Abstract
Leaf senescence is often caused by water deficit and the chimeric gene PSAG12-IPT is an auto-regulated gene delaying leaf senescence. Using in vitro leaf discs culture system, the changes of contents of chlorophylls, carotenoids, soluble protein and thiobarbituric acid reactive substance (TBARS) and antioxidant enzymes activities were investigated during leaf senescence of PSAGl2-IPT modified gerbera induced by osmotic stress compared with the control plant (wild type). Leaf discs were incubated in 20%, 40% (w/v) polyethylene glycol (PEG) 6 000 nutrient solution for 20 h under continuous light [130 µmol/(m2·s)]. The results showed that the contents of chlorophylls, carotenoids and soluble protein were decreased by osmotic stress with the decrease being more pronounced at 40% PEG, but that, at the same PEG concentration the decrease in the transgenic plants was significantly lower than that in the control plant. The activities of superoxide dismutase (SOD), catalases (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GPX) and dehydroascorbate reductase (DHAR) were stimulated by PEG treatment. However, the increases were higher in PSAG12-IPT transgenic plants than in the control plants, particularly at 40% PEG treatment. Lipid peroxidation (TBARS content) was increased by PEG treatment with the increase being much lower in transgenic plant than in the control plant. It could be concluded that the increases in the activities of antioxidant enzymes including SOD, CAT, APX, GPX and DHAR were responsible for the delay of leaf senescence induced by osmotic stress.
Keywords: Antioxidant enzymes, Gerbera, Leaf disc, Leaf senescence, Osmotic stress, PSAG12-IPT
INTRODUCTION
Leaf senescence always adversely affects higher plant production in agriculture (Dong et al., 2006). Senescence devalues ornamental plants and foliar vegetables during transportation, storage and on shelves (Li et al., 2006; Toit et al., 2004). Therefore, manipulation of leaf senescence may result in agricultural benefits. Senescence is regulated by several factors including plant hormones such as abscisic acid (ABA), ethylene, and cytokinin. ABA and ethylene have been shown to promote senescence whereas cytokinin application inhibits leaf senescence (Gan and Amasino, 1997). The IPT gene encodes a bacterial isopentenyl transferase which catalyses the rate limiting step in cytokinin biosynthesis. The IPT gene under the control of a chalcone synthase promoter has been reported to increase chlorophyll levels and stem thickness, and delay flowering onset and flower development in tobacco plants (Wang et al., 1997). In order to control leaf senescence, an autoregulatory senescence inhibition system, in which a highly senescence specific promoter SAG12 from Arabidopsis is fused to IPT, has been developed (Gan and Amasino, 1995). This fusion directs the expression of IPT in leaves at the onset of senescence and subsequently increases the cytokinin level that prevents the leaves from senescing, and high cytokinin level resulting in down regulation of the senescence specific promoter that prevents cytokinin from accumulating too high, otherwise that will interfere with normal plant development. In modified tobacco, senescence was delayed by IPT expression under control of the senescence-specific SAG12 promoter in leaves under elevated atmospheric CO2 (Ludewig and Sonnewald, 2000). The IPT gene under control of the senescence-specific SAG12 promoter from Arabidopsis also significantly delayed developmental and postharvest leaf senescence in mature heads of transgenic lettuce (McCabe et al., 2001). Among ornamentals, introduction of the IPT gene into petunia, linked to senescence-associated SAG promoters from Arabidopsis, has been reported to inhibit senescence of the lower leaves, although transgenic lines show stronger symptoms of nutritional deficiency (Jandrew and Clark, 2001).
