Abstract
Millions are exposed to ozone levels above recommended limits, impairing lung function, causing epithelial damage and inflammation, and predisposing some individuals to pneumonia, asthma, and other lung conditions. Surfactant protein A (SP A) plays a role in host defense, the regulation of inflammation, and repair of tissue damage. We tested the hypothesis that the lungs of SP-A(−/−) (KO) mice are more susceptible to ozone-induced damage. We compared the effects of ozone on KO and wild type (WT) mice on the C57BL/6 genetic background by exposing them to 2 parts/million of ozone for three or six hours and sacrificing them 0, 4, and 24 hours later. Lungs were subject to bronchoalveolar lavage (BAL) or used to measure endpoints of oxidative stress and inflammation. Despite more total protein in BAL of KO mice after a 3hr ozone exposure, WT mice had increased oxidation of protein and had oxidized SP-A dimers. In KO mice there was epithelial damage as assessed by increased LDH activity and there was increased phospholipid content. In WT mice there were more BAL PMNs and elevated macrophage inflammatory protein (MIP)-2 and monocyte chemoattractant protein (MCP)-1. Changes in MIP-2 and MCP-1 were observed in both KO and WT, however mRNA levels differed. In KO mice MIP-2 mRNA levels changed little with ozone, but in WT levels they were significantly increased. In summary, several aspects of the inflammatory response differ between WT and KO mice. These in vivo findings appear to implicate SP-A in regulating inflammation and limiting epithelial damage in response to ozone exposure.
Keywords: surfactant, SP-A, ozone, host defense, inflammation, oxidation, macrophage, pollution, injury, repair
INTRODUCTION
Millions of Americans are exposed to ozone levels exceeding the recommended National Ambient Air Quality Standards (NAAQS) limit. Exposure to ozone, a highly toxic constituent of photochemical smog, results in lung inflammation and may predispose some individuals to infection or exacerbate other lung diseases (Chauhan and Johnston, 2003; Kelly et al., 2003). Although the mechanism remains unclear, ozone-induced changes include an influx and activation of immune cells and increases in cytokines and chemokines, including MCP-1 and MIP-2 (Driscoll et al., 1993; Johnston et al., 2002; Zhao et al., 1998).
Ozone may be capable of both damaging the epithelium directly and triggering the ensuing inflammatory cascade that causes indirect damage. Pulmonary surfactant, a mixture of lipids and proteins, is situated at the air-liquid interface in the lung where it may be modified by this highly reactive oxidant. Surfactant protein A (SP-A), in addition to involvement in surfactant secretion, uptake, and metabolism by type II cells (Haagsman and Van Golde, 1991), plays an important role in innate immunity and affects several aspects of host defense function (Crouch and Wright, 2001; Floros and Phelps, 2002; Phelps, 2001; Wright, 2003), particularly the enhancement of phagocytosis of a number of microorganisms (Allen et al., 1999; Ding et al., 2004; Madan et al., 1997; Mikerov et al., 2005; Van Iwaarden et al., 1991). SP-A also augments production of reactive oxygen and nitrogen species (RONS), a mechanism to kill pathogens (Hickman-Davis et al., 2004; LeVine et al., 2002; Madan et al., 1997). Deficits in these functions in SP-A(−/−) mice makes them susceptible to pneumonia (Atochina et al., 2004; LeVine et al., 1998; LeVine et al., 2002). Evidence also indicates SP-A involvement in the regulation of inflammation, particularly with regard to lung injury and repair, by regulating proinflammatory cytokines (Alcorn and Wright, 2004; Song and Phelps, 2000), clearance of apoptotic cells (Reidy and Wright, 2003; Vandivier et al., 2002), and regulation of collagen and matrix metalloproteinases (Vazquez de Lara et al., 2000; Vazquez de Lara et al., 2003). Findings of increased macrophages and elevated matrix metalloproteinases in the SP-A(−/−)/SP-D(−/−) double knockout versus the SP-D(−/−) knockout (Hawgood et al., 2002) support the notion that SP-A is involved in macrophage regulation and in lung injury and repair.
The surfactant lipids, while primarily responsible for the reduction of surface tension, also influence immune cell behavior. They suppress activation of immune cells and inhibit production and release of cytokines (Kremlev and Phelps, 1997; Walti et al., 1997). However, both SP-A and lipids can be oxidized by ozone or endogenous RONS (Haddad et al., 1996; Kuzmenko et al., 2004; Putman et al., 1997; Uhlson et al., 2002; Wang et al., 2004; Zhu et al., 1996; Zhu et al., 1998), altering their function and potentially contributing to ozone-induced inflammation.
Experimental and clinical data suggest that the glutathione-redox cycle is important in the regulation of oxidant:antioxidant balance and the control of RONS (Rahman and MacNee, 1999; Rahman and MacNee, 2000). Lung cells contain various antioxidants and related enzymes, such as those affecting glutathione metabolism (Heffner and Repine, 1989). These substances can eliminate RONS generated during normal metabolism and changes in glutathione metabolism, and are widely recognized as a central feature of many inflammatory lung diseases (Rahman and MacNee, 1999; Rahman and MacNee, 2000).
This study assessed in vivo the role of SP-A in ozone-induced lung injury by studying the responses of C57BL/6 wild type (WT) mice and SP-A(−/−) (KO) mice on the same genetic background to ozone exposure. The data presented in this report focus on events occurring immediately after exposure to ozone and within the next 24 hours, following either a 3hr or a 6hr exposure to ozone.
MATERIALS AND METHODS
Animals
We used pathogen-free male SP-A(+/+) C57BL/6 mice (wild type; WT) (Jackson Laboratories, Bar Harbor, ME) and SP-A(−/−) (KO) mice (20-25g) on the C57BL/6 genetic background. Breeder pairs of KO mice were obtained from Dr. Samuel Hawgood (University of California, San Francisco) and propogated in the animal facility at the Penn State College of Medicine. Mice were bred and maintained under pathogen-free conditions with food and water freely available. The Institutional Animal Care and Use Committee at the Penn State College of Medicine approved this study.
Experimental Model
WT and KO mice (5-6 weeks old) were exposed to 2 parts/million (ppm) ozone or to filtered air (FA) at room temperature and 50% humidity for 3 or 6hr. Flow rates were significantly high to ensure multiple air changes per minute within the chambers. All FA and ozone exposures were conducted in parallel. A dose response study of ozone exposure (0.5, 1.0, 2.0 ppm) was performed at the 4hr time point to assess the overall response for markers under study. Although similar results were observed at the 1 ppm and 2 ppm doses for most parameters, we chose the 2 ppm dose for further study because the response was more robust for some parameters. These findings are consistent with previously reported studies (Johnston et al., 1999; Johnston et al., 2005). For each treatment condition and each time point three to six mice were used. These were put in containers with wire mesh lids and placed in the glass exposure chambers. The system accurately delivers ozone concentrations between 0.1-10ppm, as described (Umstead et al., 2002). Mice were sacrificed immediately after exposure (0hr), at 4hr, or at 24hr. Bronchoalveolar lavage (BAL) was performed in some mice and in others lungs were excised, either with or without perfusion (depending on the parameters to be studied). The lungs were then weighed and frozen immediately in liquid nitrogen.
Cell Counts and Biochemical Studies of BAL Fluid
BAL was obtained by instilling saline equal to 80% of lung vital capacity (3 times, 1.5ml total) through a tracheal cannula. Recovery was ∼90% of instilled volume and did not differ significantly between groups. BAL was centrifuged (150×g, 5min, 4°C), the cell pellet resuspended, and total and differential cell counts obtained. Cell-free BAL was frozen at −80°C for subsequent analysis. Total protein determinations were made by Micro BCA Protein Assay (Pierce Biotechnology, Rockford, IL), and total phospholipids were measured using the Phospholipids B Assay (WAKO Chemicals Inc, Richmond, VA).
Lactate dehydrogenase (LDH) activity in the cell-free BAL was determined with the CytoTox 96® Non-Radioactive Cytotoxicity assay (Promega Corporation, Madison, WI) using the manufacturer's instructions.
