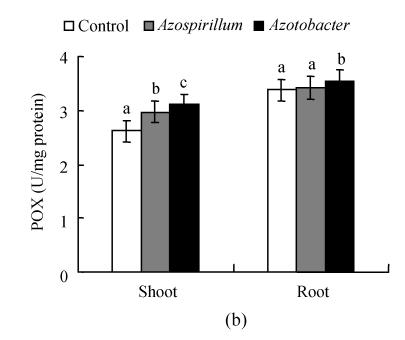
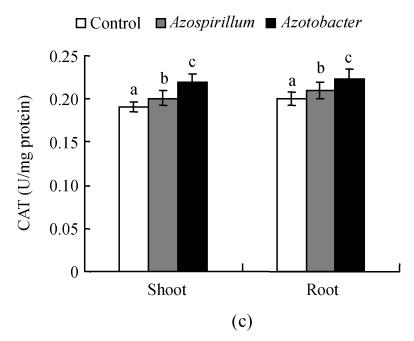
Fig. 1.
Effect of native diazotrophs on SOD (a), POX (b) and CAT (c) activities of Catharanthus roseus
Values are given as mean±SD of six experiments in each group. Bar values which are not sharing a common superscript (a, b, c) differ significantly at P≤0.05 (DMRT)
Variations are recorded in antioxidant enzyme activities under various stresses and treatments of pea (Hernandez and Almansa, 2002) and wheat (Sairam et al., 2002). Muthukumarasamy et al.(2000) showed that a reduction in POX activity in radish. We observed an increase in CAT activity in C. roseus seedlings subjected to Azotobacter and Azospirillum treatments. This result coincides with the observation in rice leaves under NaCl treatments (Lin and Kao, 2000). The changes in CAT may vary according to the intensity of stress, time of assay after the stress and induction of new isozyme(s) (Chaparzadeh et al., 2004). The level of antioxidative response depends on the species, the development and the metabolic state of the plant, as well as the duration and intensity of the stress (Reddy et al., 2004). It is well known that treatment of plants with elicitors, or attack by incompatible pathogens, causes an array of defense reactions, including the accumulation of a range of plant defensive secondary metabolites (Zhao et al., 2005). From the results it can be concluded that the application of native diazotrophs could be well used to promote growth and plants’ innate antioxidant defense potentials.
References
- 1.Abdul-Baki AA, Anderson JD. Vigour determination in soybean seed by multiple criteria. Crop Sci. 1973;13:630–633. [Google Scholar]
- 2.Beauchamp CO, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72(1-2):248–253. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Brown ME, Burlingham SK. Production of plant growth substances by Azotobacter chroococcum . J Gen Microbiol. 1968;53:135–144. doi: 10.1099/00221287-53-1-135. [DOI] [PubMed] [Google Scholar]
- 5.Chandlee JM, Scandalios JG. Analysis of variants affecting the catalase development program in maize scutellum. Theor Appl Genet. 1984;69(1):71–77. doi: 10.1007/BF00262543. [DOI] [PubMed] [Google Scholar]
- 6.Chaparzadeh N, Amico ML, Nejad RK, Izzo R, Izzo FN. Antioxidative responses of Calendula officinalis under salinity conditions. Plant Physiol Biochem. 2004;42(9):695–701. doi: 10.1016/j.plaphy.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Deka BC, Bora GC, Shadeque A. Effect of Azospirillum on growth and yield of chilli (Capsicum annuum L.) cultivar Pusa Jawala. Haryana J Hort Sci. 1992;38:41–46. [Google Scholar]
- 8.Govindarajan K, Kavitha K. Workshop on Recent Developments in Biofertilizers for Rice-Based Cropping System. Coimbatore: 2001. Studies on Azospirillum Associated with Rice Varities; pp. 9–10. [Google Scholar]
- 9.Hernandez JA, Almansa MS. Short-term effects of salt stress on antioxidant systems and leaf water relations of pea plants. Physiol Plant. 2002;115(2):251–257. doi: 10.1034/j.1399-3054.2002.1150211.x. [DOI] [PubMed] [Google Scholar]
- 10.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57(1):395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 11.ISTA (International Seed Testing Association) International rules for seed testing. Seed Sci Tech. 1976;4:52–70. [Google Scholar]
- 12.Jaleel CA, Gopi R, Lakshmanan GMA, Panneerselvam R. Triadimefon induced changes in the antioxidant metabolism and ajmalicine production in Catharanthus roseus (L.) G. Don. Plant Sci. 2006;171(2):271–276. doi: 10.1016/j.plantsci.2006.03.018. [DOI] [Google Scholar]