Environmental factors, such as drought, induce plant leaf senescence (Guo and Gan, 2005). Reactive oxygen species (ROS) have been linked to stress-induced or normal cell death (Pastori and Rio, 1997). ROS are a group of very reactive, short-lived chemicals produced during normal metabolism or after an oxidative reaction. ROS include superoxide anion, hydrogen peroxide, hydroxyl radical (Sun, 1990). ROS, at low concentrations, have been implicated in the regulation of several physiological processes such as proliferation (Shibanuma et al., 1990), differentiation (Allen and Balin, 1989), senescence (de Haan et al., 1996), and apoptosis (Mignotte and Vayssiere, 1998). At high concentrations, ROS are highly cytotoxic by inducing DNA damage, lipid peroxidation, and protein degradation (Sun, 1990). Antioxidant defense systems have been developed in aerobic cells to counteract the damaging effects of ROS. Plants possess antioxidant defense systems comprised of enzymatic and non-enzymatic components, which normally maintain ROS balance within the cell. For instance, they use a diverse array of enzymes like superoxide dismutase (SOD), catalases (CAT) and peroxidases as well as low molecular mass antioxidants (glutathione and ascorbate) to scavenge different types of ROS (Foyer et al., 1994). SOD catalyses the dismutation of superoxide to hydrogen peroxide and oxygen. However, hydrogen peroxide is also toxic to cells and has to be further detoxified by CAT and/or peroxidases to water and oxygen.
The antioxidant enzymes’ activities play an important role in scavenging ROS and therefore their improvement could increase the ability of stress tolerance and delay the senescence (Alscher et al., 2002). PSAG12-IPT is a chimeric gene associated with cytokinin synthesis and therefore inhibits leaf senescence. However, whether the changes of antioxidant enzyme activities are involved in the regulation of leaf senescence in PSAGl2-IPT modified gerbera is unknown. Using in vitro leaf discs culture system, the changes of antioxidant enzymes activities were investigated during leaf senescence of PSAGl2-IPT modified gerbera induced by osmotic stress. The objectives are to know whether antioxidant enzymes activities are involved in the regulation of leaf senescence in PSAGl2-IPT modified gerbera and clarify the physiological mechanisms of senescence delay induced by a chimeric gene PSAGl2-IPT.
MATERIALS AND METHODS
Plant materials and growth conditions
Gerbera plants, Gerbera jamesonii ‘Yuyan’, which are PSAG12-IPT transgenic plants obtained through particle bombardment transformation, and control plants, were cultured in mixed media with peat, perlite and vermiculite in the proportion of 3, 1 and 1, respectively. The plastic pot was 4 L in volume, 18 cm in diameter and 18 cm in height. The concentration of nutrient elements were as follows: 5.5 mmol/L K+, 3.0 mmol/L Ca2+, 1.0 mmol/L Mg2+, 11.25 mmol/L NO3 −, 1.5 mmol/L NH4 +, 1.25 mmol/L H2PO4 −, 1.25 mmol/L SO4 2−, 35 μmol/L Fe, 5.0 μmol/L Mn, 4.0 μmol/L Zn, 0.75 μmol/L Cu, 30 μmol/L B and 0.5 μmol/L Mo. Fe was added as Fe-EDTA. The plants were grown in a greenhouse. The air temperature was set at 20 °C (night) and 30 °C (day), relative humidity ranged from 60% to 80% and light intensity was 500 µmol/(m2·s) at noon. The photoperiod was day 14 h and night 10 h. The plant materials were cultivated under the growth conditions until flowering. Leaf discs (11 mm diameter) were prepared from fully expanded leaves using a cork borer. Leaf surface was cleaned with water and immediately transferred into a medium (5 mmol/L HEPES, 10 mmol/L KCl, 1.5 mmol/L CaCl2, pH 6.0) (Huguet-Robert et al., 2003).
Osmotic treatments
Osmotic treatments were applied by adding polyethylene glycol (PEG) 6 000 at specified concentrations, 0, 20%, 40% (w/v) to the medium (Huguet-Robert et al., 2003) and pH was readjusted to 6.0. Leaf discs were placed in Petri dishes with covers, with each containing 15 discs in 20 ml of stress medium, placed under continuous light [130 µmol/(m2·s) at the leaf discs level], shaken continuously at 20 °C and incubated for 20 h. All treatments were replicated three times concurrently with control assays performed with leaf discs incubated in flasks containing 20 ml of reference medium.
