Abstract
A fundamental challenge to the study of oxidative stress responses of Mycobacterium tuberculosis (Mtb) is to understand how the protective host molecules are sensed and relayed to control bacilli gene expression. The genetic response of Mtb to hypoxia and NO is controlled by the sensor kinases DosS and DosT and the response regulator DosR through activation of the dormancy/NO (Dos) regulon. However, the regulatory ligands of DosS and DosT and the mechanism of signal sensing were unknown. Here, we show that both DosS and DosT bind heme as a prosthetic group and that DosS is rapidly autooxidized to attain the met (Fe3+) form, whereas DosT exists in the O2-bound (oxy) form. EPR and UV-visible spectroscopy analysis showed that O2, NO, and CO are ligands of DosS and DosT. Importantly, we demonstrate that the oxidation or ligation state of the heme iron modulates DosS and DosT autokinase activity and that ferrous DosS, and deoxy DosT, show significantly increased autokinase activity compared with met DosS and oxy DosT. Our data provide direct proof that DosS functions as a redox sensor, whereas DosT functions as a hypoxia sensor, and that O2, NO, and CO are modulatory ligands of DosS and DosT. Finally, we identified a third potential dormancy signal, CO, that induces the Mtb Dos regulon. We conclude that Mtb has evolved finely tuned redox and hypoxia-mediated sensing strategies for detecting O2, NO, and CO. Data presented here establish a paradigm for understanding the mechanism of bacilli persistence.
Keywords: carbon monoxide, dormancy, nitric oxide, oxygen, persistence
Tuberculosis (TB) is a major global health burden, and current estimates suggest that one-third of the world's population (≈2 billion) is latently infected with TB (1). Latency is important largely because persistent Mycobacterium tuberculosis (Mtb) are in a state of “drug unresponsiveness” wherein the bacilli are resistant to existing antimycobacterial drugs. A major question in the TB field is: “what are the mechanisms that allow Mtb to persist in human tissues for decades without replicating, to then abruptly resume growth and cause disease?” Addressing that question is essential to the development of effective therapeutic intervention strategies. Recent evidence implicates NO as an environmental trigger of mycobacterial persistence (2–5). The latter findings are particularly interesting in light of the fact that inducible NO synthase (iNOS) and therefore NO production is crucial for protection of mice against Mtb (6), and that human macrophages in Mtb-infected tissues express iNOS (2, 7). Another factor associated with latent TB is hypoxia (8). The role of oxygen tension in TB is receiving wide attention, especially because it was demonstrated that rapid withdrawal of oxygen is lethal to Mtb, whereas a gradual depletion allows time for adaptation and bacterial survival (8). Interestingly, a significant overlap exists between the gene expression profiles of Mtb cells treated with NO and that of bacilli cultured under hypoxic conditions (3–5). These induced genes comprise the Dos regulon and are thought to play a crucial role in the shift down of Mtb to the persistent state (4, 5, 9). The Dos two-component system [originally designated Dev by Kinger and Tyagi (10)] consists of two GAF-containing sensor histidine kinases, DosS and DosT, and the cognate response regulator, DosR (9, 11) that are believed to facilitate transition to the latent form of infection. In fact, most Mtb genes up-regulated during hypoxia and NO require DosR. Nevertheless, evidence directly linking NO and hypoxia to latent TB in vivo remains circumstantial. Essential questions regarding these important sensor kinases remain unanswered. For example, how does Mtb initiate the shift down from an actively growing state to the persistent state? Also, despite extensive studies (3–5), to date, the presumed host ligands of DosS and DosT have not yet been established experimentally nor has it been determined how these sensors modulate signaling. In an accompanying paper (26), we provide evidence that Mtb WhiB3 responds to the host signals O2 and NO via its [4Fe-4S] cluster. In this article, we believe that ascertaining of host ligands of DosS and DosT and deciphering how the Dos regulon is modulated will lead to a new and better understanding of the mechanism of Mtb persistence. To address these issues, we tested the hypothesis that DosS and DosT sense O2, NO, and CO through their heme iron. We also tested the hypothesis that ligand-induced changes in their heme prosthetic group modulate autophosphorylation. To thoroughly characterize the biochemical mechanism of signal sensing, we used EPR and UV-visible spectroscopy and studied the biochemistry of DosS and DosT under defined aerobic and anaerobic conditions. Finally, we identified a third diatomic gas that induces the Mtb Dos regulon. We anticipate that the data generated in this work will lead to a better understanding of the mechanism by which Mtb enters a state of latency in humans.
