Abstract
Objective: To evaluate the effect of preimplantation genetic diagnosis (PGD) conducted for women who had Down syndrome pregnancy previously. Methods: Trisomy 21 was diagnosed by using fluorescence in site hybridization (FISH) before embryo transfer in two women who had Down syndrome pregnancies. Each received one or two PGD cycles respectively. Results: Case 1: one PGD cycle was conducted, two oocytes were fertilized and biopsied. One embryo is of trisomy 21 and the other of monosomy 21. No embryo was transferred. Case 2: two PGD cycles were conducted, in total, sixteen oocytes were fertilized and biopsied. Four embryos were tested to be normal, six of trisomy 21, and one of monosomy 21. Five had no signal. Four normal embryos were transferred but no pregnancy resulted. Conclusion: For couples who had pregnancies with Down syndrome previously, PGD can be considered, and has been shown to be an effective strategy.
Keywords: Down syndrome, Fluorescence in site hybridization (FISH), Preimplantation genetic diagnosis (PGD)
INTRODUCTION
Down syndrome (DS), also named 21 trisomy syndrome, is a chromosome disorder associated either with an extra chromosome 21 or an effective trisomy for chromosome 21. The population risk for Down syndrome is approximately 1 in 800 live births (Roizen and Patterson, 2003) and the recurrence risk after the birth of an affected child is approximately 1%~2% (Mikkelsen and Stene, 1979; Daniel et al., 1982). At present, women who had history of Down syndrome pregnancy may give birth to a normal baby with the help of prenatal diagnosis. However, they may suffer repeated pregnancy terminations following Down syndrome conception and these couples are seeking genetic diagnosis before implantation to avoid subsequent pregnancy termination (Conn et al., 1999).
Preimplantation genetic diagnosis (PGD) is a technique that was originally developed as an alternative to prenatal diagnosis for couples at high risk of transmitting a genetic defect. It involves the screening of in vitro fertilization (IVF) or intracytoplasm sperm injection (ICSI) generated embryos by the genetic analysis of one or two biopsied blastomeres, allows scientists to check specific genetic defects of the embryo so that only embryos not affected by the tested disease or balanced for the tested chromosomes will be transferred (Sermon et al., 2005). Compared to the traditional methods of prenatal diagnosis, PGD offers genetic analysis at the earliest stage in fetal development, leading to the avoidance of abnormal pregnancy and subsequent pregnancy termination.
Fluorescence in site hybridization (FISH) is the technique of choice for detecting the chromosome status in single cells for PGD, using fluorochrome-labelled DNA probes that are complementary to DNA sequences specific to individual chromosomes. It permits sexing the embryos (in case of X-linked diseases), simultaneous enumeration of up to nine chromosomes for aneuploidy screening (for the detection of abnormal numbers of chromosomes) and structural chromosome abnormalities (such as unbanlanced translocations) (Rubio et al., 2005a). FISH has been proved to be a valuable tool for the study of aneuploidy in early human embryos (Schrurs et al., 1993; Munné et al., 1998c; 2003). The number of cycles of PGD for aneuploidy screening (PGS) (Sermon et al., 2005) has been steadily increasing. However, not many reports of PGD for specific single chromosome 21 were available.
Here, we report two cases whose state of chromosome 21 was detected by using FISH in preimplantation embryos and who had Down syndrome pregnancy. At the same time, the present situation of preimplantation genetic diagnosis used for Down syndrome is discussed.
SUBJECTS AND METHODS
Ethical approval
The clinical application of PGD was licensed by the Health Ministry of People’s Republic of China. The protocol was approved by local Ethical Committee and informed written consent was obtained from both patients.
Patients
Case 1: The couple was referred for PGD after three abnormal pregnancies (one spontaneous abortion, a Down syndrome child and one termination of Down syndrome pregnancy). Maternal age was 43 years. Case 2: The couple was referred for PGD after two Down syndrome conceptions (one termination of pregnancy and a Down syndrome child). Maternal age was 30 years. Both couples had normal karyotype as proven by G-banded metaphase chromosome analysis of cultured peripheral blood lymphocytes. Both couples were counseled about the PGD procedure and the need to confirm diagnosis with prenatal diagnosis.