Enzyme extraction
For enzyme assays, leaf discs were ground with 4 ml ice-cold 50 mmol/L HEPES buffer (pH 7.8) containing 0.2 mmol/L EDTA, 2% PVPP and 2 mmol/L ascorbate. The homogenates were centrifuged at 4 °C for 20 min at 12 000×g and the resulting supernatants were used for determination of enzymatic activities (Zhu et al., 2000). All spectrophotometric analyses were conducted on a SHIMADZU UV-2410PC spectrophotometer.
Determination of enzymatic activities
SOD activity was assayed by measuring its ability to inhibit the photochemical reduction of nitroblue tetrazolium (Stewart and Bewley, 1980). CAT activity was measured using the reduction in absorbance at 240 nm due to the decrease of extinction of H2O2 (Patra et al., 1978). Guaiacol peroxidase (GPX) activity was measured by the increase in absorbance at 470 nm due to guaiacol oxidation (Nickel and Cunningham, 1969). Ascorbate peroxidase (APX) activity was measured by the decrease in absorbance at 290 nm as ascorbate was oxidized (Nakano and Asada, 1981). The assay of dehydroascorbate reductase (DHAR) activity was carried out by measuring the increase in absorbance at 265 nm due to reduced ascorbate formation (Nakano and Asada, 1981).
Determination of lipid peroxidation
Lipid peroxidation was estimated by measuring the concentration of thiobarbituric acid reactive substances (TBARS) (Shalata and Tal, 1998), the reaction mixture in a total volume of 4 ml containing 1 ml of extracts, 3 ml of 2% (w/v) TBA (2-thiobarbituric acid) made in 20% trichloroacetic acid (TCA). The mixture was heated at 95 °C for 30 min and then quickly cooled on ice. After centrifugation at 10 000×g for 10 min, the absorbance of the supernatant was measured at 532 nm. A correction of non-specific turbidity was made by subtracting the absorbance value taken at 600 nm. The lipid peroxides were expressed as (nmol TBARS)/(g DW) by using an extinction coefficient of 155 (mmol/L)−1·cm−1 (Shalata and Tal, 1998).
Determination of soluble protein content
Soluble protein concentration was measured using bovine serum albumin as standard at 595 nm according to the method of Bradford (1976).
Determination of chlorophylls and carotenoids content
Contents of chlorophyll (a and b) and carotenoids were determined in alkaline acetone extracts (Lichtenthaler, 1987).
RESULTS
Chlorophylls and carotenoids
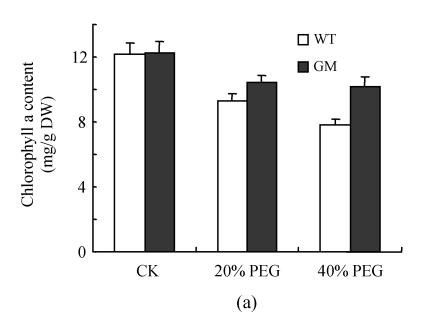
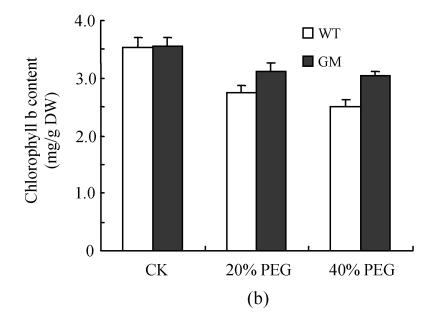
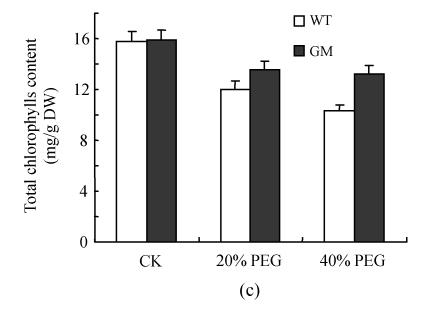
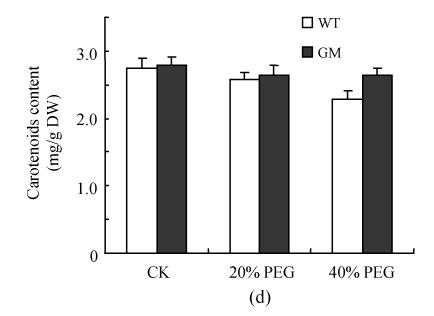
Fig.1 indicates that the contents of chlorophyll a, chlorophyll b and total chlorophylls in leaf discs were significantly decreased by osmotic stress (incubation in PEG solution) with the decrease being more pronounced in 40% PEG than in 20% PEG. However, at the same PEG concentration the decrease in transgenic plants was significantly lower than that in the control plants, especially at 40% PEG. The contents of carotenoids in leaf discs were reduced by PEG treatment, especially in the control plants at 40% PEG treatment.