Results
Mtb DosS and DosT Are Heme Proteins.
To investigate the mechanism of signal sensing and relay of the Mtb Dos regulon, we initiated a thorough biochemical characterization of DosS and DosT and found that both purified preparations yielded a red color, suggesting the presence of heme.
We exploited UV-visible spectroscopy and the pyridine hemochromogen assay to conclusively establish that both DosS and DosT are heme proteins and to determine the type and oxidation state of the heme. For DosS, the sharp Soret (409 nm), α (575 nm), and β (536 nm) bands, in addition to another weak band at ≈640 nm, are indicative of a hexa-coordinated, high-spin heme protein [supporting information (SI) Fig. 8] and is in support of a previous study (12). For DosT, the Soret (412 nm), α (575 nm), and β (538 nm) bands are indicative of a hexa-coordinated heme protein (SI Fig. 8 and SI Text, note 1). The pyridine hemochromogen assay demonstrated that both native proteins bind approximately equimolar amounts of heme b (SI Fig. 9). These results demonstrate that the GAF-containing proteins DosS and DosT bind heme as a prosthetic group (see SI Text, note 2).
DosS is in the Met (Fe3+) Form.
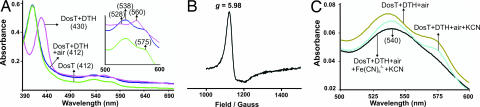
Heme-based sensor proteins are key regulators of adaptive responses to ligands such as O2 and NO and are generally comprised of a heme-containing sensory domain that modulates the histidine kinase domain of the same protein. Bacterial heme sensor kinases typically bind O2 and are in the oxy form (13). To dissect the mechanism of the heme iron-mediated ligand sensing of DosS, we used EPR and UV-visible spectroscopy and examined the redox state of the heme iron and whether O2 is a ligand of DosS. First, we compared the absorption spectra of aerobically purified DosS with that of dithionite (DTH)-exposed DosS. This exposure caused a shift in the Soret band from 409 to 430 nm, and the α and β bands converged into a new peak at 557 nm (Fig. 1A), which resembles the deoxy form of FixL and hemoglobin (14). Importantly, when this reduced sample was exposed to atmospheric oxygen, the absorption spectrum rapidly reverted back within seconds to that of native DosS (Fig. 1A, SI Fig. 10, and SI Text, note 3).
Fig. 1.
Characterization of DosS by EPR and UV-visible spectroscopy. (A) UV-visible characterization of DosS (3 μM in 20 mM Tris, pH 7.5) as purified, exposed to DTH or treated with DTH and reexposed to air. (Inset) Enlarged image of the 500- to 600-nm region. (B) EPR spectroscopy analysis of air-exposed DosS (6 μM in 20 mM Tris, pH 7.5). (C) UV-visible spectrum of DosS as purified, exposed to air after DTH treatment, the former treated with KCN or Fe(CN)63−. Note the characteristic met-CN peak at 540 nm. Numbers in parentheses indicate absorption maximum in nanometers.
To conclusively determine the heme-iron oxidation status, we used EPR spectroscopy and demonstrated that O2-exposed DosS gave a strong axial feature centered at g = 5.98, which is indicative of Fe3+ high-spin (S = 5/2) species (Fig. 1B and SI Text, note 4). These data provide conclusive evidence that, upon air exposure, reduced DosS is rapidly oxidized by O2 to generate met DosS.
As an additional verification that the DosS heme iron is oxidized rather than ferrous O2-bound, the oxidation status of the DosS heme iron after air exposure was determined by other independent techniques. Because the met (Fe3+) form of heme reacts with CN− to produce a met–CN− complex characterized by single peak at 540 nm, the spectral properties of the CN− complex with met heme proteins are routinely used to determine the oxidation status of heme iron. Reduced DosS was exposed to atmospheric oxygen for 30–120 s and then exposed to an excess of KCN. This process caused the convergence of α and β bands into a single peak at 540 nm, which is indicative of a complex between met DosS and KCN, a finding that substantiates that the DosS heme iron is in the met form (Fig. 1C). Finally, air-exposed DosS was treated with an excess of the oxidant Fe(CN)63− and no change was observed in the spectral properties of DosS (Fig. 1C). Taken together, these data demonstrate that DosS exists in the met form and that ferrous DosS is rapidly oxidized by O2 to generate met DosS.
