Abstract
Researchers broadly agree that the best way to halt the AIDS epidemic is to develop a vaccine against HIV. But despite huge investment a vaccine is proving elusive, as Alison Tonks explains
The AIDS pandemic is now more than 25 years old, and for most of its history, scientists have been searching for an effective vaccine against HIV. There have been many false dawns, dashed hopes, and disappointments along the way as evangelical rhetoric has eventually given way to a more pragmatic acceptance that a vaccine would be great, and may even be possible, but it won't be on offer at a clinic near you any time soon. The most optimistic experts predict it will be at least another 10 years before any kind of vaccine is available1; the most pessimistic say it could take 50. Even then, the first vaccines will probably be only partially effective.2 Why is such an important task taking so long?
The trouble with HIV
HIV is one of the most complex viruses ever identified, and it's extremely good at evading any immune mediated strategy directed against it. HIV is already genetically diverse—there are currently nine genetic subtypes (or clades) of HIV1, the most prevalent strain—and new forms are emerging all the time. HIV mutates rapidly so scientists are trying to hit a constantly moving target.3 4 Any successful vaccine must be effective against multiple subtypes and will need constant surveillance and modification to keep it ahead of the inevitable steady stream of new variants.
HIV has a full menu of other defences. Critical surface proteins that help HIV enter human cells are protected by a layer of sugary molecules called N-linked glycans and by the ability to change shape during the process of infection. It's also a retrovirus, inserting its own genetic material into human cells quickly and efficiently. Once established, infection permanently weakens the body's defences.
An HIV vaccine would have only a few days or weeks to prevent HIV from establishing a permanent foothold in the body.3 That's asking a lot for any vaccine, but with HIV scientists are in uncharted waters. There are no existing vaccines against retrovirus infections in humans, and there are no cases of natural immunity to help guide vaccine development. So far, over 60 million people have been infected with HIV worldwide. Not one has managed to clear the virus completely, even after successful treatment with antiretroviral drugs. We don't know what a successful immune response against HIV looks like. Scientists are still trying to characterise the elusive “immune correlates of protection”—the specific immune responses that a vaccine must stimulate to successfully prevent infection.
Traditional techniques don't work
Vaccinologists' traditional weapons seem useless against such challenges. Live attenuated (or inactivated) vaccines, which have been so successful in the past against smallpox, polio, and measles, are not an option against HIV because of the theoretical possibilities of infection or shedding of live virus.4 5 6
Another traditional vaccine strategy—raising neutralising antibodies to disable the virus and prevent infection—has also run aground. Back in the mid-1980s scientists found and characterised one of the surface glycoproteins that helps HIV gain entry to human cells. The protein is called gp120, and later a genetically engineered version became the basis for the first vaccine to reach an advanced stage of human testing (AIDSVax, VaxGen).
Researchers thought the gp120 vaccine would induce neutralising antibodies that would bind the invading virus and stop HIV entering cells, protecting the vaccinated person from infection. But in seminal trials published in 2005 and 2006, the vaccine failed to protect either gay men or injecting drug users despite producing antibodies in 90% of those vaccinated.7 8 The antibodies simply weren't versatile enough to cope with HIV's genetic diversity.
Progress is further hampered by the lack of a reliable animal model to road test candidate vaccines. Monkeys with simian immunodeficiency virus (SIV) are the lab rats of HIV vaccine research, but important differences between monkeys with SIV and humans with HIV have misled researchers at least once. The gp120 vaccine worked well in chimpanzees.3 When it comes to testing HIV vaccines, only humans will do.
What are the options?
Although the failure of the gp120 vaccine was not an unexpected disappointment, researchers remain doggedly determined to find new immunogens that work better (or even work at all). A vaccine that induces broadly neutralising antibodies is still the holy grail of HIV vaccine research, because scientists believe it is the only sure fire way of preventing HIV infection. “We know these kind of vaccines are the key to preventing HIV, and we also know they are achievable in the long run,” says Wayne Koff, senior vice president of research and development at the International AIDS Vaccine Initiative, “We've already isolated monoclonal antibodies with broadly neutralizing capabilities, and we're expecting further real advances within the next few years.” But scientists like Dr Koff are still at the basic science stage of their research. They have had to go back to the laboratory to work backwards from these antibodies to find the immunogens that might stimulate their production. And it's slow going. No clinical trials are on the horizon.
Researchers have turned instead to the cell mediated arm of the immune system, the T lymphocytes that can find and destroy cells infected with HIV. Even in natural infections this component of the immune system can control viral replication for at least a few years.5 A vaccine that stimulates T cells would not prevent infection in the traditional sense but might at least suppress the infection long enough to delay or even prevent the onset of AIDS, reduce patients' dependence on antiretroviral drugs, and help stop the virus spreading.