Fig. 1.
Effects of osmotic stress on the contents of chlorophyll a (a), chlorophyll b (b), total chlorophylls (c) and carotenoids (d) in leaf discs of PSAG12-IPT transgenic (gerbera modified, GM) and control (wild type, WT) gerbera plants. Data are the mean±SD of three replicates
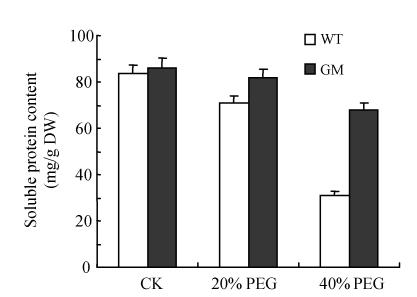
Soluble protein content
The soluble protein content in leaf discs was decreased by PEG treatment with the decrease at 40% PEG being higher than that at 20% PEG. However, the decrease induced by PEG in modified plants was significantly lower than that in the control plants, especially at 40% PEG (Fig.2).
Fig. 2.
Effects of osmotic stress on soluble protein content in leaf discs of PSAG12-IPT transgenic (GM) and control (WT) gerbera plants. Data are the mean±SD of three replicates
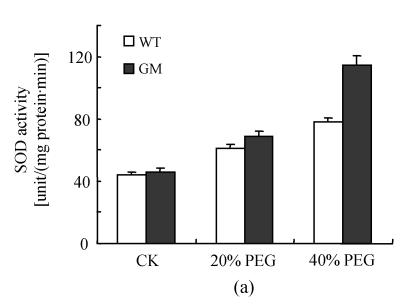
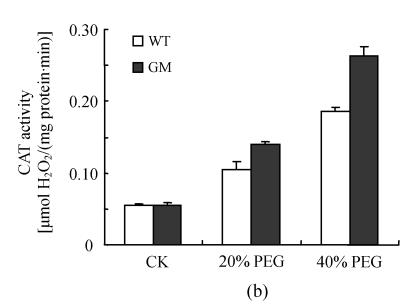
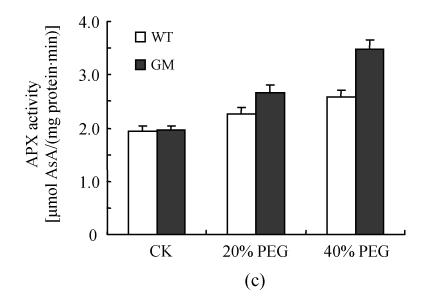
Antioxidant enzyme activities
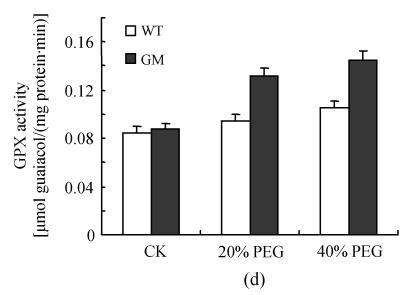
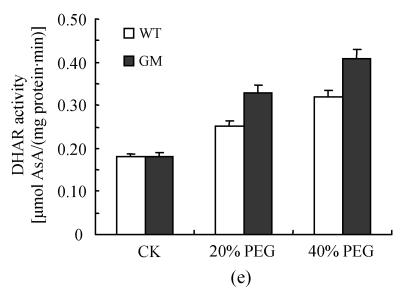
After osmotic stress, the changes of activities of SOD, CAT, APX, GPX and DHAR had similar tendency. The activities of SOD, CAT, APX, GPX and DHAR were stimulated by PEG treatment. However, the increases were higher in PSAG12-IPT transgenic plants than in the control plants, particularly at 40% PEG treatment (Fig.3).