DosT Is in the Oxy (O2-Bound) Form.
Having established that DosS is in the met form, we next sought to determine whether exposure of DosT to oxygen converts its heme iron to the met or the O2-bound (oxy) form. To do so, we recorded the absorption spectrum of aerobically purified DosT and compared it with DTH-exposed DosT. DTH exposure of DosT caused a red shift in Soret bands from 412 to 430 nm, whereas the α and β bands produced a major and minor peak at 560 and 528 nm, respectively (Fig. 2A). When DTH-treated DosT was reexposed to air, the absorption spectrum rapidly reverted back to that of aerobically purified DosT (Fig. 2A and SI Text, note 3). This behavior is consistent with the deoxy form reported for the Escherichia coli direct oxygen sensor (EcDOS) (15). To accurately determine the redox state of the heme iron, we used EPR spectroscopy and demonstrated that air-exposed DosT generated no signal, suggesting the absence of a paramagnetic iron species. However, treatment of air-exposed DosT with the Fe(CN)63− generated a sharp axial signal centered at g = 5.98, demonstrating that oxidized DosT contains a paramagnetic high-spin Fe3+ heme species (Fig. 2B). The latter finding strongly suggests that air-exposed DosT is in the oxygen-bound (oxy) form similar to that of NifL, HemAT, and EcDOS. Exposing DosT to air for 4 days at room temperature did not change the spectral properties (results not shown) and may indicate it is an unusually stable O2-bound heme iron protein. To further confirm that DosT is in the oxy form, DosT was constantly stirred in a sealed cuvette for 2 h under a constant flow of argon gas to allow replacement of bound oxygen, and the absorption spectrum was recorded. The spectrum was virtually identical to the DTH-exposed DosT spectrum (data not shown), strongly suggesting that DTH or deoxygenating by argon gas converts oxy DosT (Fe2+–O2) into deoxy DosT (Fe2+). To further corroborate that DosT is in the oxy form, we treated air-exposed DosT (up to 48 h at room temperature) with an excess of KCN, but did not observe any change in the absorption spectrum, demonstrating that DosT contains ferrous iron and is not capable of forming a complex with CN−. However, when air-exposed DosT was first treated with an excess of Fe(CN)63− followed by KCN, a peak at 540 nm was generated. This finding indicated that DosT contains ferrous heme that can be oxidized by Fe(CN)63− to generate a met heme specie, which can then react with CN− (Fig. 2C) to form a met CN− complex. In sum, the spectroscopy data conclusively demonstrate that native air-exposed DosT is in the stable, oxy form.
Fig. 2.
Characterization of DosT using EPR and UV-visible spectroscopy. (A) UV-visible spectroscopy characterization of DosT (3 μM in 20 mM Tris, pH 7.5) as purified, exposed to DTH, followed by air exposure. (Inset) An enlarged image of the 500- to 600-nm region. (B) EPR spectroscopy analysis of purified DosT (6 μM) after treatment with Fe(CN)63−. No EPR signal was detected with purified DosT without Fe(CN)63− exposure. (C) UV-visible spectrum of DosT exposed to air followed by DTH treatment, the former treated with KCN, and exposed to Fe(CN)63− followed by KCN. Numbers in parentheses indicate absorption maximum in nanometers.
NO and CO Are Ligands of DosS and DosT.
Despite the fact that it has been known for some time that DosS and DosT are sensor kinases (9, 11), surprisingly no ligand for either protein had been identified. On the other hand, it is well known that heme-based sensors commonly bind diatomic gases such as O2, NO, and CO (13). NO has been shown to be essential for the control of acute and chronic infection of Mtb (2, 6), but there is to date no evidence to implicate CO in the virulence of Mtb.