Materials and methods
Ovarian stimulation, fertilization and embryo culture: ICSI was conducted as described by van Steirteghem et al.(1993) to avoid sperm contamination. Briefly, patients underwent superovulation and oocyte retrieval was conducted by vaginal ultrasound guided aspiration. Oocytes were subjected to ICSI and assessed for normal fertilization after 17 h. Embryos were cultured in vitro until 44 h post-insemination when the majority had reached the 6~10 cell stage.
Biopsy and slide preparation: On day 3 the embryos biopsy procedure was performed after decompaction in Ca/Mg-free Scandinavian Embryo Medium (Science Scandinavia) and acidified Tyrode’s solution for zona drilling. One blastomere was biopsied from each embryo. After biopsy, embryos were immediately returned to normal culture conditions. Each single blastomere was transferred to poly-L-lysine slides in spreading solution (0.01 mol/L HCl, 0.1% Tween 20) that was gently agitated until lysis occurred and all nuclei were clear of cytoplasm. The slides were left to air dry for ~20 min, washed in PBS for 5 min and dehydrated through an ethanol series (70%, 80%, 100% at 5 min each).
FISH: FISH was conducted as Munné et al.(1998a) described. Biopsied blastomeres were hybridized with probe of locus-specific indicator (LSI) 21 (SpectrumOrange) provide by Vysis Inc. (Illinois, USA) and LSI (13q34) (SpectrumGreen) was used as control. FISH signals were visualized using an Olympus-AX fluorescence microscope. Only embryos with normal chromosomal complement were transferred on day 4 of culture while those exhibiting chromosomal abnormalities were excluded.
RESULTS
Case 1
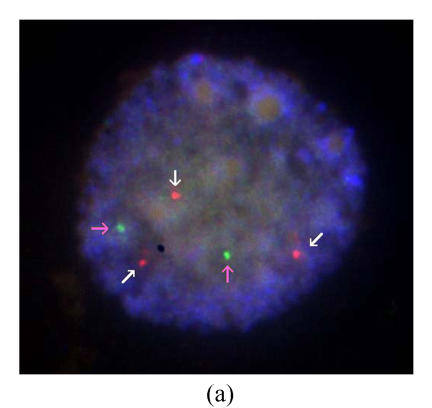
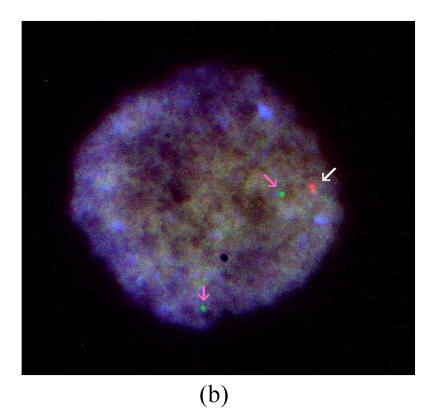
One PGD cycle was conducted. Three oocytes were obtained, of which two were fertilized normally and biopsied on day 3. FISH results showed that one is of trisomy 21, and the other is of monosomy 21. No embryo was transferred (Figs.1a and 1b).
Fig. 1.