Fig. 3.
Effects of osmotic stress on SOD (a), CAT (b), APX (c), GPX (d) and DHAR (e) activities in leaf discs of PSAG12-IPT transgenic (GM) and control (WT) gerbera plants. Data are the mean±SD of three replicates
Lipid peroxidation
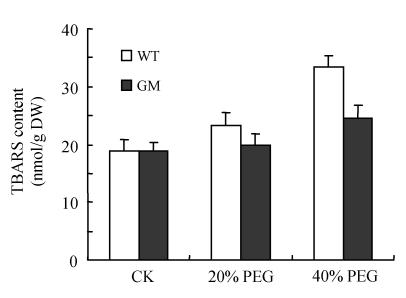
As an indicator of lipid peroxidation, TBARS concentration was measured. The TBARS concentration in the leaf discs was increased by osmotic stress with the increase being higher at 40% PEG than at 20% PEG. However, this increase was significantly higher in the control plants than in transgenic plants (Fig.4).
Fig. 4.
Effects of osmotic stress on TBARS content in leaf discs of PSAG12-IPT transgenic (GM) and control (WT) gerbera plants. Data are the mean±SD of three replicates
DISCUSSION
Genotypic differences in drought tolerance could be attributed to the ability of plants to growth (Turtola et al., 2006). Leaf senescence can be affected by a complex array of factors and manipulating any one of these factors will result in either promotion or prevention of leaf senescence, for example, water deficit promotes leaf senescence (Thomas et al., 1995). In many studies, PEG 6 000 is often used as an inducer of water deficit (osmotic stress) and promotes plant senescence (Behera et al., 2003). In the present experiment, adding PEG in the incubation medium of leaf discs induced leaf senescence, indicated by the considerable decrease of chlorophylls and soluble protein content (Figs.1 and 2). However, the decreases of chlorophylls and soluble protein content in PSAG12-IPT modified gerbera plants were significantly reduced compared to the control plants, indicating that leaf senescence was significantly delayed by the particle bombardment transformation of the chimeric gene PSAG12-IPT.
Even under optimal conditions, many metabolic processes produce ROS. The production of toxic oxygen derivatives is increased as a result of all types of abiotic or biotic stresses. Plants possess efficient systems for scavenging active oxygen species that protect them from destructive oxidative reactions (Foyer et al., 1994). As part of this system, antioxidant enzymes are key elements in the defense mechanisms. Many changes have been observed in the activities of antioxidant enzymes in plants under osmotic stress. Superoxide dismutase and ascorbate peroxidase activities were found to be enhanced in osmotically stressed leaf discs of rape plants compared with the control (Aziz and Larher, 1998). An increase in ascorbate peroxidase, superoxide dismutase and glutathione reductase activities was also observed in the drought tolerant wheat cultivar when the leaves were subjected to osmotic stress in vitro (Lascano et al., 2001). The results of the present experiment showed that the activities of SOD, CAT, APX, GPX and DHAR in leaf discs were stimulated by PEG treatment (osmotic stress) with the increases in leaf discs of PSAG12-IPT transgenic plants being higher than those of control plants, particularly at 40% PEG treatment. Higher activities of SOD, CAT, APX, GPX and DHAR in leaf discs of PSAG12-IPT transgenic plants with PEG treatment coincided with decrease in the TBARS concentration, suggesting that oxidative damage induced by osmotic stress could be alleviated by transformation of the chimeric gene PSAG12-IPT. Such protection system against oxidative stress may play a crucial role in preventing leaves from senescence induced by osmotic stress. This result was consistent with the observation of Dertinger et al.(2003). They found that the activities of antioxidatve enzymes (ascorbate peroxidase and glutathione reductase) in old leaves of PSAG12-IPT modified tobacco plants were higher than in wild-type leaves.