Cytokine ELISAs
MCP-1 and MIP-2 were measured in BAL fluid by ELISA assay (R&D systems, McKinley Place, NE). These ELISA kits had detection limits of 1.5 pg/ml for MIP-2 and 2 pg/ml for MCP-1. Briefly, cell-free BAL (500μl) was lyophilized, reconstituted to 100μl with assay diluent, assays performed, and read at 450nm. The five-fold concentration of the BAL fluid did not appear to adversely affect the ELISAs.
SP-A Dot Blot
SP-A levels in the BAL were determined by protein dot blot (Phelps et al., 2004). Protein dot blotting was done by diluting 25 μl of the cell-free BAL fluid in 975 μl of 0.02 M tris-buffered saline (pH 7.5; TBS) solution and applying 200 μl aliquots to Bio-Rad Trans Blot transfer medium (nitrocellulose, 0.45 μm) under vacuum in a 96 well dot blot apparatus (Bio-Rad, Hercules, CA). Blots were blocked overnight in 0.01 M Na/K phosphate buffered saline (pH 7.2; PBS) containing 1% bovine serum albumin (BSA). Following blocking, blots were incubated in a solution containing polyclonal rabbit anti-SP-A IgG (1:10,000) in PBS with 1% BSA, 0.05% Tween-20 for 1 hour at RT and washed 3 times for 10 minutes with PBS, 0.5% Tween-20. The blots were then incubated for 1 hour at RT with secondary antibody (goat anti-rabbit IgG HRP conjugate; 1:25,000) (BioRad) and then washed as in the previous step. Antibody binding was detected by enhanced chemiluminescence (ECL). Blots were incubated for 1 minute with 10 ml of each ECL solution (PerkinElmer Life Sciences, Boston, MA). They were then wrapped in cellophane, the excess solution was removed, and the blot exposed to Kodak X-Omat XAR film (Eastman Kodak Co., Rochester, NY). The film was developed and SP-A levels were quantified by laser densitometry.
Semi-Quantitative RT-PCR for SP-A, MCP-1, and MIP-2 mRNA Expression
RNA (2μg) from frozen pulverized lung (Vazquez de Lara et al., 2003) was denatured for 10min at 70°C and reverse transcribed with Maloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA) for 10min at 25°C, 60min at 42°C, and 5min at 95°C. A gradient RoboCycler PCR System (Stratagene, La Jolla, CA) was used for PCR with the following primer pairs:
SP-A Forward 5'-GACGTTTGTGTTGGAAGCCCTGG-3':
SP-A Reverse 5'-GGTACCAGTTGGTGTAGTTCACAG-3';
MCP-1 Forward 5'-CGGTTTTGGAAAAGTGGTTG-3':
MCP-1 Reverse 5'-CCTACGGGATCTGAAAGACG-3';
MIP-2 Forward 5'-CCCTGCCAAGGGTTGACTTC-3':
MIP-2 Reverse 5'-GGCACATCAGGTACGATCCAG-3';
18S rRNA served as an internal standard (QuantumRNA™ 18S Internal standard, Ambion, Austin, TX). Product sizes for SP-A, MCP-1, MIP-2, and 18S are 570, 300, 287, and 489 bp, respectively. PCR conditions were: initial step 2min at 95°C followed by 21 cycles for SP-A, 29 cycles for MCP-1, and 34 cycles for MIP-2 30sec at 95°C, 1min at 55°C for all 4 sets of primer pairs and 72°C for 10min. From each PCR mixture 10 μl were mixed with 1μL loading buffer (50% glycerol, 1mM EDTA-Na2, 0.04% bromophenol blue, 0.04% xylene cyanole) and run on 1.2% agarose gels, stained in ethidium bromide, and band intensity evaluated using Gel Analyzing Imager (Kodak ID Image Analysis Software, Rochester, NY).
Detection of Oxidized Protein and oxidized SP-A in BAL
Oxidized proteins were detected using OxyBlot Oxidized Protein Detection Kit (Intergen, Purchase, NY) as described (Umstead et al., 2002) with some modifications. Briefly, 25 μl of BAL samples were denatured by adding an equal volume of 12% SDS. Samples were then derivatized with 2.5 μl of 10× 2, 4-dinitriphenylhydrazine (DNPH) solution and incubated for 10 min at room temperature. Derivatization was stopped with the addition of 25 μl of neutralization solution. Samples were then analyzed by dot blot. Aliquots containing the DNPH-derivatized proteins were brought up to a volume of 500 μl with 0.01 M phosphate buffer (pH 7.2) and 200 μl of each sample was blotted onto nitrocellulose by vacuum using a 96-well dot-blot apparatus (Bio-Rad). Immunodetection of oxidized proteins was performed according to the manufacturer's instructions, although the rabbit anti-DNP and goat anti-rabbit IgG (HRP-conjugated) antibodies supplied were used at half the recommended concentrations. ECL was used to detect antibody binding, blots were exposed to XAR film (Eastman Kodak Co., Rochester, NY), and band intensity quantified by laser densitometry.
Oxidized SP-A protein was detected by the method of Robinson et al with some modifications (Robinson et al., 1999). Briefly 200 μl aliquots of BAL samples were concentrated by using ULTRAFREE® MC centrifugal filtration device (Millipore Corporation, Bedford, MA, USA) with a molecular weight cutoff limit of 10,000. Protein samples were then subjected to electrophoresis on 12.5% SDS polyacrylamide gels, the separated proteins transferred to polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) in a buffer of 25 mM Tris base, 192 mM glycine, 20% methanol and the membrane dehydrated for 1 minute with 100% methanol. The membrane was washed for 5 minutes in 0.02 M Tris (pH 7.5) with 20% methanol, 5 minute with 2 N hydrochloric acid (HCl), and then treated with 100 μg DNPH/ml 2 N HCl for 5 minutes. The membrane was again washed 3 times with 2 N HCl, 7 times with 100% methanol, and one time with 0.02 M TBS (5 minutes each wash). The membrane was blocked overnight in 5% powdered milk (Carnation) in 0.02 M TBS, 0.05% Tween-20. All the post-transfer steps were done in 100 ml of solution at room temperature. Immunodetection of oxidized proteins was done, as described above for BAL protein dot blots.
Preparation of Lung Homogenates
Enzyme assays were done using perfused, pulverized, homogenized lung tissue in 0.1M sodium phosphate buffer (pH 7.4) to make a 10% homogenate. Post-mitochondrial supernatant (PMS) was obtained by centrifuging the homogenate at 8,000×g for 20min at 4°C, after which the supernatant was removed and stored at −80°C until assayed. For GSH assay, pulverized tissue was homogenized in 5% m-phosphoric acid to make a 10% homogenate, incubated on ice for 10min, vortexed vigorously, centrifuged at 8,000×g at 4°C for 10min, and the supernatant removed and frozen until assayed. Protein determinations of supernatants were done using Bio-Rad Protein Assay (Bio-Rad, Hercules, CA).
Estimation of Reduced and Oxidized Glutathione
Total and oxidized glutathione content were determined by the enzymatic recycling method (Baker et al., 1990) with some modifications. The freshly prepared assay mixture consisting of 175 μl of 0.3 mM NADPH in 0.1 M phosphate buffer (pH 7.4) with 1 mM EDTA and 25 μl of glutathione reductase (10 U/ml) was placed in a microtiter plate and 50 μl of lung supernatant from the homogenate was added and incubated for 10 minutes at room temperature. Twenty-five μl of Ellman's reagent (6 mM 5,5”-dithio-bis(2-nitrobenzoic acid)) was added to start the reaction in a total volume of 275 μl and the absorbance of the sample immediately read in a SPECTRA Fluor Plus microtiter ELISA plate reader at 405 nm for 6 minutes at intervals of 120 sec to determine the change in absorbance/min. These values were used to obtain levels of total GSH. Oxidized glutathione was measured after the masking of reduced glutathione in the reaction mixture with 2μl of 97% 2-vinylpyridine and 3μl of 14% triethanolamine (98% 2, 2', 2”-nitroethanol diluted with 6 volumes of water). After 1hr at room temperature the mixture was analyzed as described above. Glutathione standards were prepared over a range of 25-500nM/well.
Enzyme Assays
Glutathione peroxidase, glutathione reductase, and glutathione S-transferase were measured in PMS of lung homogenate by microtiter methods with minor modifications (McFarland et al., 1999).