Experts agree that this kind of vaccine, even a partially effective one, is the best we can hope for in the medium term. “The idea of using vaccines to control rather than prevent an infection is not so outrageous,” says Andrew McMichael, director of the Weatherall Institute of Molecular Medicine at Oxford University and leading AIDS researcher. “Many of us are infected with Epstein-Barr virus, for example. But our immune system keeps the virus well under control so for most of us it's harmless. If we could get to get to a situation with HIV that mimics that natural control of Epstein-Barr virus, that would be great.”
Two T cell vaccines are already being tested in placebo controlled trials. The biggest, a phase III trial based in Thailand, has already randomised over 16 000 uninfected volunteers.9 Half of them have been given a combined vaccine designed to induce both T cells and neutralising antibodies. Researchers hope the combined strategy, called “prime and boost,” will work better than either strategy alone. But this approach is controversial, according to Professor McMichael, partly because researchers are using the failed gp20 subunit vaccine as part of the package.
Perhaps more hopeful is a recombinant adenovirus vector vaccine that carries three harmless HIV genes in to human cells. The genes produce foreign proteins that stimulate a cell mediated immune response. This vaccine, which is manufactured by Merck, is being tested in 6000 uninfected volunteers in two trials. “These trials are critical,” says Professor McMichael. “Any clear evidence of an effect would be enormously encouraging because it's the first vaccine to test the concept of protective cell mediated immunity alone in humans. A similar vaccine worked well in monkeys, suppressing replication of simian immunodeficiency virus, so I'm hopeful.” Preliminary results are expected in the next two or three years.
Vaccine options
Live attenuated or whole killed HIV—Safety fears have halted development of vaccines containing whole HIV
Subunit vaccines use harmless HIV proteins or peptides to induce an antibody response. The failed gp20 vaccine was the first. No other subunit vaccines are close to being tested in clinical trials
DNA vaccines use isolated HIV genes to induce a cell mediated immune response. The genes produce foreign HIV proteins when incorporated into human cells. DNA vaccines are currently in phase I trials
Recombinant vector vaccines use viral vectors to transport isolated HIV genes in to human cells. Viral vectors are weakened viruses, so do not cause infection. Most vaccines under development use this technique, including the only two in large scale clinical trials: a canary pox viral vector vaccine and an adenovirus vector vaccine containing the genes gag, pol, and nef
Combination vaccines— Researchers hope combinations of different designs, strategies, or immunogens will produce a broader, more powerful, or more durable immune response
At the very least, repeated disappointments and setbacks have taught this determined research community a valuable lesson in humility. The buzz surrounding the two leading candidate vaccines remains muted. They have learnt to expect less. “The history of vaccine research is defined by successes built on failures,” notes Dr Koff. “Just look at malaria. Developing vaccines has always been a slow iterative process. Learning what doesn't work enables scientists to focus on what eventually does work.”
Dr Gary Nabel, director of the US National Institutes of Health's Vaccine Research Centre is equally cautious: “This is going to be a long road. I think that these initial studies will hopefully allow us to put a stake in the ground and say that it is possible to generate immunity and tell us what mechanisms may be most effective. And then it will be up to us to refine that going down the road. We need to dig in for the long haul.”
Will a vaccine be worth the effort?
The vaccine research community is in harmony over this question. “Vaccines would be the best way to control HIV,” says Professor McMichael. “Even a partially effective vaccine would be a start, something we can build on.” On the other side of the Atlantic, Anthony Fauci and Margaret Johnston from the US National Institute of Allergy and Infectious Diseases agree that a vaccine would be an “enormously valuable tool” in the worldwide effort to control a pandemic that still infects an estimated 14 000 people every day.3
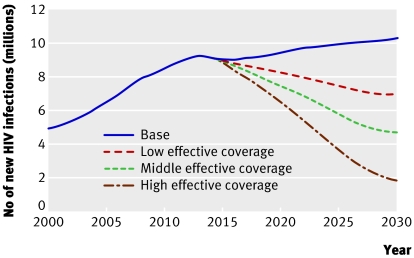
It's hard to know with any certainty how a vaccine would change the pandemic's trajectory. But the International AIDS Vaccine Initiative, a not for profit organisation devoted to finding a vaccine, estimates that a vaccine licensed in 2015 could prevent between one tenth and one half of the projected 150 million new infections expected between 2015 and 2030.10 Without one, the number of new infections each year would increase from around 6 million today to around 10 million by 2030 (fig 1).10
Fig 1 Effect of different vaccine scenarios on new infections in adults and children living in low and middle income countries (adapted from Stover J10)