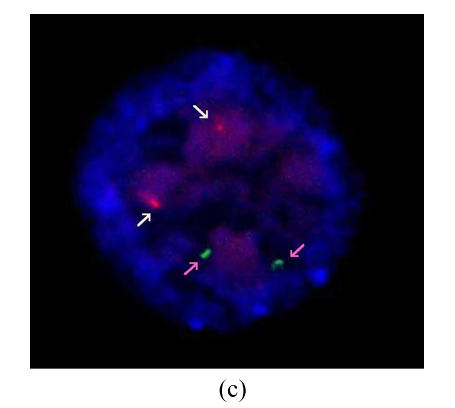
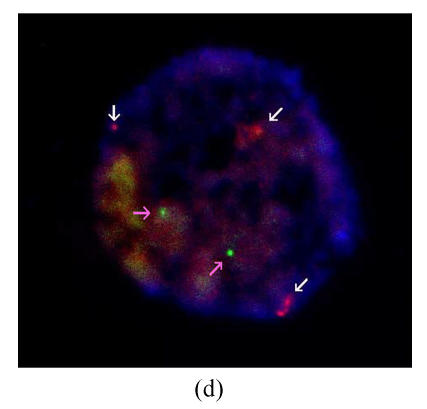
Results of FISH analysis of biopsied blastomeres from preimplantation embryos using chromosome 21 (orange, white arrow) and chromosome 13 (green, pink arrow) probes. Case 1: (a) trisomy 21 (three orange signals), (b) monosomy 21 (one orange signal); Case 2: (c) normal for chromosome 21 (two orange signals), (d) trisomy 21 (three orange signals). All of the blastomeres are normal for chromosome 13 (two green signals)
Case 2
Two PGD cycles were conducted. In the first cycle, ten oocytes were obtained, of which eight were fertilized normally and biopsied on day 3. Two gave a normal/balanced signal pattern, and were transferred on day 4. Three embryos were proved to be trisomy 21, and one of monosomy 21. The other two embryos were inconclusive because of hybridization failure. No pregnancy resulted. In the second cycle, seventeen oocytes were obtained, fourteen were fertilized normally, and eight biopsied on day 3. Two gave a normal/balanced signal pattern, and were transferred on day 4. Three embryos were proved to be trisomy 21. The other three embryos were inconclusive. No pregnancy resulted (Figs.1c and 1d).
DISCUSSION
Down syndrome is classically characterized by birth defects and mental retardation. In conventional prenatal diagnosis, definitive diagnosis of Down syndrome requires amniocentesis, chorionic villus sampling or umbilical blood sampling. Efforts have been made to reduce the risk of prenatal diagnosis, including obtaining fetal cells from maternal blood (Ho et al., 2003; Yang et al., 2003; 2006), applying molecular genetic techniques to broaden the range of identifiable genetic disorders (Solassol et al., 2003; Yang et al., 2006) and raising the detection rate of noninvasive prenatal screening to reduce the false-positive rate in the hope of reducing the examine rate of invasive procedure (Cicero et al., 2003; Muller et al., 2003; Nicolaides, 2004; Spencer et al., 2003). However, invasive diagnosis is unavoidably combined with some complications and there are many religious controversies over whether to terminate the affected pregnancy. Moreover, the termination of pregnancy in the event of Down syndrome is not acceptable for some couples and there is growing demand for PGD diagnosis of Down syndrome.
PGD is an alternative to prenatal diagnosis combined with assisted reproductive technology (ART) and genetic diagnosis technology, which offers genetic analysis at the earliest stage in fetal development, allows unaffected embryos to be identified and transferred to the uterus (Munné et al., 2004). PGD avoids not only pregnancy termination when the fetus is proved affected by prenatal diagnosis, but also potential complications of invasive procedures, such as abortion, hemorrhage and uterus infection. Moreover, PGD can solve the political and ethical controversy of whether to terminate the affected pregnancy. Since Handyside et al.(1990) reported the first established pregnancy using PGD in 1990, the PGD cycles have been stably increasing worldwide (Geraedts et al., 2000; Harper et al., 2006; Sermon et al., 2005), and PGD has been extensively applied in sex-linked disorders, single gene disease, autosomal abnormalities, and aneuploid detection of women with advanced age. Therefore, PGD has been a practical option for avoiding the birth of affected children, representing an important complement to traditional prenatal diagnosis (Kuliev and Verlinsky, 2004).
PGS was applied primarily to infertile women with the following indications: advanced maternal age (AMA) (Kahraman et al., 2000), recurrent implantation failure (RIF) (Caglar et al., 2005; Taranissi et al., 2005) and recurrent miscarriage (RM) (Rubio et al., 2005b). PGD for numerical chromosome abnormalities has four potential benefits: (1) to prevent trisomic offspring by analyzing chromosomes 13, 16, 18, 21 and 22 (Kuliev et al., 2003); (2) to reduce spontaneous abortions (Munné et al., 1998b; Rubio et al., 2003); (3) to reduce multiple pregnancy by minimizing the number of embryos necessary for replacement and successful pregnancy (Munné, 2002); and (4) to improve implantation. Many cycles of PGS were performed worldwide using FISH probes for chromosome 13, 18, 21 and others. However, only 4 reports of 5 cases of PGD for specific chromosome 21 are available (Conn et al., 1998; 1999; Scriven et al., 2001; Luo et al., 2002). All cases had history of Down syndrome pregnancy. PGD was conducted using cleavage stage embryo biopsy and FISH analysis for chromosome 21. Each couple underwent one or two treatment cycles. The case information and results are summarized in Table 1.
