Abstract
In an attempt to investigate the effects of treatment of human leishmaniasis, the cytokines produced by peripheral blood mononuclear cells (PBMCs) of patients with cutaneous leishmaniasis (CL) and mucocutaneous leishmaniasis (MCL) under treatment with amphotericin B were determined during the active disease from cocultures of cells and Leishmania (Viannia) braziliensis antigens. PBMC of these patients exhibited a nonsignificant marginal increased production of TNF-α upon antigen stimulation. However, under the same antigenic stimulus, patients with active MCL presented higher IFN-γ production compared to patients with CL. This increased IFN-γ production was accompanied by a drastically augmented IL-12 synthesis from cells of MCL patients. The highlighted T cell responses could be relevant for sound control measures of protozoan infections with emphasis on the combined usage of immunoenhancing agents and antiprotozoal drugs.
1. INTRODUCTION
Leishmaniasis is a vector-borne disease caused by obligate intramacrophage protozoan parasites of the genus Leishmania [1, 2]. The infecting Leishmania species determines the clinical presentation of disease, of which there are three dominant clinical forms: cutaneous leishmaniasis (CL), mucocutaneous leishmaniasis (MCL), and visceral leishmaniasis [1, 2]. In Bolivia, the etiological agent of both, CL and MCL is Leishmania (Viannia) braziliensis, formerly known as the L. braziliensis complex [3]. While CL is characterized by single or multiple ulcerated dermal lesions, MCL which develops as a complication of L. (V.) braziliensis CL in 5%–20% of patients [4] from parasite dissemination to the upper respiratory tract mucosa, involving the nasal, pharyngeal, and laryngeal mucosa, leads to extensive tissue destruction [5, 6]. CL either heals spontaneously or promptly responds to antimonial therapy but MCL usually evolves chronically and is difficult to treat [7]. Then, amphotericin B (amB) is an alternative for patients who fail to respond to pentavalent antimonial therapy.
It has been known that amB potentiates the antimicrobial and tumoricidal activities of macrophages [8], either directly [9] or via induction of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), as well as generation of a respiratory burst [10, 11]. Apart from these effects, little is known about the mechanisms associated with the efficacy of this compound in the treatment of MCL. Therefore, it was of interest to determine the participation of other soluble factors, apart from TNF-α, in amB-treated mucocutaneous leishmaniasis, keeping in mind that activation of the infected macrophages to kill intracellular parasites is carried out through a cell-mediated response that requires the classic features of antigen presentation and production of IL-12 by macrophages and activation of TH1 lymphocytes with production of interferon-γ (IFN-γ) to activate the macrophages. The present study was aimed at elucidating the participation of critical soluble factors associated with amB treatment that could alleviate, in future, the collateral effects of this arduous treatment by combining immunochemotherapy with lower doses of drug.
In the present investigation, we present evidence for an exacerbated TH1 immune response in MCL treated with amB, manifested by an elevated synthesis of IFN-γ which directly relates to a great increase in IL-12 production.
2. MATERIALS AND METHODS
Patients
Twenty four leishmaniasis patients were included in this study, 12 with CL and 12 with MCL, including male and female, average age 30 years old. All of them acquired the disease in the Yungas Valley of La Paz Department, an endemic area for L (V.) braziliensis infection. Patients included in the study presented clinical features compatible with CL or MCL, were positive in both the Montenegro skin test and the serology for L. (V.) braziliensis antibodies (indirect immunofluorescence). At the moment of taking the blood samples, MCL patients were being treated with amphotericin B, at a dose of 1 mg/kg/day by infusion till a total dose of 1 to 3 grams, and had received mean doses of 7.5 (5–10 doses). CL patients were not receiving treatment when blood samples were taken. Informed consent was obtained from each participating donor before taking blood samples.
Antigens
The parasite lysate (ALb) utilized for cytokine production was obtained from an L. (V.) braziliensis strain (MHOM/BR/75/2903). The promastigotes were resuspended in phosphate-buffered saline (PBS) pH 7.2, at a concentration of 1 × 108 parasites per mL, and soluble antigens were prepared through seven cycles of freezing (−70°C) and thawing (37°C) the parasite suspension. This material was assayed for protein content, aliquoted, and stored at −70°C until used.