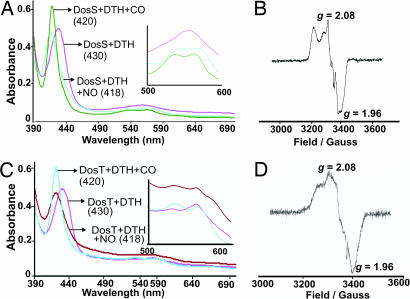
To determine whether NO and CO are ligands of DosS or DosT, we used absorption and EPR spectroscopy and compared the spectral properties of DosS and DosT independently exposed to NO or CO. Because heme nitrosylation and carbonylation requires binding of NO or CO to deoxy ferrous heme iron, DosS and DosT were first treated with DTH and then exposed to either the NO generator proline NONOate, or alternatively, to CO.
The absorption spectroscopy results strongly suggest that ferrous DosS treated with NO and CO generated heme-nitrosyl and heme-carbonyl DosS species as indicated by the changes in spectral properties (e.g., the shift in the Soret and appearance of distinct α and β bands) compared with unexposed ferrous DosS (Fig. 3A). These changes are highly characteristic of heme–NO and heme–CO complexes (16, 17). To further validate that NO binds the heme iron of DosS, we used EPR spectroscopy. DosS was treated with DTH to generate ferrous DosS, and then exposed to proline NONOate. A highly characteristic nitrosyl-heme EPR spectrum was obtained, demonstrating that NO binds DosS (Fig. 3B). These experiments confirmed that only ferrous-DosS binds NO, which leads to the formation of a characteristic nitrosyl–heme complex (Fig. 3B and SI Text, note 5).
Fig. 3.
NO and CO are ligands of DosS or DosT. (A) UV-visible spectroscopy characterization of DosS exposed to DTH and exposed to NO or CO. (B) EPR spectroscopy analysis of DosS (6 μM) treated with DTH followed by NO. (C) DosT exposed to DTH and exposed to NO or CO. (D) EPR spectroscopy analysis of DosT (6 μM) treated with DTH followed by NO. Numbers in parentheses indicate absorption maximum in nanometers.
To demonstrate that NO and CO are ligands of DosT, deoxy DosT was independently exposed to NO or CO. The spectral changes observed were highly characteristic of the formation of nitrosylated and carbonylated heme species (Fig. 3C) (16, 17). To further confirm that NO is a ligand of DosT, we used EPR to analyze deoxy DosT exposed to proline NONOate. Highly characteristic nitrosyl-heme EPR spectra were obtained, demonstrating that NO binds deoxy DosT (Fig. 3D). In addition, oxy DosT exposed to proline NONOate did not generate met DosT (SI Text, note 6 and SI Fig. 11). These data conclusively demonstrated that NO is a ligand of DosT.
As an initial step toward examining whether NO and CO can generate heme–nitrosyl or heme–carbonyl complexes in the presence of O2, we independently treated oxy DosT with CO or NO. Intriguingly, CO-exposed, but not NO-exposed, oxy DosT caused a characteristic change in electronic absorption spectra (SI Fig. 11), which is highly indicative of a heme–carbonyl complex (16, 17). In sum, these data conclusively demonstrate that NO and CO are ligands of ferrous DosS and deoxy DosT. Finally, the data also suggest that binding of NO or CO is influenced by the redox state of DosS heme iron and the ligation state of DosT.
DosS Is a Redox Sensor.
Having identified O2, NO, and CO as ligands for DosS and DosT, we sought to examine whether they were regulatory ligands capable of affecting autophosphorylation of the histidine kinases.