Table 1.
The case information and PGD results
| Reference | Case | Maternal age | Pregnancy times (DS pregnancies) | Parental karyotypes | Cycle | Oocytes |
Biopsy diagnosis | Transferred | Outcome | ||
| Collected | Fertilized | Biopsied | |||||||||
| Conn et al. (1998) | 1a | 31 | 4 (3) | 45, XX, der (13; 21) (q10; q10) | 1 | 11 | 8 | 6 | 2 normal | Yes | NP |
| 2 monosomy 21, 13 | No | ||||||||||
| 1 monosomy 21 | No | ||||||||||
| 1 monosomy 13 | No | ||||||||||
| 2 | 14 | 10 | 5 | 2 monosomy 21, 13 | No | ||||||
| 1 tetrasomy 21 | No | ||||||||||
| 1 trisomy 21 | No | ||||||||||
| 1 unconclusive | No | ||||||||||
| Conn et al. (1999) | 2 | 36 | 4 (3) | Normal | 1 | 31 (total) | 13 (total) | 2 | 2 trisomy 21 | No | NP |
| 2 | 3 | 2 normal | Yes | ||||||||
| 1 trisomy 21 | No | ||||||||||
| 3 | 32 | 5 (1) | 46, XX, t (6;21) (q13; 22.3) | 1 | 32 (total) | 11 (total) | 4 | 1 normal | Yes | ABP | |
| 2 trisomy 21 | No | ||||||||||
| 1 monosomy 21 | No | ||||||||||
| 2 | 0 | ||||||||||
| Scriven et al.(2001) | 4b | 39 | 2 (1) | 45, XX, der (14; 21) (q10; q10) | 1 | 11 | 7 | 5 | 3 normal | Yes | NP |
| 1 normal | No | ||||||||||
| 1 trisomy 21, 14 | No | ||||||||||
| 2 | 15 | 10 | 9 | 3 normal | Yes | OHC | |||||
| 1 trisomy 21 | No | ||||||||||
| 1 monosomy 21 | No | ||||||||||
| 1 trisomy 14 | No | ||||||||||
| 1 monosomy 14 | No | ||||||||||
| 2 unconclusive | No | ||||||||||
| Luo et al. (2002) | 5 | 30 | 1 (1) | Normal | 1 | 14 | 13 | 8 | 3 normal | Yes | OHC |
| 3 normal | No | ||||||||||
| 1 trisomy 21 | No | ||||||||||
| 1 no signal | No | ||||||||||
| Author’s cases | 6 (case 1) | 43 | 3 (2) | Normal | 1 | 3 | 2 | 2 | 1 trisomy 21 | No | |
| 1 monosomy 21 | No | ||||||||||
| 7 (case 2) | 30 | 2 (2) | Normal | 1 | 10 | 8 | 8 | 2 normal | Yes | NP | |
| 3 trisomy 21 | No | ||||||||||
| 1 monosomy 21 | No | ||||||||||
| 2 unconclusive | No | ||||||||||
| 2 | 17 | 14 | 8 | 2 normal | Yes | NP | |||||
| 3 trisomy 21 | No | ||||||||||
| 3 unconclusive | No | ||||||||||
Probes: Locus-specific indicator (LSI) 13 and 21
Probes: LSI 21 and a biotinylated 14q subtelomere probe
NP: No pregnancy; OHC: One healthy child; ABP: A biochemical pregnancy
The PGD cycles summarized here were conducted for women who had pregnancies of Down syndrome previously. In total of above summarized reports and ours, 60 embryos were biopsied. Of them, only 22 (36.7%) were tested to be normal for chromosome 21, 18 (30.0%) transferred, 2 clinical pregnancies resulted, and two healthy children were born and no Down syndrome pregnancy occurred. In addition, the FISH results of surplus embryos (not biopsied because of fewer than six cells on day 3) showed that the abnormality rate was as high as 81.8% (18/22) (Conn et al., 1998; 1999; Cozzi et al., 1999; Scriven et al., 2001; Luo et al., 2002). These data indicate that applying PGD for couples with history of Down syndrome effectively avoided affected pregnancy, although the pregnancy rate is low. The data here also imply that successful pregnancy has more possibility occurring in couples with high rate of normal embryos (50% and more), as suggested by Scriven et al.(2001), Conn et al.(1998; 1999) and Luo et al.(2002).