In summary, leaf senescence under osmotic stress was delayed by the transformation of PSAG12-IPT gene in gerbera. The increases in the activities of antioxidant enzymes including SOD, CAT, APX, GPX and DHAR may be responsible for the delay of leaf senescence, indicated by the reduction of the decreases in chlorophylls and soluble protein content and the increases in TBARS content in leaf discs under osmotic stress.
References
- 1.Allen RG, Balin AK. Oxidative influence on development and differentiation: an overview of a free radical theory of development. Free Radic Biol Med. 1989;6(6):631–661. doi: 10.1016/0891-5849(89)90071-3. [DOI] [PubMed] [Google Scholar]
- 2.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases in controlling oxidative stress in plants. J Exp Bot. 2002;53(372):1331–1341. doi: 10.1093/jexbot/53.372.1331. [DOI] [PubMed] [Google Scholar]
- 3.Aziz A, Larher F. Osmotic stress induced changes in lipid composition and peroxidation in leaf discs of Brassica napus L. J Plant Physiol. 1998;153(5):754–762. [Google Scholar]
- 4.Behera SK, Nayak L, Biswal B. Senescing leaves possess potential for stress adaptation: the developing leaves acclimated to high light exhibit increased tolerance to osmotic stress during senescence. J Plant Physiol. 2003;160(2):125–131. doi: 10.1078/0176-1617-00791. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantitation of micogram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.de Haan JB, Cristiano F, Iannello R, Bladier C, Kelner MJ, Kola I. Elevation in the ratio of Cu/Zn-superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum Mol Genet. 1996;5(2):283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 7.Dertinger U, Schaz U, Schulz ED. Age-dependence of the antioxidative system in tobacco with enhanced glutathione reductase activity or senescence-induced production of cytokinins. Physiol Plant. 2003;119(1):19–29. doi: 10.1034/j.1399-3054.2003.00095.x. [DOI] [Google Scholar]
- 8.Dong HZ, Li WJ, Tang W, Li ZH, Zhang DM, Niu YH. Yield, quality and leaf senescence of cotton grown at varying planting dates and plant densities in the Yellow River Valley of China. Field Crops Res. 2006;98(2-3):106–115. doi: 10.1016/j.fcr.2005.12.008. [DOI] [Google Scholar]
- 9.Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92(4):696–717. doi: 10.1034/j.1399-3054.1994.920422.x. [DOI] [Google Scholar]
- 10.Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270(5244):1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- 11.Gan S, Amasino RM. Making sense of senescence. Plant Physiol. 1997;113(2):313–319. doi: 10.1104/pp.113.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo YF, Gan SS. Leaf senescence: signals, execution, and regulation. Current Topics in Developmental Biology. 2005;71:83–112. doi: 10.1016/S0070-2153(05)71003-6. [DOI] [PubMed] [Google Scholar]
- 13.Huguet-Robert V, Sulpice R, Lefort C, Maerskalck V, Emery N, Larher FR. The suppression of osmoinduced proline response of Brassica napus L. var oleifera leaf discs by polyunsaturated fatty acids and methyljasmonate. Plant Sci. 2003;164(1):119–127. doi: 10.1016/S0168-9452(02)00343-6. [DOI] [Google Scholar]
- 14.Jandrew J, Clark DG. Selectively induced nutrient deficiency in transgenic PSAG12-IPT, PSAG13-IPT and PSAG12-Kn1 petunias. HortSci. 2001;36:518–519. [Google Scholar]
- 15.Lascano HR, Antonicelli GE, Luna CM, Melchiorre MN, Gómez LD, Racca RW, Trippi VS, Casano LM. Antioxidant system response of different wheat cultivars under drought: field and in vitro studies. Aust J Plant Physiol. 2001;28(11):1095–1102. [Google Scholar]
- 16.Li WX, Zhang M, Yu HQ. Study on hypobaric storage of green asparagus. J Food Eng. 2006;73(3):225–230. doi: 10.1016/j.jfoodeng.2005.01.024. [DOI] [Google Scholar]