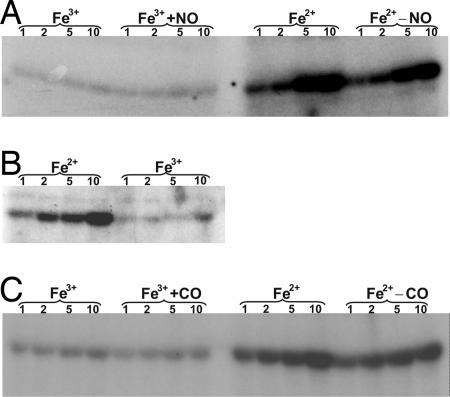
A met DosS sample was divided into two aliquots inside an anaerobic glove box. One aliquot was reduced with DTH, whereas the other sample was left untreated. Both were then examined in the autokinase assay. Results showed that ferrous DosS possessed much higher autokinase activity than did met DosS (Fig. 4A) and suggest that ferrous DosS is in the “on” or active state (SI Text, note 7). As a further validation that the redox state of DosS heme iron modulates autokinase activity, we used Fe(CN)63− rather than O2 to oxidize DTH-reduced DosS and analyzed its effect on autokinase activity. Similar to met DosS autokinase activity, we found that the oxidation of ferrous DosS heme iron by Fe(CN)63− to the ferric form also inhibited DosS kinase activity (Fig. 4B). These data suggest that ferric DosS is in the “off” or inactive state.
Fig. 4.
O2, NO, and CO are regulatory ligands of DosS. (A) Effect of O2 and NO on DosS autokinase activity. Purified DosS (Fe3+) was exposed to DTH to generate the Fe2+ form and analyzed in the autokinase assay. Note the significant difference in autokinase activity between met and ferrous DosS. DosS (Fe3+) or DTH-reduced DosS (Fe2+) were exposed to proline NONOate and are indicated by the Fe3++NO and Fe2+−NO labels, respectively. (B) Effect of the heme-iron redox state on DosS autokinase activity. DosS was exposed to DTH (Fe2+) followed by treatment with Fe(CN)63− to generate met DosS. (C) Met DosS was exposed to DTH to generate the ferrous form. Both forms were exposed to CO and are indicated by the Fe3++CO and Fe2+−CO labels, respectively. Numbers indicate minutes of incubation.
Next, we assessed whether NO influenced DosS autokinase activity. This is an important experiment, largely because of the role of NO in protection against Mtb infection and because NO induces expression of the Dos regulon (3–5). DosS was anaerobically reduced with DTH, exposed to proline NONOate, and used in the autokinase reactions. Notably, nitrosylation of DosS showed autokinase activity comparable to levels of active ferrous DosS autokinase activity (Fig. 4A). These results suggest that NO directly binds ferrous DosS through the formation of nitrosyl–heme complex and is consistent with its role in regulation of the Mtb Dos regulon.
Heme proteins are used by some bacteria, for example, Rhodospirillum rubrum (18), to sense CO. We therefore examined whether CO had any effect on DosS autokinase activity. We found that exposure of met DosS to CO did not generate any further autokinase activity. However, reduction of DosS with DTH followed by CO exposure producing CO–DosS showed autokinase activity comparable with that of reduced DosS (Fig. 4C).
In sum, these data demonstrated that molecular O2 rapidly oxidizes ferrous DosS to met DosS, which dramatically decreases its autokinase activity. As a result, we conclude that DosS is a redox sensor. As opposed to the role of environmental O2, which functions as an off switch, host-generated ligands such as NO or CO may function to maintain stable NO–DosS or CO–DosS complexes in the “locked” or active state. Lastly, the biochemistry of DosS strongly suggests that DosS can directly modulate the expression of the Mtb Dos regulon in response to hypoxia, NO, and CO.
DosT Is a Hypoxia Sensor.
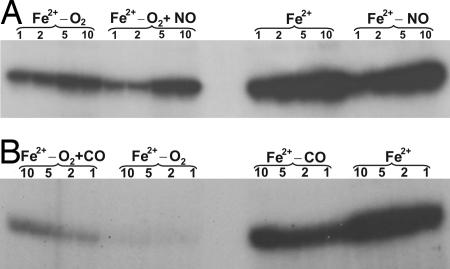
Having established that NO and CO are potential modulatory ligands of DosS, we next sought to determine the effect of these ligands on DosT autokinase activity. An oxy DosT sample was transferred to the glove box and divided into two aliquots. One aliquot was treated with DTH to remove dissolved O2 and the O2 bound to DosT (deoxy), whereas the other samples were left untreated (oxy). The results demonstrated that deoxy DosT had significantly increased autokinase activity as compared with inactive oxy DosT (Fig. 5A), strongly suggesting that the O2 bound to DosT inhibits autokinase activity (SI Text, note 8).
Fig. 5.