Women who have a history of Down syndrome pregnancy are at high risk of recurrent Down syndrome (Hook, 1992). Data from livebirths and from amniocenteses in Europe (Stene et al., 1984; Warburton et al., 1987) showed that for women with a history of trisomy 21, there is an increased recurrence risk of trisomy 21, but not other trisomy. Although a wider selection of commercial probes is now becoming available, the development of suitable probe combinations for detecting multiple chromosome abnormality is expensive (Conn et al., 1999). Therefore, for patients with a history of Down syndrome pregnancy, applying PGD for chromosome 21 is practicable (Scriven et al., 2001; Luo et al., 2002).
The cause of recurrent Down syndrome is complex. Although studies have shown that most of recurrent trisomy 21 pregnancies may be the result of chance alone (Panaqalos et al., 1992), the possibility of parental translocation and gonadal mosaicism has important implications for recurrence risk. Other evidence suggests that women with a diminished ovarian reserve may have a higher risk of a trisomic conception, so that biological ovarian age may be a better indicator of trisomy risk than chronological age (van Montfrans et al., 2001). Recently, many suggestions for the mechanisms of non-age-related risks (absolute excess risk) have been put forward, indicating that women who experienced a Down syndrome pregnancy at a young age have a greater absolute excess risk of recurrence than those whose first Down syndrome pregnancy was at an older age (Morris et al., 2005). Thus, the causative reasons for high recurrence of Down syndrome in our cases and in Luo et al.(2002)’s in which both parents had a normal chromosome complement are needed for further analysis.
In conclusion, PGD for trisomy 21 with FISH avoids affected pregnancy and offers favorable results. Although standard prenatal diagnosis remains the method of choice for aneuploidy, for couples who had pregnancies with Down syndrome previously, PGD can also be considered, and has been shown to be an effective strategy.
Footnotes
Project supported by the National Basic Research Program of China (Nos. 2006CB944006 and 2006CB504004), the Key Research Program of Zhejiang Province, China (No. 2006C13078) and the Bureau of Science and Technology of Hangzhou, China (No. 20061123B03)
References
- 1.Caglar GS, Asimakopoulos B, Nikolettos N, Diedrich K, Al-Hasani S. Preimplantation genetic diagnosis for aneuploidy screening in repeated implantation failure. Reprod Biomed Online. 2005;10(3):381–388. doi: 10.1016/s1472-6483(10)61800-7. [DOI] [PubMed] [Google Scholar]
- 2.Cicero S, Bindra R, Rembouskos G, Spencer K, Nicolaides KH. Integrated ultrasound and biochemical screening for trisomy 21 using fetal nuchal translucency, absent fetal nasal bone, free beta-hCG and PAPP-A at 11 to 14 weeks. Prenat Diagn. 2003;23(4):306–310. doi: 10.1002/pd.588. [DOI] [PubMed] [Google Scholar]
- 3.Conn CM, Harper JC, Winston RM, Delhanty JD. Infertile couples with Robertsonian translocations: preimplantation genetic analysis of embryos reveals chaotic cleavage divisions. Hum Genet. 1998;102(1):117–123. doi: 10.1007/s004390050663. [DOI] [PubMed] [Google Scholar]
- 4.Conn CM, Cozzi J, Harper JC, Winston RM, Delhanty JD. Preimplantation genetic diagnosis for couples at high risk of Down syndrome pregnancy owing to parental translocation or mosaicism. J Med Genet. 1999;36(1):45–50. [PMC free article] [PubMed] [Google Scholar]