Culture of PBMC
PBMCs were purified by centrifugation (400 g, 20°C, 45 minute) over a mixture of Ficoll Hypaque at a density of 1.077 (Sigma, St. Louis, Mo, USA). After washings with serum free medium, the cells were resuspended at the desired concentration in RPMI medium containing 10% heat-inactivated human AB serum (Sigma), 100 IU of penicillin per mL, and 100 μg of streptomycin per mL (complete medium). Fresh PBMCs were cultured in duplicate in 24 well plates at a final concentration of 1.25 × 106 cells/mL in 2 mL complete medium for 3days (37°C, 5% CO2), in the absence or presence of ALb, at a final protein concentration of 15 μg/mL.
Cytokine assays
Aliquots of cell-free supernatants from ALb in vitro-stimulated PBMC cultures were assayed for TNF-α, IFN-γ, and IL-12 by means of solid phase sandwich enzyme linked immunosorbent assays (ELISAs) (BioSource Europe, Belgium). All samples were tested in duplicate and cytokine concentrations were determined by comparison to standard curves. The sensitivity of each assay was as follows: TNF-α, 3 pg/mL; IFN-γ, 0.03 IU/mL; and IL-12, 1.5 pg/mL.
Statistical analysis
Statistical analysis was performed by the Wilcoxon nonparametric test using the Systat software, version 10.2 (Systat Software Inc., Richmond, Calif, USA). The level of significance was set at P < .05.
3. RESULTS AND DISCUSSION
The course of MCL has been associated with an unmodulated high production of the proinflammatory cytokines IFN-γ and TNF-α [12]. Considering the high activity of amB in the treatment of MCL, we decided to compare specific cytokine production between PBMC from CL and MCL patients, through an in vitro cell culture approach with ALb, that would recreate the status of patients' immune response.
Cytokine production by PBMCs from CL and MCL patients
The response of PBMC induced by ALb stimulation was evaluated in terms of TNF-α, IFN-γ, and IL-12 production, at 3 days of culture. While a 72-hour culture period has proven sufficient to stimulate production of TNF-α and IFN-γ in patients' PBMCs stimulated with leishmanial antigens [12], IL-12 production is specifically stimulated as early as 24 hours of PBMC culture from MCL patients [13].
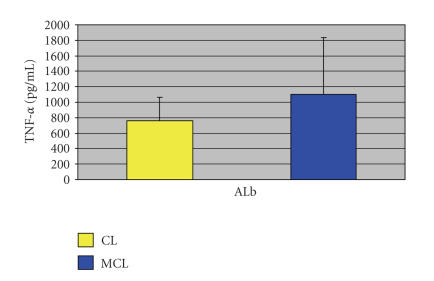
Whatever the patient group, TNF-α, IFN-γ, and IL-12 were released at similar background levels. Therefore, in this study, the levels of cytokine released in unstimulated PBMC cultures did not reflect an activated state from contact in vivo with parasite antigens. Figure 1 reveals a nonsignificant slight increase in the production of TNF-α in MCL (1104 ± 732 pg/mL), comparing with CL (760 ± 307 pg/mL) patients (P = .1).
Figure 1.
TNF-α production in cell-free supernatants of CL and MCL patients' PBMCs measured by ELISA upon ALb stimulation.
The reduced liberation of TNF-α in the supernatants of MCL patients is surprising considering that amB has been associated with its production [14] but it also reflects the beneficial effect of this drug in MCL as it has been reported that refractory mucosal leishmaniasis can be successfully treated through a combination of pentavalent antimony plus pentoxifylline, an inhibitor of TNF-α production [15]. Alternatively to the activation of macrophage microbicidal capacity through the induction of proinflammatory cytokines, amB can also exert its effect intracellularly. Intracellular accumulation of the drug in monocytes augmented the capacity of the cells to kill ingested Candida albicans [16]. However, whether this mechanism of action is in fact operating in MCL treatment remains to be confirmed.
Contrary to the production of TNF-α, samples from MCL patients had the capacity to significantly augment synthesis of IFN-γ with regard patients with CL (17.1 ± 11.5 versus 4.2 ± 3.2 IU/m) [P < .05] (Figure 2). Furthermore, the observation of background levels of IL-4 despite stimulation with ALb (not shown) evokes a TH1-type immune response.