Statistical Methods
Data were analyzed by t-test (Sigma Stat; SPSS; Chicago, IL).
RESULTS
Behavioral Observations
Mice exposed to ozone behaved differently from those in FA. Soon after ozone exposure the fur ruffles, 30min to 1hr later the ozone-exposed mice become less active, curl up, and apparently sleep through the exposure. Their activity returns to normal within 1hr after exposure. Mice exposed to FA are active throughout the exposure. Both WT and KO mice behaved similarly under both exposure conditions (3 and 6hr). Moreover, a relatively consistent pattern of response in most of the parameters measured was observed for both exposure times.
Total Protein, Oxidized Protein, and oxidized SP-A
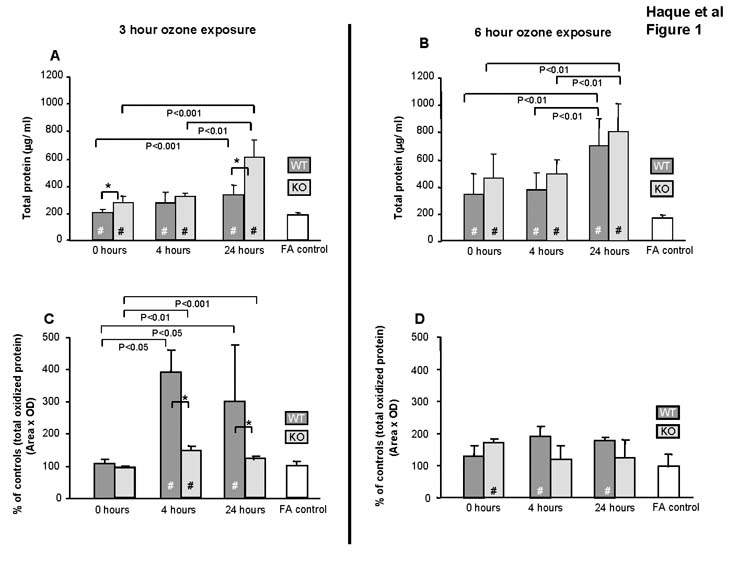
Exposure to ozone in both WT and KO mice resulted in significant increases in BAL total protein at all time points compared to FA-exposed controls for both ozone exposure protocols (Fig. 1A and 1B). In general, the increases tended to be larger in KO mice than in WT (n=6/group), with significant differences between WT and KO (Fig 1A) at 0 and 24hr points (p<0.05). There were no differences between FA-treated WT and KO mice for any time points.
Figure 1.
Effect of ozone on total protein and total oxidized protein content in BAL. Amounts of total protein (Fig. 1A and 1B) and oxidized protein (Fig. 1C and 1D) in cell-free BAL (n=6/group) were quantified after a 3hr and 6hr exposure to FA or ozone. Values (mean ± SD) for WT (dark gray bars), KO (light gray bars), and FA negative control (open bars) are shown for various post-exposure recovery times. FA values are represented by a single bar because no differences were seen in KO and WT mice for the various time points. All ozone-exposed values that are significantly different from FA controls are indicated by a (#) within the bar. In all panels differences (p≤0.05) between WT and KO after ozone-exposure are indicated by asterisks (*) and connecting bars. All other pair-wise comparisons are noted by connecting bars with the p value for each comparison shown under each connecting bar. In Fig. 1A and 1B all ozone-treated samples differ (p≤0.05) from FA. In Fig. 1C and 1D FA values at each time point are set at 100% and values in ozone-exposed mice are expressed as % of control.
The degree of oxidation of BAL protein was determined by measuring carbonyl content (Fig. 1C). The levels of oxidized protein in WT and KO mice did not significantly differ from the FA control immediately following ozone exposure, but significant differences from FA control at 4 and 24hr points were observed. Oxidation of total protein was lower in KO mice versus WT at both 4 and 24hr (p<0.05). No significant differences were observed between WT and KO after a 6hr exposure (Fig 1D).
After ozone, SP-A levels in BAL from WT mice were unchanged, but SP-A mRNA levels were significantly higher at 4hr (not shown). As expected, similar analyses on samples from KO mice detected neither SP-A protein nor mRNA.
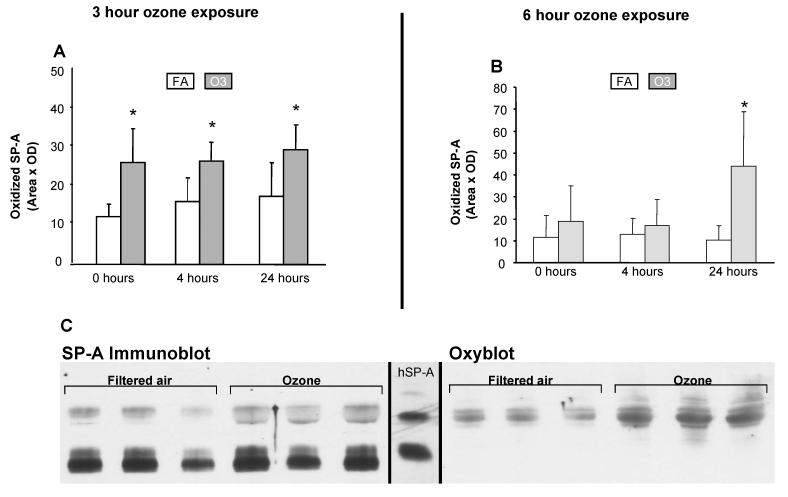
Ozone exposure significantly increased levels of oxidation of SP-A at 4hr after exposure (Fig. 2A and 2C-oxyblot), as observed with total oxidized BAL protein (Fig. 1C). However, increases in oxidized SP-A were observed immediately following ozone exposure, as well as at 4 and 24hr following a 3hr ozone exposure (Fig. 2A), and at 24hr for the 6hr ozone exposure (Fig. 2B). Figure 2C depicts a representative Western blot used for quantitation of the 4hr point following a 3hr ozone exposure. The ECL-exposed film of an SP-A immunoblot is shown where both SP-A monomer and dimer are detected by a polyclonal SP-A antibody with little difference in total SP-A (monomer + dimer) between samples exposed to FA or ozone. An OxyBlot of an ECL-exposed film of an identically prepared blot demonstrates that only the dimeric form of SP-A is detected even though the replicate immunoblot confirms the presence of ample amounts of monomeric SP-A. Moreover, the amount of oxidation of dimeric SPA increases after ozone exposure. A similar pattern was observed for all time points where a significant difference in SP-A oxidation was detected (Fig. 2C).
Figure 2.
Effect of ozone on oxidation of SP-A. Densitometric measurements of oxidized SP-A in BAL of WT mice exposed to ozone or FA are shown for 0, 4, and 24hr points after a 3hr (Fig. 2A) or a 6hr ozone exposure. Asterisks (*) indicate significant differences (p≤0.05) between FA and ozone-exposed samples (n=6/group). Representative Western blots of SP-A (SP-A Immunoblot) and oxidized SP-A (Oxyblot) are shown for the 4hr point after a 3hr ozone exposure (Fig. 2B). SDS gel electrophoresis was performed on concentrated samples from 200 μl of BAL fluid. The blots are immunostained with SP-A antiserum (SP-A immunoblot) or with the OxyBlot kit (Oxyblot). The SP-A bands on OxyBlot were identified by comparison with an identical SP-A immunoblot. A reference lane containing immunostained human alveolar proteinosis SP-A (hSP-A) with bands of monomeric and dimeric SP-A is shown between panels.