- 13.Jaleel CA, Gopi R, Sankar B, Manivannan P, Kishorekumar A, Sridharan R, Panneerselvam R. Studies on germination, seedling vigour, lipid peroxidation and proline metabolism in Catharanthus roseus seedlings under salt stress. South African Journal of Botany. 2007;73(2):190–195. doi: 10.1016/j.sajb.2006.11.001. [DOI] [Google Scholar]
- 14.Kar A, Choudhary BK, Bandyopadhyay NG. Comparative evaluation of hypoglycemic activity of some Indian medicinal plants in alloxan diabetic rats. J Ethnopharmacol. 2003;84(1):105–108. doi: 10.1016/S0378-8741(02)00144-7. [DOI] [PubMed] [Google Scholar]
- 15.Kumar KB, Khan PA. Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Ind J Exp Bot. 1982;20:412–416. [PubMed] [Google Scholar]
- 16.Lakshmanan A, Govindarajan K, Kumar K. Effect of seed treatment with native diazotrophs on the seedling parameters of Senna and Ashwagandha. Crop Res. 2005;30(1):119–123. [Google Scholar]
- 17.Lin CC, Kao CH. Effect of NaCl stress on H2O2 metabolism in rice leaves. Plant Growth Regul. 2000;30(2):151–155. doi: 10.1023/A:1006345126589. [DOI] [Google Scholar]
- 18.Magnotta M, Murata J, Chen J, de Luca V. Identification of a low vindoline accumulating cultivar of Catharanthus roseus (L.) G. Don. by alkaloid and enzymatic profiling. Phytochemistry. 2006;67(16):1758–1764. doi: 10.1016/j.phytochem.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Muthukumarasamy M, Dutta Gupta S, Panneerselvam R. Enhancement of peroxidase, polyphenol oxidase and superoxide dismutase activities by triadimefon in NaCl stressed Raphanus sativus L. Biol Plant. 2000;43(2):317–320. doi: 10.1023/A:1002741302485. [DOI] [Google Scholar]
- 20.Nurnberger T, Colling C, Hahlbrock K, Jabs T, Renelt A, Sacks WR, Scheel D. Perception and transduction of an elicitor signal in cultured parsley cells. Biochem Soc Symp. 1994;60:173–182. [PubMed] [Google Scholar]
- 21.Prochazkova D, Sairam RK, Srivastava GC, Singh DV. Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci. 2001;161(4):765–771. doi: 10.1016/S0168-9452(01)00462-9. [DOI] [Google Scholar]
- 22.Reddy AR, Chiatanya KV, Vivekanandan M. Draught induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161(11):1189–1202. doi: 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Sairam RK, Veerabhadra Rao K, Srivastava GC. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002;163(5):1037–1046. doi: 10.1016/S0168-9452(02)00278-9. [DOI] [Google Scholar]
- 24.Somssich IE, Hahlbrock K. Pathogen defence in plant—a paradigm of biological complexity. Trends Plant Sci. 1998;3(3):86–90. doi: 10.1016/S1360-1385(98)01199-6. [DOI] [Google Scholar]
- 25.Verpoorte R, Contin A, Memelink J. Biotechnology for the production of plant secondary metabolites. Phytochem Rev. 2002;1(1):13–25. doi: 10.1023/A:1015871916833. [DOI] [Google Scholar]
- 26.Vranova E, Inze D, van Brensegem F. Signal transduction during oxidative stress. J Exp Bot. 2002;53(372):1227–1236. doi: 10.1093/jexbot/53.372.1227. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe I, Barraquio WL. Low levels of fixed nitrogen are required for isolation of free-living nitrogen fixing organisms from rice roots. Nature. 1979;277(5697):565–566. doi: 10.1038/277565a0. [DOI] [Google Scholar]
- 28.Zhao J, Lawrence T, Davis C, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23(4):283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]