Figure 2.
IFN-γ production in cell-free supernatants of CL and MCL patients' PBMCs measured by ELISA upon ALb stimulation.
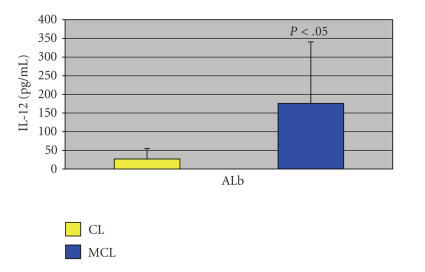
Additionally, and in direct correlation to the increased production of IFN-γ, there was a much higher concentration of IL-12 when comparing MCL (175.7 ± 164.8 pg/mL) and CL (26.8 ± 28.2 pg/mL) patient cytokine responses (P < .05) (Figure 3). Future studies in vitro with amB will seek to verify the cellular source of IL-12 considering that, in human, peripheral blood monocyte/macrophages are the main producers of IL-12 [17]. A previous study [18] reporting suppression of IL-12 production by murine macrophages infected with L. mexicana amastigotes on interaction with TH1 cells is of particular interest in the context of this study as it adds incentive to unveil through the present experimental system (ALb stimulation) the intracellular signals, likely set in motion by amB treatment, to increase production of IL-12, one of the two cytokines most clearly needed for protection in leishmaniasis.
Figure 3.
IL-12 production in cell-free supernatants of CL and MCL patients' PBMCs measured by ELISA upon ALb stimulation.
In general, the present results are reminiscent of a previous investigation on the use of a recombinant leishmanial antigen from Leishmania braziliensis [13]. Apart from the production of IL-2, this antigen elicited also the production of IFN-γ dependent on IL-12, from PBMCs of patients with mucosal and cutaneous leishmaniasis. By analogy to our observations, it could be postulated that amB treatment would favor, preferentially, processing of antigens inducing TH1-type immune responses, whereby stimulation of antigen-presenting cells by IFN-γ leads to IL-12 production, potentiating in this manner, a positive feedback loop. Even though amB killing of Leishmania parasites does not require a host immune response [19], we reason that similar targeting of the TH1-cell mechanism might increase its efficacy and permit lower doses to be used with compatible activities.
An important aspect to consider in the present study is that cytokines are being compared in two patients groups differing by two parameters, MCL versus CL and treated versus untreated patients. Hence, there would be the possibility that the increase in IFN-γ and IL-12 relates to the different clinical forms rather than to amB treatment. However, it is interesting to note that samples from three MCL patients, not under treatment and excluded from this study, had the capacity to produce lower levels of IFN-γ compared with treated patients, and this production was not associated with an increased release of IL-12.
Therefore, manipulation of the host's immune response in favor of the TH1-cell-associated mechanism may provide the opportunity to use amB-sparing regimens with lower doses of drug, fewer injections, and/or a shorter treatment duration, avoiding toxicity associated with the cumulative dose. Future studies will seek to improve understanding on the mechanisms of action of amB at the cellular level, particularly those associated with increased IL-12 production.
ACKNOWLEDGMENTS
Funding for this work was provided by Sustainable Sciences Institute, San Francisco, California, and by the French Embassy in La Paz, Bolivia.
References
- 1.Herwaldt BL. Leishmaniasis. The Lancet. 1999;354(9185):1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 2.Dedet JP. Les Leishmanioses, In: Ellipses (France and Maghreb countries) and Edicef-Hachette (International) eds. AUPELF-UREF Médecine tropicale collection. Paris, 2003: 13–245.