Total phospholipids and LDH activity in BAL
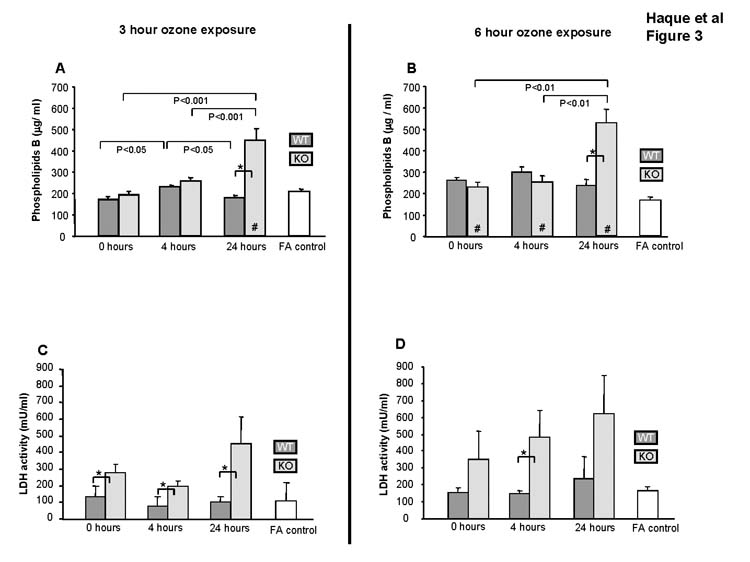
Total BAL phospholipids (n=3/group) following a 3hr ozone exposure were quantified by Phospholipids B assay (Fig. 3A). In WT mice ozone exposure did not affect phospholipids compared to FA at any time point. In KO mice phospholipid levels were significantly increased at 24hr after exposure (p<0.05). Analysis of BAL for lipid peroxidation failed to identify any consistent pattern resulting from ozone exposure (not shown). Similar observations were made for the 6hr ozone exposure protocol (Fig. 3B).
Figure 3.
Effect of ozone on BAL. The levels of phospholipids (Fig. 3A and 3B) and LDH (Fig. 3C and 3D) in BAL were determined (n=3/group). Values (mean ± SD) for WT (dark gray bars), KO (light gray bars), and FA negative control (open bars) are shown for various recovery times following a 3hr and a 6 hr ozone exposure. FA values are represented by a single bar because no differences were seen in FA controls from KO and WT mice for the various time points. All ozone-exposed values that are significantly different from FA controls are indicated by a (#) within the bar. In all panels differences (p≤0.05) between WT and KO after ozone-exposure are indicated by asterisks (*) and connecting bars. All other pair-wise comparisons are noted by connecting bars with the p value for each comparison shown under each connecting bar.
To assess the impact of ozone on tissue damage, we measured LDH in BAL (Fig. 3C). Following ozone exposure no changes were observed in WT mice versus FA, but in KO mice LDH levels were elevated compared to WT (p<0.05) for all time points of the 3hr ozone exposure protocol (Fig. 3C). Similar trends were observed for the 6hr protocol (Fig. 3D).
BAL Cells
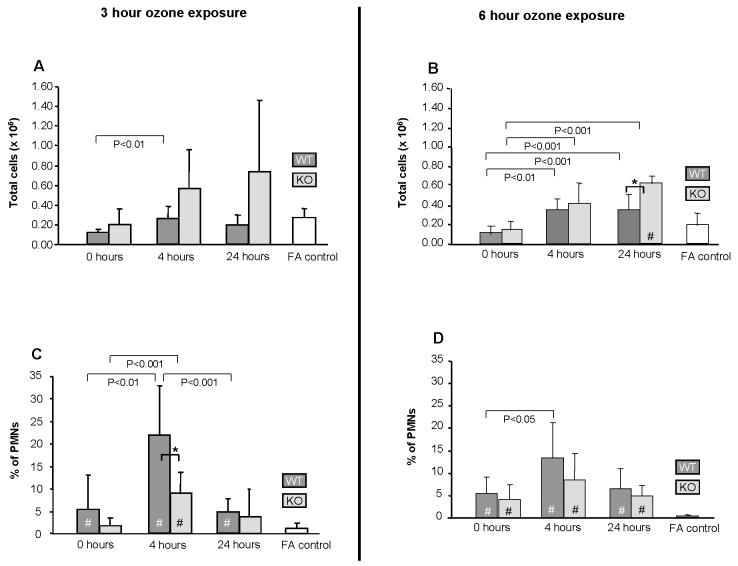
The total number of cells (Fig. 4A) in BAL from WT and KO mice was similar (n=6/group). Although ozone exposure at 2ppm for 3hr (Fig. 4A) or 6hr (Fig. 4B) had little effect on total cells in WT mice, there was a trend toward increased cells in KO mice. However, these changes achieved significance only at 24hr following a 6hr exposure (Fig. 4B).
Figure 4.
Effect of ozone on the recovery of total cells and PMNs in BAL. Total (Fig. 4A and 4B) and PMN (Fig. 4C and 4D) cell counts are shown (n=6/group). Values (mean ± SD) for WT (dark gray bars), KO (light gray bars), and FA negative control (open bars) are shown for various recovery times following a 3hr ozone exposure. FA values are represented by a single bar because no differences were seen in FA controls from KO and WT mice for the various time points. All ozone-exposed values that are significantly different from FA controls are indicated by a (#) within the bar. In all panels differences (p≤0.05) between WT and KO after ozone-exposure are indicated by asterisks (*) and connecting bars. All other pair-wise comparisons are noted by connecting bars with the p value for each comparison shown under each connecting bar.
Despite the lack of consistent change in total cells in BAL after ozone exposure, differential cell counts revealed a significant increase in the percentage of PMNs in ozone-exposed mice versus FA-exposed mice at most time points following either ozone exposure (3 or 6hr). As expected, the increase in PMNs after ozone is mirrored by a significant decrease in monocytes/macrophages at most points (not shown). The maximum increase in PMNs was 4hr after ozone exposure. In WT mice PMNs were 21.8% and 13.4% of total cells in the 3 and 6hr ozone-exposed groups, respectively, versus less than 1% in either corresponding FA group. The increases in PMNs in ozone-exposed KO mice at 0 and 4hr points were 40-60% below those in WT mice with the greatest difference (p<0.05) occurring 4hr after a 3hr exposure (Fig. 4C). The percent of PMNs was similar between WT and KO at the 24hr time point (Fig. 4C). There were no significant differences in monocytes/macrophages observed between WT and KO mice exposed to either FA or ozone at the time points studied. After a 6hr exposure PMNs were 25-36% below the WT, with the greatest difference also occurring at the 4hr point (Fig. 4D).
Effect of Ozone on the Production of Cytokines
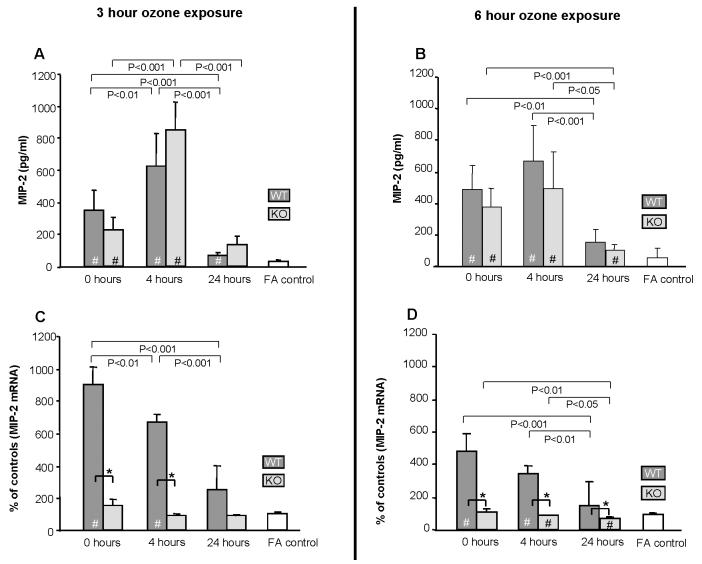
MIP-2 was detected in all samples (n=6/group) and significantly elevated (p<0.05) versus the FA control immediately after ozone exposure in almost all time points examined in both WT and KO (Fig. 5A, 5B). The levels continued to increase and were much higher at 4hr after exposure. By 24hr, MIP-2 levels were markedly reduced and almost back to normal. No significant differences were observed between WT and KO.
Figure 5.