- 5.Cozzi J, Conn CM, Harper J, Winston RM, Rindl M, Farndon PA, Delhanty JD. A trisomic germ cell line and precocious chromatid segregation leads to recurrent trisomy 21 conception. Hum Genet. 1999;104(1):23–28. doi: 10.1007/s004390050905. [DOI] [PubMed] [Google Scholar]
- 6.Daniel A, Stewart L, Saville T, Brookwell R, Paul H, Purvis-Smith S, Lam-Po-Tang PR. Prenatal diagnosis in 3000 women for chromosome X-linked and metabolic disorders. Am J Med Genet. 1982;11(1):61–75. doi: 10.1002/ajmg.1320110109. [DOI] [PubMed] [Google Scholar]
- 7.Geraedts J, Handyside A, Harper J, Liebaers I, Sermon K, Staessen C, Thornhill A, Viville S, Wilton L. ESHRE PGD Consortium Steering Committee: ESHRE Preimplantation Genetic Diagnosis (PGD) Consortium: data collection II (May 2000) Hum Reprod. 2000;15(12):2673–2683. doi: 10.1093/humrep/15.12.2673. [DOI] [PubMed] [Google Scholar]
- 8.Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from boipsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. 1990;344(6268):768–770. doi: 10.1038/344768a0. [DOI] [PubMed] [Google Scholar]
- 9.Harper JC, Boelaert K, Geraedts J, Harton G, Kearns WG, Moutou C, Muntjewerff N, Repping S, SenGupta S, Scriven PN. ESHRE PGD consortium data collection V: cycles form January to December 2002 with pregnancy follow-up to October 2003. Hum Reprod. 2006;21(1):3–21. doi: 10.1093/humrep/dei292. [DOI] [PubMed] [Google Scholar]
- 10.Ho SS, O′Donoghue K, Choolani M. Fetal cells in maternal blood: state of the art for non-invasive prenatal diagnosis. Annu Acad Med Singapore. 2003;32(5):597–603. quiz 604. [PubMed] [Google Scholar]
- 11.Hook EB. Prevalence, Risks and Recurrence. In: Brock DJH, Rodeck CH, Ferguson-Smith MA, editors. Prenatal Diagnosis and Screening. Edinburgh: Churchill Livingston; 1992. pp. 351–392. [Google Scholar]
- 12.Kahraman S, Bahce M, Samli H, Imirzalioglu N, Yakisn K, Cengiz G, Donmez E. Healthy births and ongoing pregnancies obtained by preimplantation genetic diagnosis in patients with advanced maternal age and recurrent implantation failure. Hum Reprod. 2000;15(9):2003–2007. doi: 10.1093/humrep/15.9.2003. [DOI] [PubMed] [Google Scholar]
- 13.Kuliev A, Verlinsky Y. Thirteen years’ experience of preimplantation diagnosis: report of the Fifth International Symposium on Preimplantation Genetics. Reprod Biomed Online. 2004;8(2):229–235. doi: 10.1016/s1472-6483(10)60521-4. [DOI] [PubMed] [Google Scholar]
- 14.Kuliev A, Cieslak J, Ilkevitch Y, Verlinsky Y. Chromosomal abnormalities in a series of 6733 human oocytes in preimplantation diagnosis for age-related aneuploidies. Reprod Biomed Online. 2003;6(1):54–59. doi: 10.1016/s1472-6483(10)62055-x. [DOI] [PubMed] [Google Scholar]
- 15.Luo C, Xing FQ, Luo SQ. Normal pregnancy in a woman having had a child with trisomy 21 syndrome before: application of preimplantation genetic diagnosis. Di Yi Jun Yi Da Xue Xue Bao. 2002;22(3):269–271. (in Chinese) [PubMed] [Google Scholar]
- 16.Mikkelsen M, Stene J. Previous Child with Down Syndrome and Other Chromosome Aberrations. In: Murken JD, Stengel-Rutkwski S, Schwinger E, editors. Proceedings of the 3rd European Conference on Prenatal Diagnosis of Genetic Disorders. Dtuggart: Enke; 1979. pp. 22–29. [Google Scholar]