- 3.Desjeux P, Quilici M, Lapierre J. On 113 cases of cutaneous leishmaniasis and mucocutaneous leishmaniasis observed in Bolivia. Sero-immunologic study of 71 cases. Bulletin de la Société de Pathologie Exotique et de ses Filiales. 1974;67(4):387–395. [PubMed] [Google Scholar]
- 4.David C, Dimier-David L, Vargas F, Torrez M, Dedet JP. Fifteen years of cutaneous and mucocutaneous leishmaniasis in Bolivia: a retrospective study. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87(1):7–9. doi: 10.1016/0035-9203(93)90398-a. [DOI] [PubMed] [Google Scholar]
- 5.Castes M, Agnelli A, Rondon AJ. Mechanisms associated with immunoregulation in human American cutaneous leishmaniasis. Clinical and Experimental Immunology. 1984;57(2):279–286. [PMC free article] [PubMed] [Google Scholar]
- 6.Convit J, Ulrich M, Fernandez CT, et al. The clinical and immunological spectrum of American cutaneous leishmaniasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87(4):444–448. doi: 10.1016/0035-9203(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 7.Marsden PD. Mucosal leishmaniasis (“espundia” Escomel, 1911) Transactions of the Royal Society of Tropical Medicine and Hygiene. 1986;80(6):859–876. doi: 10.1016/0035-9203(86)90243-9. [DOI] [PubMed] [Google Scholar]
- 8.Perfect JR, Granger DL, Durack DT. Effects of antifungal agents and γ interferon on macrophage cytotoxicity for fungi and tumor cells. Journal of Infectious Diseases. 1987;156(2):316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- 9.Berman JD, Wyler DJ. An in vitro model for investigation of chemotherapeutic agents in leishmaniasis. Journal of Infectious Diseases. 1980;142(1):83–86. doi: 10.1093/infdis/142.1.83. [DOI] [PubMed] [Google Scholar]
- 10.Wolf JE, Massof SE. In vivo activation of macrophage oxidative burst activity by cytokines and amphotericin B. Infection and Immunity. 1990;58(5):1296–1300. doi: 10.1128/iai.58.5.1296-1300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vonk AG, Netea MG, Denecker NEJ, Verschueren ICMM, van der Meer JWM, Kullberg BJ. Modulation of the pro- and anti-inflammatory cytokine balance by amphotericin B. Journal of Antimicrobial Chemotherapy. 1998;42(4):469–474. doi: 10.1093/jac/42.4.469. [DOI] [PubMed] [Google Scholar]
- 12.Bacellar O, Lessa H, Schriefer A, et al. Up-regulation of Th1-type responses in mucosal leishmaniasis patients. Infection and Immunity. 2002;70(12):6734–6740. doi: 10.1128/IAI.70.12.6734-6740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skeiky YAW, Guderian JA, Benson DR, et al. A recombinant Leishmania antigen that stimulates human peripheral blood mononuclear cells to express a Th1-type cytokine profile and to produce interleukin 12. Journal of Experimental Medicine. 1995;181(4):1527–1537. doi: 10.1084/jem.181.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chia JKS, Pollack M. Amphotericin B induces tumor necrosis factor production by murine macrophages. Journal of Infectious Diseases. 1989;159(1):113–116. doi: 10.1093/infdis/159.1.113. [DOI] [PubMed] [Google Scholar]
- 15.Lessa HA, Machado P, Lima F, et al. Successful treatment of refractory mucosal leishmaniasis with pentoxifylline plus antimony. American Journal of Tropical Medicine and Hygiene. 2001;65(2):87–89. doi: 10.4269/ajtmh.2001.65.87. [DOI] [PubMed] [Google Scholar]
- 16.Martin E, Stuben A, Gorz A, Weller U, Bhakdi S. Novel aspect of amphotericin B action: accumulation in human monocytes potentiates killing of phagocytosed Candida albicans. Antimicrobial Agents and Chemotherapy. 1994;38(1):13–22. doi: 10.1128/aac.38.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Andrea A, Rengaraju M, Valiante NM, et al. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. Journal of Experimental Medicine. 1992;176(5):1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinheber N, Wolfram M, Harbecke D, Aebischer T. Phagocytosis of Leishmania mexicana amastigotes by macrophages leads to a sustained suppression of IL-12 production. European Journal of Immunology. 1998;28(8):2467–2477. doi: 10.1002/(SICI)1521-4141(199808)28:08<2467::AID-IMMU2467>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Murray HW, Hariprashad J, Fichtl RE. Treatment of experimental visceral leishmaniasis in a T-cell-deficient host: response to amphotericin B and pentamidine. Antimicrobial Agents and Chemotherapy. 1993;37(7):1504–1505. doi: 10.1128/aac.37.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]