A vaccine would have the biggest effect in low and middle income countries. But, no one still believes, if they ever did, that a vaccine alone will be enough. A partially effective vaccine could even accelerate the pandemic by taking the brakes off high risk behaviours. Existing control measures including education, condoms, clean needle exchanges, and widely available antiretroviral drugs will become more, not less, important should such a vaccine ever get a licence.3
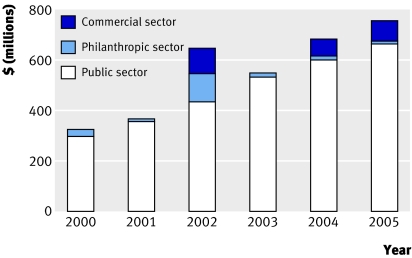
The belief in an HIV vaccine is so powerful that the search is fast becoming a global research industry. The past decade has seen a bewildering proliferation of collaborations, consortiums, agencies, and organisations dedicated to the effort and now led by the Global HIV Vaccine Enterprise (box).11 Predictably, funding for research is failing to keep up with demand. The drugs industry is only just dipping its toe in the water. Most of the money spent to date has come from the public sector (with the US giving the lion's share), with regular top-ups from the Bill and Melinda Gates Foundation (fig 2).12 The enterprise estimates that to fully implement its strategy will cost $1.2bn (£600m; €90m) a year.13 In 2006, the global spend outside the commercial sector totalled only $684m.14
Fig 2 Funding sources for research into HIV vaccine (adapted from AIDS vaccine blueprint 200612)
Key organisations in HIV vaccine research
International AIDS Vaccine Initiative (www.iavi.org)
AIDS Vaccine Advocacy Coalition (www.avac.org)
Center for HIV-AIDS Vaccine Immunology (www.chavi.org)
Joint United Nations Programme on HIV/AIDS at (www.unaids.org)
HIV Vaccine Trials Network (www.hvtn.org)
Collaboration for AIDS Vaccine Discovery (www.cavd.org)
Vaccine Research Center at the National Institutes of Health (www.vrc.nih.gov/VRC)
EuroVacc Foundation (www.eurovacc.org)
Global HIV Vaccine Enterprise (www.hivvaccineenterprise.org)
Professor McMichael thinks the enterprise is doing a great job of setting the research agenda and coordinating the worldwide effort to find a vaccine. “But we still need a bit of room at the edges for innovation and free thinking. A small part of the global funding should still go to individuals working outside the mainstream. One good idea may be all it takes.”
HIV vaccine research has come a long way since the wildly overoptimistic predictions made by desperate politicians in the mid-1980s. The world waited over a century for a vaccine against typhoid once the causative agent had been identified. Later, it took nearly half a century to develop vaccines against polio and measles.5 HIV has rewritten the rule book since then, and researchers have had to start once again from scratch.
“The search for an AIDS vaccine is a far greater challenge than sending a man to the moon,” wrote Mike Powell and Mitchell Warren, president and executive director of the AIDS Vaccine Advocacy Coalition in their 2006 report. “When it came down to the space race, we knew where we were; we knew where the moon was; and we knew, roughly, how to get there. It was, essentially, an engineering problem. When it comes to an AIDS vaccine, we don't know where the moon is—yet. But that doesn't stop us from aiming for the heavens.”15
Competing interests: None declared.
References
- 1.Will there be an HIV vaccine in the next 10 years? Nat Med 2007;13:518-9. [Google Scholar]
- 2.Day M. AIDS expert doubts vaccine will be found in near future. BMJ 2007;334:1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston MI, Fauci AS. An HIV vaccine—evolving concepts. N Engl J Med 2007;356:2073-81. [DOI] [PubMed] [Google Scholar]
- 4.Gallo RC. The end or the beginning of the drive to an HIV preventive vaccine: a view from over 20 years. Lancet 2005;366:1894-8. [DOI] [PubMed] [Google Scholar]
- 5.Markel H. The search for effective HIV vaccines. N Engl J Med 2005;353:753-7. [DOI] [PubMed] [Google Scholar]
- 6.Horton R. AIDS: the elusive vaccine. New York Review of Books 2004;51(14). www.nybooks.com/articles/17400
- 7.rgp120 HIV Vaccine Study Group. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005;191:654-65. [DOI] [PubMed] [Google Scholar]
- 8.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind placebo controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006;194:1661-71. [DOI] [PubMed] [Google Scholar]
- 9.Department of Diseases Control, Thailand Ministry of Public Health. Prime-boost HIV vaccine phase III trial. www.primeboost3.org/press_eng_160505.htm
- 10.Stover J. Estimating the global impact of an AIDS vaccine New York: International AIDS Vaccine Initiative, 2005.
- 11.Global HIV Vaccine Enterprise. About the enterprise www.hivvaccineenterprise.org/index.html
- 12.International AIDS Vaccine Initiative. AIDS vaccine blueprint 2006 New York: IAVI, 2006.
- 13.Coordinating Committee of the Global HIV/AIDS Vaccine Enterprise. Global HIV/AIDS Vaccine Enterprise: scientific strategic plan. PLoS Med 2005;2:e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher K, Lamourelle G, Harrison P, Warren M, Finley B, Gandhi G et al. Adding it all up: funding for HIV vaccine and microbicide development, 2000 to 2005 Geneva: HIV Vaccines and Microbicides Resource Tracking Working Group, 2006
- 15.AIDS Vaccine Advocacy Coalition. AIDS vaccines: the next frontiers New York: AVAC, 2006. www.avac.org/pdf/reports/2006_Report/AVAC_Report_2006_single.pdf