- 17.Morris JK, Mutton DE, Alberman E. Recurrences of free trisomy 21: analysis of data from the National Down Syndrome Cytogenetic Register. Prenat Diagn. 2005;25(12):1120–1128. doi: 10.1002/pd.1292. [DOI] [PubMed] [Google Scholar]
- 18.Muller F, Benattar C, Audibert F, Roussel N, Dreux S, Cuckle H. First-trimester screening for Down syndrome in France combining fetal nuchal translucency measurement and biochemical markers. Prenat Diagn. 2003;23(10):833–836. doi: 10.1002/pd.700. [DOI] [PubMed] [Google Scholar]
- 19.Munné S. Preimplantation genetic diagnosis of numerical and structural chromosome abnormalities. Reprod Biomed Online. 2002;4(2):183–196. doi: 10.1016/s1472-6483(10)61938-4. [DOI] [PubMed] [Google Scholar]
- 20.Munné S, Fung J, Cassel MJ, Marquez C, Weier HU. Preimplantation genetic analysis of translocations: case-specific probes for interphase cell analysis. Hum Genet. 1998;102(6):663–674. doi: 10.1007/s004390050759. [DOI] [PubMed] [Google Scholar]
- 21.Munné S, Magli C, Bahce M, Fung J, Legator M, Morrison L, Cohert J, Gianaroli L. Preimplantation diagnosis of the aneuploidies most commonly found in spontaneous abortions and live births: XY, 13, 14, 15, 16, 18, 21, 22. Prenat Diagn. 1998;18(13):1459–1466. doi: 10.1002/(SICI)1097-0223(199812)18:13<1459::AID-PD514>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Munné S, Marquez C, Reing A, Garrisi J, Alikani M. Chromosome abnormalities in embryos obtained after conventional in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 1998;69(5):904–908. doi: 10.1016/S0015-0282(98)00039-9. [DOI] [PubMed] [Google Scholar]
- 23.Munné S, Sandalinas M, Escudero T, Velilla E, Walmsley R, Sadowy S, Cohen J, Sable D. Improved implantation after preimplantation genetic diagnosis of aneuploidy. Reprod Biomed Online. 2003;7(1):91–97. doi: 10.1016/s1472-6483(10)61735-x. [DOI] [PubMed] [Google Scholar]
- 24.Munné S, Escudero T, Colls P, Xuezhong Z, Oter M, Garrisi M, Barnes F, Zouves C, Werlin L, Maqli C, et al. Predictability of preimplantation genetic diagnosis of aneuploidy and translocations on prospective attempts. Reprod Biomed Online. 2004;9(6):645–651. doi: 10.1016/s1472-6483(10)61775-0. [DOI] [PubMed] [Google Scholar]
- 25.Nicolaides KH. Nuchal translucency and other first-trimester sonographic markers of chromosomal abnormalities. Am J Obstet Gynecol. 2004;191(1):45–67. doi: 10.1016/j.ajog.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 26.Panaqalos CG, Talbot CCJr, Lewis JG, Adelsberqer PA, Petersen MB, Serre JL, Rethore MO, de Blois MC, Parent P, Schinzel AA. DNA polymorphism analysis in families with recurrence of free trisomy 21. Am J Hum Genet. 1992;51(5):1015–1027. [PMC free article] [PubMed] [Google Scholar]
- 27.Roizen NJ, Patterson D. Down’s syndrome. Lancet. 2003;361(9365):1281–1289. doi: 10.1016/S0140-6736(03)12987-X. [DOI] [PubMed] [Google Scholar]
- 28.Rubio C, Simon C, Vidal F, Rodrigo L, Pehlivan J, Remohi J, Pellicer A. Chromosomal abnormalities and embryo development in recurrent miscarriage couples. Hum Reprod. 2003;18(1):182–188. doi: 10.1093/humrep/deg015. [DOI] [PubMed] [Google Scholar]
- 29.Rubio C, Rodrigo L, Perez-Cano I, Mercader A, Mateu E, Buendia P, Remohi J, Simon C, Pellicer A. FISH screening of aneuploidies in preimplantation embryos to improve IVF outcome. Reprod Biomed Online. 2005;11(4):497–506. doi: 10.1016/s1472-6483(10)61146-7. [DOI] [PubMed] [Google Scholar]