Effect of ozone on MIP-2 protein levels in BAL and mRNA in lung. MIP-2 protein (n=6/group) was assayed by ELISA (Fig. 5A and 5B) and mRNA (n=3) was determined by quantitative RT-PCR (Fig. 5C and 5D). mRNA values were corrected using 18S ribosomal RNA as internal standard and expressed as % of control for each time point. Values (mean ± SD) for WT (dark gray bars), KO (light gray bars), and FA negative control (open bars) are shown for various recovery times. FA values are represented by a single bar because no differences were seen in FA controls from KO and WT mice for the various time points. All ozone-exposed values that are significantly different from FA controls are indicated by a (#) within the bar. In all panels differences (p≤0.05) between WT and KO after ozone-exposure are indicated by asterisks (*) and connecting bars. All other pair-wise comparisons are noted by connecting bars with the p value for each comparison shown under each connecting bar.
Levels of MIP-2 mRNA were significantly higher in WT ozone-exposed mice than in FA controls at nearly all time points (Fig. 5C, 5D). The greatest differences were immediately after exposure and although somewhat lower, remained significantly elevated compared to FA control at 4hr post-exposure. At the 24hr point, the differences compared to FA control, although not significant (Fig. 5C) exhibited a trend similar to that observed in the 6hr ozone exposure protocol (Fig. 5D; p<0.05). In KO mice, conversely, there was no significant increase in MIP-2 mRNA after ozone exposure. On the contrary, at 4 and 24hr after 6hr of ozone (Fig. 5D) there was a small, but significant, decrease in MIP-2 mRNA in KO mice. Levels of MIP-2 mRNA were significantly lower at 0 and 4hr points in ozone-treated KO mice (p<0.05) versus WT (Fig. 5C), as well as at 24hr (Fig. 5D).
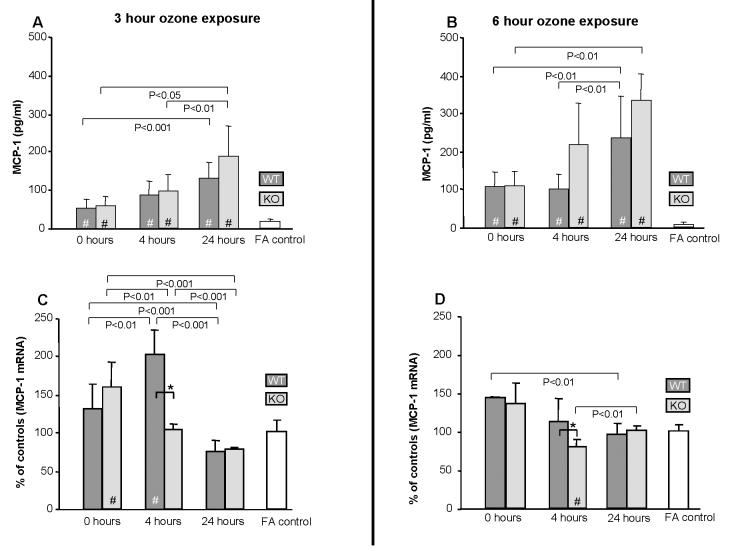
MCP-1 was detected in all samples and followed a different pattern than MIP-2 (Fig. 6A, 6B). It was significantly elevated in ozone-exposed mice compared to FA at all points in both time exposure groups (p<0.05), with similar patterns being observed in WT and KO mice. No differences between WT and KO mice were observed. Levels of MCP-1 mRNA were significantly (p<0.05) higher in WT mice only at 4hr following a 3hr (Fig. 6C) or 6hr (Fig. 6D) ozone exposure when compared to FA or to KO mice.
Figure 6.
Effect of ozone on MCP-1 protein levels in BAL and mRNA in lung. MCP-1 protein (n=6/group) was assayed by ELISA (Fig. 6A and 6B) and mRNA (n=3) was determined by quantitative RT-PCR (Fig. 6C and 6D). mRNA values were corrected using 18S ribosomal RNA as internal standard and expressed as % of control for each time point. Values (mean ± SD) for WT (dark gray bars), KO (light gray bars), and FA negative control (open bars) are shown for various recovery times. FA values are represented by a single bar because no differences were seen in FA controls from KO and WT mice for the various time points. All ozone-exposed values that are significantly different from FA controls are indicated by a (#) within the bar. In all panels differences (p≤0.05) between WT and KO after ozone-exposure are indicated by asterisks (*) and connecting bars. All other pair-wise comparisons are noted by connecting bars with the p value for each comparison shown under each connecting bar.
Glutathione Metabolism
Various endpoints related to glutathione metabolism were measured. Levels of reduced and oxidized glutathione were assayed, and total glutathione and the ratio of reduced to oxidized glutathione calculated. Levels of total glutathione were unaffected by ozone exposure. In the 3hr WT exposure group there were small, but significant, increases in reduced glutathione at 4 and 24hr after ozone (not shown). However, similar changes were not observed in the 6hr exposure WT or in the KO mice. Oxidized glutathione levels were elevated at some time points with no observable consistent change. Total glutathione and the ratio of oxidized to reduced glutathione changed little.
Levels of activity of glutathione S-transferase, glutathione reductase, and glutathione peroxidase were not altered in either mouse strain after ozone exposure (not shown).
DISCUSSION
SP-A is important for several immune cell functions, the regulation of inflammation, and in processes related to lung injury and repair. The surfactant lipids, in addition to their role in surface tension reduction, can suppress some SP-A actions (Kremlev et al., 1997; Kremlev and Phelps, 1994; Phelps, 2001; Song and Phelps, 2000). We studied KO and WT mice with the same genetic background to assess the in vivo role of SP-A in the regulation of inflammation and oxidative stress induced by acute ozone exposure. We found many similarities in responses, but also some pronounced differences. These included differences in differential cell counts (% PMNs), total and oxidized BAL protein, cytokine regulation, and phospholipids levels. We speculate that these differences occur because immune cells from the KO mice are functionally impaired by SP-A's absence and we propose the following scenario: Ozone exposure causes tissue damage and protein exudation, resulting in an inflammatory response which then aids in the removal of damaged tissue and exudate by immune cells (primarily macrophages) and subsequent repair of tissue damage. Lack of SP-A compromises these processes. Moreover, we observed that ozone exposure (2 ppm) for 3hr or 6hr overall shows similar results, although the response to the 6hr exposure may in some cases be considerably blunted, perhaps due to various competing mechanisms, the result of a longer exposure to the injurious agent, and a probable indirect action of ozone-induced mediators at longer exposure times.
The higher levels of oxidized protein in WT versus KO mice indicate that there may be increased RONS production in the WT following a 3hr ozone exposure. Because KO mice have increased levels of protein in BAL, a likely inflammatory stimulus, we speculate that the ability to mount an effective response is compromised in KO lungs, as assessed by several parameters. Moreover, because no differences in oxidized protein were observed immediately following ozone exposure indicates that oxidation may not be a direct result of ozone exposure, but possibly a secondary process resulting from RONS production by activated immune cells.
Furthermore, macrophages that have not been primed by SP-A may have a reduced ability to produce RONS and hence the lower levels of oxidized protein in the KO mice. The increases in oxidized protein in WT mice at 4hr after the 3hr ozone exposure, along with the deficits in immune cell function and in the regulation of RONS production in KO mice (Atochina et al., 2004; Hickman-Davis et al., 2004; Korfhagen, 2001; LeVine et al., 2002) support this notion.
An alternative explanation could be that strain-specific differences exist in other oxidant regulatory mechanisms. This seems less probable because both WT and KO mice shared the same genetic background and because our findings indicated no differences between WT and KO mice in the levels of glutathione and enzymes affecting its metabolism. The glutathione system is either: a) not important in lung defense from acute ozone exposure and/or its sequelae; b) the time points studied were not optimal to detect glutathione-related changes; or c) these responses are tightly compartmentalized in the lung and thus changes were undetectable in the whole BAL and/or unfractionated lung tissue. Other studies of pollutant-induced lung injury (Behndig et al., 2006; Plopper et al., 1998) support the third possibility.