- 17.Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol. 1987;148:350–382. [Google Scholar]
- 18.Ludewig F, Sonnewald U. High CO2-mediated down-regulation of photosynthetic gene transcripts is caused by accelerated leaf senescence rather than sugar accumulation. FEBS Letters. 2000;479(1-2):19–24. doi: 10.1016/S0014-5793(00)01873-1. [DOI] [PubMed] [Google Scholar]
- 19.McCabe MS, Garratt LC, Schepers F, Jordi WJRM, Stoopen GM, Davelaar E, van Rhijn JHA, Power JB, Davey MR. Effects of PSAG12-IPT gene expression on development and senescence in transgenic lettuce. Plant Physiol. 2001;127(2):505–516. doi: 10.1104/pp.127.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mignotte B, Vayssiere JL. Mitochondria and apoptosis. Eur J Biochem. 1998;252(1):1–15. doi: 10.1046/j.1432-1327.1998.2520001.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakano Y, Asada K. Hydrogen peroxide scanvenged by ascorbated specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981;22(5):867–880. [Google Scholar]
- 22.Nickel RS, Cunningham BA. Improved peroxidase assay method using Ieuco 2,3,6-trichlcroindophenol and application to comparative measurements of peroxidase catalysis. Anal Biochem. 1969;27(2):292–299. doi: 10.1016/0003-2697(69)90035-9. [DOI] [PubMed] [Google Scholar]
- 23.Pastori GM, Rio LA. Natural senescence of pea leaves, an activated oxygen-mediated function for peroxisomes. Plant Physiol. 1997;113(2):411–418. doi: 10.1104/pp.113.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patra HL, Kar M, Mishre D. Catalase activity in leaves and cotyledons during plant development and senescence. Biochem Physiol. 1978;172(4):385–390. [Google Scholar]
- 25.Shalata A, Tal M. The effect of salt stress on lipid peroxidation and antioxidants in the leaf of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii . Physiol Plant. 1998;104(2):169–174. doi: 10.1034/j.1399-3054.1998.1040204.x. [DOI] [Google Scholar]
- 26.Shibanuma M, Kuroki T, Nose K. Stimulation by hydrogen peroxide of DNA synthesis, competence family gene expression and phosphorylation of a specific protein in quiescent Balb/3T3 cells. Oncogene. 1990;5(7):1025–1032. [PubMed] [Google Scholar]
- 27.Stewart RC, Bewley JD. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980;65(2):245–248. doi: 10.1104/pp.65.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8(6):583–599. doi: 10.1016/0891-5849(90)90156-D. [DOI] [PubMed] [Google Scholar]
- 29.Thomas JC, Smigoli AC, Bohnert HJ. Light-inducible expression of ipt from Agrobacterium tumefaciens results in cytokinin accumulation and osmotic stress symptoms in transgenic tobacco. Plant Mol Biol. 1995;27(2):225–235. doi: 10.1007/BF00020179. [DOI] [PubMed] [Google Scholar]
- 30.Toit ES, Robbertse PJ, Niederwieser JG. Temperature regime during bulb production affects foliage and flower quality of Lachenalia cv. Ronina pot plants. Scientia Horticulturae. 2004;102(4):441–448. [Google Scholar]
- 31.Turtola S, Rousi M, Pusenius J, Yamaji K, Heiska S, Tirkkonen V, Meier B, Riitta JT. Genotypic variation in drought response of willows grown under ambient and enhanced UV-B radiation. Environmental and Experimental Botany. 2006;56(1):80–86. doi: 10.1016/j.envexpbot.2005.01.007. [DOI] [Google Scholar]
- 32.Wang J, Letham DS, Cornish E, Stevenson KR. Studies of cytokinin action and metabolism using tobacco plants expressing either the ipt or the GUS gene controlled by a chalcone synthase promoter I developmental features of the transgenic plants. Plant Physiol. 1997;24(5):661–672. [Google Scholar]
- 33.Zhu ZJ, Gerendas J, Bendixen R, Schinner K, Tabrizi H, Sattelmacher B, Hansen UP. Different tolerance to light stress in NO3 − and NH4 +-grown Phaseolus vulgaris L. Plant Biol. 2000;2(5):558–570. doi: 10.1055/s-2000-7498. [DOI] [Google Scholar]