- 30.Rubio C, Pehlivan T, Rodrigo L, Simon C, Remohi J, Pellicer A. Embryo aneuploidy screening for unexplained recurrent miscarriage: a minireview. Am J Reprod Immunol. 2005;53(4):159–165. doi: 10.1111/j.1600-0897.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- 31.Schrurs BM, Winston RM, Handyside AH. Preimplantation diagnosis of aneuploidy using fluorescent in situ hybridization: evaluation using a chromosome 18-specifid probe. Hum Reprod. 1993;8(2):296–301. doi: 10.1093/oxfordjournals.humrep.a138040. [DOI] [PubMed] [Google Scholar]
- 32.Scriven PN, Flinter FA, Braude PR, Ogilvie CM. Robertsonian translocations reproductive risks and indications for preimplantation genetic diagnosis. Hum Reprod. 2001;16(11):2267–2273. doi: 10.1093/humrep/16.11.2267. [DOI] [PubMed] [Google Scholar]
- 33.Sermon K, Moutou C, Harper J, Geraedts J, Scriven P, Wilton L, Magli MC, Michiels A, Viville S, de Die C. ESHRE PGD consortium data collection IV: May-December 2001. Hum Reprod. 2005;20(1):19–34. doi: 10.1093/humrep/deh552. [DOI] [PubMed] [Google Scholar]
- 34.Solassol J, Rahil H, Sapin V, Lemery D, Dastugue B, Boespflug-Tanguy O, Creveaux I. Detection of trisomy 21 by quantitative fluorescent-polymerase chain reaction in uncultured amniocytes. Prenat Diagn. 2003;23(4):287–291. doi: 10.1002/pd.579. [DOI] [PubMed] [Google Scholar]
- 35.Spencer K, Spencer CE, Power M, Dawson C, Nicolaides KH. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years prospective experience. BJOG. 2003;110(3):281–286. doi: 10.1046/j.1471-0528.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- 36.Stene J, Stene E, Mikkelsen M. Risk for chromosome abnormality at amniocentesis following a child with a non-inherited chromosome aberration. A European Collaborative Study on Prenatal Diagnoses 1981. Prenat Diagn. 1984;4(7):81–95. doi: 10.1002/pd.1970040707. [DOI] [PubMed] [Google Scholar]
- 37.Taranissi M, El-Toukhy T, Gorgy A, Verlinsky Y. Influence of maternal age on the outcome of PGD for aneuploidy screening in patients with recurrent implantation failure. Reprod Biomed Online. 2005;10(5):628–632. doi: 10.1016/s1472-6483(10)61670-7. [DOI] [PubMed] [Google Scholar]
- 38.van Montfrans JM, Lambalk CB, van Hooff MHA, Vugt JMG. Are elevated FSH concentrations in the pre-conceptional period a risk factor for Down’s syndrome pregnancies? Hum Reprod. 2001;16(6):1270–1273. doi: 10.1093/humrep/16.6.1270. [DOI] [PubMed] [Google Scholar]
- 39.van Steirteghem AC, Nagy Z, Joris H, Liu J, Staessen C, Smitz J, Wisanto A, Devroey P. High fertilization and implantation rates after intracytoplasmic sperm injection. Hum Reprod. 1993;8(7):1061–1066. doi: 10.1093/oxfordjournals.humrep.a138192. [DOI] [PubMed] [Google Scholar]
- 40.Warburton D, Kline J, Stein Z, Hutzler M, Chin A, Hassold T. Does the karyotype of a spontaneous abortion predict the karyotype of a subsequent abortion? Evidence from 273 women with two karyotyped sponstaneous abortions. Am J Hum Genet. 1987;41(3):465–483. [PMC free article] [PubMed] [Google Scholar]
- 41.Yang YH, Kim SH, Yang ES, Kim SK, Kim IK, Park YW, Cho JS, Lee YH. Prenatal diagnosis of fetal trisomy 21 from maternal peripheral blood. Yonsei Med J. 2003;44(2):181–186. doi: 10.3349/ymj.2003.44.2.181. [DOI] [PubMed] [Google Scholar]
- 42.Yang YH, Yang ES, Kwon JY, Kim IK, Park YW. Prenatal diagnosis of trisomy 21 with fetal cells in maternal blood using comparative genomic hybridization. Fetal Diagn Ther. 2006;21(1):125–133. doi: 10.1159/000089062. [DOI] [PubMed] [Google Scholar]