Typically oxidation of protein that may lead to protein dysfunction is viewed as a consequence of excess RONS. Another aspect of oxidation is that it “tags” proteins for clearance or elimination (Stadtman and Levine, 2000). In this context, oxidation of total BAL protein would be considered a step in its removal from the alveolus and the higher levels of oxidized protein in WT mice may indicate a more active repair process. There is also growing evidence that oxidants play an important signaling role (Chatterjee and Fisher, 2004; Martindale and Holbrook, 2002; Thannickal and Fanburg, 2000). A recent paper has examined lung injury following allogeneic bone marrow transplantation (Milla et al., 2004). Although the injury was thought to be mediated by RONS, the injury was more pronounced in mice lacking myeloperoxidase, an important generator of RONS (Chatterjee and Fisher, 2004; Milla et al., 2004). These findings illustrate that while excess RONS may cause tissue damage, these same molecules may also have important roles in maintaining homeostasis and limiting tissue damage. Moreover, the fact that significant increases in LDH in BAL were observed in KO versus WT mice following ozone exposure supports the possibility that SP-A provides protection against excess RONS and their ability to damage tissue.
Oxidized SP-A was significantly elevated immediately after ozone exposure, whereas the increases in oxidation of total BAL protein were somewhat delayed following a 3hr ozone exposure. This could indicate that SPA is more susceptible to oxidative modification than other BAL proteins, as suggested (Bridges et al., 2000; Kuzmenko et al., 2004). Moreover, the content of oxidized SP-A after a 6hr ozone exposure period increased at the 24hr time point even though no significant changes in the total oxidized protein were detected. These observations together indicate that the increased susceptibility of SP-A to oxidation may be a defense or scavenging mechanism for elevated RONS.
The data also show a relationship between oxidation and non-reducible dimerization. The oxidative modification primarily affects the non-reducible dimeric form of SP-A. This form is present in the FA-exposed samples following electrophoresis in reducing gels. Several studies have shown that various SP-A functions are adversely affected by oxidation (Huang et al., 2004; Wang et al., 2002; Wang et al., 2004) or nitration (Davis et al., 2002; Haddad et al., 1996; Zhu et al., 1996; Zhu et al., 1998). Although, the oxidative modification of SP-A with ozone exposure and the likely resultant loss of some SP-A functions may lessen the differences between WT and KO mice, the fact that several differences were observed between WT and KO suggests that SP-A plays some role in regulating the response to ozone.
Following ozone exposure no differences in BAL phospholipids were observed in WT mice, but increased levels were found in KO mice at the later time points. The absence of lipid changes in WT mice may be due to SP-A's presence because SP-A inhibits surfactant phospholipid secretion after LPS-induced lung injury (Quintero et al., 2002). Although in vitro ozone exposure of SP-A or SP-A variants affects their biophysical properties and reduces their ability to inhibit surfactant secretion (Oosting et al., 1991a; Oosting et al., 1991b; Selman et al., 2003; Wang et al., 2004), the in vivo impact of ozone on SP-A properties and the consequences of this on SP-A function are not entirely known. Thus, although SP-A oxidation occurs soon after ozone exposure, there are either sufficient amounts of unoxidized SP-A remaining to inhibit phospholipid secretion or the oxidized SP-A still retains some inhibitory function, whereas in the KO mouse, inhibition by SP-A does not occur and phospholipids levels increase. The OxyBlot data, where SP-A dimers (but not monomers) were oxidized, suggest a link between oxidation and dimerization, and confirm that unoxidized SP-A is present after ozone exposure. Also, ozone-induced epithelial damage in KO mice, as assessed by increased LDH, may contribute to the observed phospholipid increase in BAL.
Although SP-A was not increased in response to ozone, published studies indicate that its presence may be necessary in order for immune cells to respond appropriately to injury or proinflammatory stimuli (Hickman-Davis et al., 2004; LeVine et al., 2002). The stimulus in this case was ozone-induced tissue damage, and the role of SP-A in response to this stimulus was identified with both stimulatory and inhibitory activities. At 3hr and/or 6hr of ozone exposure increased tissue damage in KO mice was observed as assessed by increased BAL total protein and increased LDH levels. An increased inflammatory response was also observed in WT mice, with a significantly increased neutrophil influx and in the levels of oxidation of BAL protein, but with little change of these parameters in KO mice. A prompt initiation of an inflammatory response is essential to limit tissue damage and trigger repair processes. Published studies have reported that SP-A has both stimulatory (Kremlev et al., 1997; Song and Phelps, 2000) and inhibitory effects (Alcorn and Wright, 2004; McIntosh et al., 1996) on immune cells. These seemingly dichotomous effects of SP-A on immune cells appear to be reconciled by a report that attributes different binding mechanisms of SP-A on the subsequent occurrence of different responses (Gardai et al., 2003).
Thus, the possibility of defects in various host defense mechanisms in KO mice is a more likely explanation of the observed differences between WT and KO mice. The increased levels of PMNs in WT mice may support this postulate. Although differences in MIP-2, a neutrophil chemoattractant produced by macrophages, were not observed between WT and KO mice, regulatory differences were quite evident by mRNA measurements. KO mice showed no major changes in MIP-2 mRNA levels in response to ozone, whereas WT mice showed significant differences in ozone-induced mRNA levels. These observations, along with less consistent changes in MCP-1 protein (a monocyte/macrophage chemoattractant and activator) and its mRNA levels, indicate that KO macrophages may function suboptimally versus WT macrophages.
MIP-2 and MCP-1 are produced by a number of different cells including neutrophils, epithelial cells, and macrophages. The lack of differences in MIP-2 and MCP-1 protein levels versus the presence of differences in MIP-2 and MCP-1 mRNA levels between WT and KO mice provide challenges for interpretation, although this may not be an unusual occurrence. The following may underlie this paradoxical observation: 1) It may be in part due to differences in the proportion of cell types in BAL and their respective regulatory mechanisms versus the lung tissue used for cytokine mRNA measurement. 2) Release of preformed cytokine from damaged epithelial cells in KO mice may contribute to the increased levels seen in KO mice. Higher LDH levels were observed in KO mice following ozone exposure, whereas no significant change was observed in LDH activity in WT mice. 3) A discordance between protein and mRNA levels has been observed in other comparisons where only a subset showed a significant correlation between protein and RNA content (Chen et al., 2002; Gygi et al., 1999). Regulation of translational and posttranslational modifications and other processes may account for the lack of correlation between MIP-2 and MCP-1 protein and mRNA levels. Alternatively, strong negative correlations between protein and mRNA values (Chen et al., 2002) may be a consequence of unknown regulatory mechanisms or to negative feedback regulation.
In summary, for the first time, we show a role for innate host defense and SP-A, in particular, in ozone-induced lung injury. Ozone causes tissue damage and protein exudation resulting in an inflammatory response, which is a necessary precursor for the subsequent removal of damaged tissue and its eventual repair. We postulate that in WT mice the immune cells are primed by SP-A and respond rapidly, presumably via the production of RONS to oxidatively modify and enhance the removal of the protein exudate in the alveolus, thereby facilitating the removal of the inflammatory stimulus and triggering the initiation of repair mechanisms. In the absence of SP-A, the immune cells are not primed, rendering them less capable of mounting an effective inflammatory response as assessed by decreased PMNs and oxidized protein in KO mice. The lack of a robust response to an inflammatory stimulus prolongs exposure to that stimulus and delays initiation of repair. Increased phospholipid levels and epithelial cell damage in KO mice further indicate a role for SP-A in these processes. Thus, SP-A may serve as a scavenger of RONS and play a role in lung homeostasis and in the control of inflammation and tissue repair following ozone exposure.
ACKNOWLEDGEMENTS
Supported partly by ES009882-04A1 from the National Institute of Environmental Health Sciences (JF), by Philip Morris Incorporated (DSP), and under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds (DSP); The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alcorn JF, Wright JR. Surfactant protein A inhibits alveolar macrophage cytokine production by CD14-independent pathway. Am J Physiol Lung Cell Mol Physiol. 2004;286:L129–L136. doi: 10.1152/ajplung.00427.2002. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Harbeck R, Smith B, Voelker DR, Mason RJ. Binding of rat and human surfactant proteins A and D to Aspergillus fumigatus conidia. Infect.Immun. 1999;67:4563–4569. doi: 10.1128/iai.67.9.4563-4569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atochina EN, Beck JM, Preston AM, Haczku A, Tomer Y, Scanlon ST, Fusaro T, Casey J, Hawgood S, Gow AJ, Beers MF. Enhanced lung injury and delayed clearance of Pneumocystis carinii in surfactant protein A-deficient mice: attenuation of cytokine responses and reactive oxygen-nitrogen species. Infect.Immun. 2004;72:6002–6011. doi: 10.1128/IAI.72.10.6002-6011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker MA, Cerniglia GJ, Zaman A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Analytical Biochemistry. 1990;190:360–365. doi: 10.1016/0003-2697(90)90208-q. [DOI] [PubMed] [Google Scholar]
- Behndig AF, Mudway IS, Brown JL, Stenfors N, Helleday R, Duggan ST, Wilson SJ, Boman C, Cassee FR, Frew AJ, Kelly FJ, Sandstrom T, Blomberg A. Airway antioxidant and inflammatory responses to diesel exhaust exposure in healthy humans. Eur.Respir.J. 2006;27:359–365. doi: 10.1183/09031936.06.00136904. [DOI] [PubMed] [Google Scholar]
- Bridges JP, Davis HW, Damodarasamy M, Kuroki Y, Howles G, Hui DY, McCormack FX. Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J.Biol.Chem. 2000;275:38848–38855. doi: 10.1074/jbc.M005322200. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Fisher AB. ROS to the rescue. Am J Physiol Lung Cell Mol Physiol. 2004;287:L704–L705. doi: 10.1152/ajplung.00182.2004. [DOI] [PubMed] [Google Scholar]
- Chauhan AJ, Johnston SL. Air pollution and infection in respiratory illness. Br.Med.Bull. 2003;68:95–112. doi: 10.1093/bmb/ldg022. [DOI] [PubMed] [Google Scholar]
- Chen G, Gharib TG, Huang CC, Taylor JM, Misek DE, Kardia SL, Giordano TJ, Iannettoni MD, Orringer MB, Hanash SM, Beer DG. Discordant protein and mRNA expression in lung adenocarcinomas. Mol Cell Proteomics. 2002;1:304–313. doi: 10.1074/mcp.m200008-mcp200. [DOI] [PubMed] [Google Scholar]
- Crouch EC, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu.Rev.Physiol. 2001;63:521–554. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- Davis IC, Zhu S, Sampson JB, Crow JP, Matalon S. Inhibition of human surfactant protein A function by oxidation intermediates of nitrite. Free Radic.Biol.Med. 2002;33:1703–1713. doi: 10.1016/s0891-5849(02)01170-x. [DOI] [PubMed] [Google Scholar]
- Ding J, Umstead TM, Floros J, Phelps DS. Factors affecting SP-A-mediated phagocytosis in human monocytic cell lines. Respiratory Medicine. 2004;98:637–650. doi: 10.1016/j.rmed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Driscoll KE, Simpson L, Carter J, Hassenbein D, Leikauf GD. Ozone inhalation stimulates expression of a neutrophil chemotactic protein, macrophage inflammatory protein 2. Toxicol.Appl.Pharmacol. 1993;119:306–309. doi: 10.1006/taap.1993.1074. [DOI] [PubMed] [Google Scholar]
- Floros J, Phelps DS. Pulmonary surfactant protein A; structure, expression, and its role in innate host defense. In: Nakos G, Lekka M, editors. Update of intensive care medicine. University of Ioannina; Ioannina, Greece: 2002. pp. 87–102. [Google Scholar]
- Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsman HP, Van Golde LMG. Synthesis and assembly of lung surfactant. Annu.Rev.Physiol. 1991;53:441–464. doi: 10.1146/annurev.ph.53.030191.002301. [DOI] [PubMed] [Google Scholar]
- Haddad IY, Zhu S, Ischiropoulos H, Matalon S. Nitration of surfactant protein A results in decreased ability to aggregate lipids. Am.J.Physiol.(Lung Cell.Mol.Physiol.) 1996;14:L281–L288. doi: 10.1152/ajplung.1996.270.2.L281. [DOI] [PubMed] [Google Scholar]
- Hawgood S, Ochs M, Jung A, Akiyama J, Allen L, Brown C, Edmondson J, Levitt S, Carlson E, Gillespie AM, Villar A, Epstein CJ, Poulain FR. Sequential targeted deficiency of SP-A and -D leads to progressive alveolar lipoproteinosis and emphysema. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1002–L1010. doi: 10.1152/ajplung.00118.2002. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Repine JE. Pulmonary strategies of antioxidant defense. American Review of Respiratory Disease. 1989;140:531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Hickman-Davis JM, Gibbs-Erwin J, Lindsey JR, Matalon S. Role of surfactant protein-a in nitric oxide production and Mycoplasma killing in congenic C57BL/6 mice. Am J Respir.Cell Mol Biol. 2004;30:319–325. doi: 10.1165/rcmb.2003-0246OC. [DOI] [PubMed] [Google Scholar]
- Huang W, Wang G, Phelps DS, Al Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L546–L553. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Oberdorster G, Gelein R, Finkelstein JN. Endotoxin potentiates ozone-induced pulmonary chemokine and inflammatory responses. Exp.Lung Res. 2002;28:419–433. doi: 10.1080/01902140290092029. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Stripp BR, Reynolds SD, Avissar NE, Reed CK, Finkelstein JN. Inflammatory and antioxidant gene expression in C57BL/6J mice after lethal and sublethal ozone exposures. Exp.Lung Res. 1999;25:81–97. doi: 10.1080/019021499270448. [DOI] [PubMed] [Google Scholar]
- Johnston RA, Schwartzman IN, Flynt L, Shore SA. Role of interleukin-6 in murine airway responses to ozone. Am J Physiol Lung Cell Mol Physiol. 2005;288:L390–L397. doi: 10.1152/ajplung.00007.2004. [DOI] [PubMed] [Google Scholar]
- Kelly FJ, Dunster C, Mudway I. Air pollution and the elderly: oxidant/antioxidant issues worth consideration. Eur.Respir.J Suppl. 2003;40:70s–75s. doi: 10.1183/09031936.03.00402903. [DOI] [PubMed] [Google Scholar]
- Korfhagen TR. Surfactant protein A (SP-A)-mediated bacterial clearance: SP-A and cystic fibrosis. Am J Respir.Cell Mol Biol. 2001;25:668–672. doi: 10.1165/ajrcmb.25.6.f221. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Phelps DS. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1994;267:L712–L719. doi: 10.1152/ajplung.1994.267.6.L712. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Phelps DS. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1997;272:L1070–L1077. doi: 10.1152/ajplung.1997.272.6.L1070. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Umstead TM, Phelps DS. Surfactant protein A regulates cytokine production in the monocytic cell line THP-1. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1997;272:L996–L1004. doi: 10.1152/ajplung.1997.272.5.L996. [DOI] [PubMed] [Google Scholar]
- Kuzmenko AI, Wu H, Bridges JP, McCormack FX. Surfactant lipid peroxidation damages surfactant protein A and inhibits interactions with phospholipid vesicles. J Lipid Res. 2004;45:1061–1068. doi: 10.1194/jlr.M300360-JLR200. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Hartshorn K, Elliott J, Whitsett J, Korfhagen T. Absence of SP-A modulates innate and adaptive defense responses to pulmonary influenza infection. Am J Physiol Lung Cell Mol Physiol. 2002;282:L563–L572. doi: 10.1152/ajplung.00280.2001. [DOI] [PubMed] [Google Scholar]
- LeVine AM, Kurak KE, Bruno MD, Stark JM, Whitsett JA, Korfhagen TR. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am J Respir.Cell Mol Biol. 1998;19:700–708. doi: 10.1165/ajrcmb.19.4.3254. [DOI] [PubMed] [Google Scholar]
- Madan T, Eggleton P, Kishore U, Strong P, Aggrawal SS, Sarma PU, Reid KBM. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect.Immun. 1997;65:3171–3179. doi: 10.1128/iai.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- McFarland VA, Inouye LS, Lutz CH, Jarvis AS, Clarke JU, McCant DD. Biomarkers of oxidative stress and genotoxicity in livers of field-collected brown bullhead, Ameiurus nebulosus. Arch.Environ.Contam Toxicol. 1999;37:236–241. doi: 10.1007/s002449900510. [DOI] [PubMed] [Google Scholar]
- McIntosh JC, Mervin-Blake S, Conner E, Wright JR. Surfactant protein A protects growing cells and reduces TNF-a activity from LPS-stimulated macrophages. American Journal of Physiology - Lung Cellular and Molecular Physiology. 1996;271:L310–L319. doi: 10.1152/ajplung.1996.271.2.L310. [DOI] [PubMed] [Google Scholar]
- Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005;288:L150–L158. doi: 10.1152/ajplung.00135.2004. [DOI] [PubMed] [Google Scholar]
- Milla C, Yang S, Cornfield DN, Brennan ML, Hazen SL, Panoskaltsis-Mortari A, Blazar BR, Haddad IY. Myeloperoxidase deficiency enhances inflammation after allogeneic marrow transplantation. Am J Physiol Lung Cell Mol Physiol. 2004;287:L706–L714. doi: 10.1152/ajplung.00015.2004. [DOI] [PubMed] [Google Scholar]
- Oosting RS, Van Golde LMG, Verhoef J, Van Bree L. Species differences in impairment and recovery of alveolar macrophage functions following single and repeated ozone exposures. Toxicol.Appl.Pharmacol. 1991a;110:170–178. doi: 10.1016/0041-008x(91)90299-t. [DOI] [PubMed] [Google Scholar]
- Oosting RS, van Greevenbroek MJ, Verhoef J, Van Golde LMG, Haagsman HP. Structural and functional changes of surfactant protein A induced by ozone. Am.J.Physiol.(Lung Cell.Mol.Physiol.) 1991b;261:L77–L83. doi: 10.1152/ajplung.1991.261.2.L77. [DOI] [PubMed] [Google Scholar]
- Phelps DS. Surfactant regulation of host defense function in the lung: A question of balance. Pediatric Pathology and Molecular Medicine. 2001;20:269–292. [PubMed] [Google Scholar]
- Phelps DS, Umstead TM, Mejia M, Carrillo G, Pardo A, Selman M. Increased surfactant protein-a levels in patients with newly diagnosed idiopathic pulmonary fibrosis. Chest. 2004;125:617–625. doi: 10.1378/chest.125.2.617. [DOI] [PubMed] [Google Scholar]
- Plopper CG, Hatch GE, Wong V, Duan X, Weir AJ, Tarkington BK, Devlin RB, Becker S, Buckpitt AR. Relationship of inhaled ozone concentration to acute tracheobronchial epithelial injury, site-specific ozone dose, and glutathione depletion in rhesus monkeys. Am J Respir.Cell Mol Biol. 1998;19:387–399. doi: 10.1165/ajrcmb.19.3.3183. [DOI] [PubMed] [Google Scholar]
- Putman E, Van Golde LMG, Haagsman HP. Toxic oxidant species and their impact on the pulmonary surfactant system. Lung. 1997;175:75–103. doi: 10.1007/pl00007561. [DOI] [PubMed] [Google Scholar]
- Quintero OA, Korfhagen TR, Wright JR. Surfactant protein A regulates surfactant phospholipid clearance after LPS-induced injury in vivo. Am.J.Physiol Lung Cell Mol.Physiol. 2002;283:L76–L85. doi: 10.1152/ajplung.00418.2001. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Lung glutathione and oxidative stress: implications in cigarette smoke-induced airway disease. Am.J.Physiol.(Lung Cell.Mol.Physiol.) 1999;21:L1067–L1088. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Regulation of redox glutathione levels and gene transcription in lung inflammation: therapeutic approaches. Free Radic.Biol.Med. 2000;28:1405–1420. doi: 10.1016/s0891-5849(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Reidy MF, Wright JR. Surfactant protein A enhances apoptotic cell uptake and TGF-beta1 release by inflammatory alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2003;285:L854–L861. doi: 10.1152/ajplung.00439.2002. [DOI] [PubMed] [Google Scholar]
- Robinson CE, Keshavarzian A, Pasco DS, Frommel TO, Winship DH, Holmes EW. Determination of protein carbonyl groups by immunoblotting. Analytical Biochemistry. 1999;266:48–57. doi: 10.1006/abio.1998.2932. [DOI] [PubMed] [Google Scholar]
- Selman M, Lin HM, Montano M, Jenkins AL, Estrada A, Lin Z, Wang G, DiAngelo SL, Guo X, Umstead TM, Lang CM, Pardo A, Phelps DS, Floros J. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum.Genet. 2003;113:542–550. doi: 10.1007/s00439-003-1015-4. [DOI] [PubMed] [Google Scholar]
- Song M, Phelps DS. Comparison of SP-A and LPS effects on the THP-1 monocytic cell line. Am.J.Physiol Lung Cell Mol.Physiol. 2000;279:L110–L117. doi: 10.1152/ajplung.2000.279.1.L110. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Levine RL. Protein oxidation. Annals of the New York Academy of Sciences. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- Uhlson C, Harrison K, Allen CB, Ahmad S, White CW, Murphy RC. Oxidized phospholipids derived from ozone-treated lung surfactant extract reduce macrophage and epithelial cell viability. Chem.Res.Toxicol. 2002;15:896–906. doi: 10.1021/tx010183i. [DOI] [PubMed] [Google Scholar]
- Umstead TM, Phelps DS, Wang G, Floros J, Tarkington BK. In vitro exposure of proteins to ozone. Toxicology Mechanisms and Methods. 2002;12:1–16. doi: 10.1080/15376510209167932. [DOI] [PubMed] [Google Scholar]
- Van Iwaarden JF, Van Strijp JAG, Ebskamp MJM, Welmers AC, Verhoef J, Van Golde LMG. Surfactant protein A is opsonin in phagocytosis of herpes simplex virus type 1 by rat alveolar macrophages. Am.J.Physiol.(Lung Cell.Mol.Physiol.) 1991;261:L204–L209. doi: 10.1152/ajplung.1991.261.2.L204. [DOI] [PubMed] [Google Scholar]
- Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and C1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J.Immunol. 2002;169:3978–3986. doi: 10.4049/jimmunol.169.7.3978. [DOI] [PubMed] [Google Scholar]
- Vazquez de Lara LG, Becerril C, Montano M, Ramos C, Maldonado V, Melendez J, Phelps DS, Pardo A, Selman M. Surfactant components modulate fibroblast apoptosis and type I collagen and collagenase-1 expression. Am.J.Physiol Lung Cell Mol.Physiol. 2000;279:L950–L957. doi: 10.1152/ajplung.2000.279.5.L950. [DOI] [PubMed] [Google Scholar]
- Vazquez de Lara LG, Umstead TM, Davis SE, Phelps DS. Surfactant protein A increases matrix metalloproteinase-9 production by THP-1 cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L899–L906. doi: 10.1152/ajplung.00082.2003. [DOI] [PubMed] [Google Scholar]
- Walti H, Polla BS, Bachelet M. Modified natural porcine surfactant inhibits superoxide anions and proinflammatory mediators released by resting and stimulated human monocytes. Pediatr.Res. 1997;41:114–119. doi: 10.1203/00006450-199701000-00018. [DOI] [PubMed] [Google Scholar]
- Wang G, Bates-Kenney SR, Tao JQ, Phelps DS, Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry. 2004;43:4227–4239. doi: 10.1021/bi036023i. [DOI] [PubMed] [Google Scholar]
- Wang G, Umstead TM, Phelps DS, Al Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ.Health Perspect. 2002;110:79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JR. Pulmonary surfactant: a front line of lung host defense. J Clin.Invest. 2003;111:1453–1455. doi: 10.1172/JCI18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Simpson LG, Driscoll KE, Leikauf GD. Chemokine regulation of ozone-induced neutrophil and monocyte inflammation. Am J Physiol. 1998;274:L39–L46. doi: 10.1152/ajplung.1998.274.1.L39. [DOI] [PubMed] [Google Scholar]
- Zhu S, Haddad IY, Matalon S. Nitration of surfactant protein A (SP-A) tyrosine residues results in decreased mannose binding ability. Archives of Biochemistry and Biophysics. 1996;333:282–290. doi: 10.1006/abbi.1996.0392. [DOI] [PubMed] [Google Scholar]
- Zhu S, Kachel DL, Martin WJ, Matalon S. Nitrated SP-A does not enhance adherence of Pneumocystis carinii to alveolar macrophages. Am J Physiol. 1998;275:L1031–L1039. doi: 10.1152/ajplung.1998.275.6.L1031. [DOI] [PubMed] [Google Scholar]