O2, NO, and CO are regulatory ligands of DosT. (A) Effect of O2 and NO on DosT autokinase activity. To remove bound O2 and generate the deoxy form (Fe2+), oxy DosT (Fe2+−O2) was deoxygenated, transferred to the glove box, exposed to DTH, and analyzed in the autokinase assay. To study the effect of NO on autokinase activity, oxy DosT (Fe2+−O2) and deoxy DosT (Fe2+) was exposed to proline NONOate and are indicated by the Fe2+−O2 +NO and Fe2+−NO labels, respectively. (B) To study the effect of CO on autokinase activity, oxy DosT (Fe2+−O2) and deoxy DosT (Fe2+) were exposed to CO for 30 s and are indicate by the Fe2+−O2 +CO and Fe2+−CO labels, respectively. Note that partial substitution of oxygen bound to DosT by CO (performed in the presence of air) leads to increased autokinase activity, albeit not as much as the heme-carbonyl and deoxy DosT samples.
Next, we assessed the effect of NO on oxy and deoxy DosT. The samples were independently exposed to proline NONOate and analyzed for autokinase activity. NO–DosT autokinase activity was found to be comparable to that of deoxy DosT (Fig. 5A), suggesting that ligands, as well as the ligation state, can modulate autokinase activity. Importantly, as is the case for NO–DosS, these data are consistent with a role for NO–DosT in modulating expression of the Mtb Dos regulon.
Because the spectroscopic data (SI Fig. 11) suggested that CO can bind DosT in the presence of O2, we analyzed whether CO had any effect on oxy DosT autokinase activity. Oxy DosT was divided (on the bench) into two aliquots, one was exposed to CO and the other was left untreated. Oxy DosT treated with CO showed enhanced autokinase activity compared with inactive oxy DosT (Fig. 5B), further supporting our spectroscopy data that CO can replace bound O2 to generate a heme–CO complex. Next, an oxy DosT sample was deoxygenated with argon gas and transferred to the glove box where it was reduced with DTH. The sample was divided into two aliquots, one was treated with CO and the other was left untreated; both were analyzed for autokinase activity. The data demonstrated that deoxy DosT showed the highest autokinase activity, followed by NO–DosT, CO–DosT, and oxy DosT (Fig. 5B).
Taken together, the spectroscopic and autokinase results demonstrate that O2 bound to DosT inhibits autokinase activity, whereas deoxy DosT enhances autokinase activity. Accordingly, we conclude that DosT is an O2 sensor. In addition, similar to DosS, the results suggest that NO or CO can keep DosT in a locked state by altering the DosT heme ligation state by forming stable CO–DosT or NO–DosT complexes.
CO Induces Expression of the Mtb Dos Regulon.
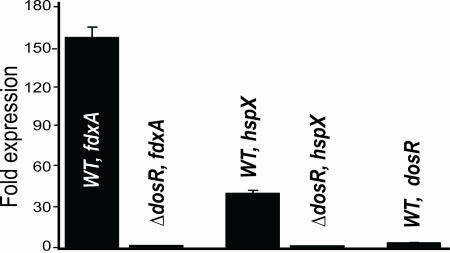
Having established conclusive biochemical evidence that CO is capable of reacting with DosS and DosT, we now sought to test the hypothesis that CO is able to induce the Mtb Dos regulon. We used the CO-releasing molecule tricarbonylchlororuthenium(II) dimer (CORM-2) or CO dissolved in PBS to generate 50 μM CO in 7H9 (SI Text). Logarithmically cultured WT Mtb and Mtb dosR mutant (Mtb ΔdosR) cells were independently treated with 50 μM CO for 3 h, and Mtb dosR (Rv3133c), hspX (Rv2031c), and fdxA (Rv2007c) expression was examined by real-time PCR. The relative expression of the three genes were enhanced ≈3.8- to 160-fold in CO-treated WT Mtb, but not in CO-treated Mtb ΔdosR (Fig. 6) or untreated cells, strongly suggesting that the Mtb Dos regulon is induced by CO and mediated via DosR. Importantly, because CO is generated in human lungs (19) by heme oxygenase 1 (HO-1), we strongly anticipate that these findings will have significant implications for understanding Mtb persistence and suggest a complex synergism between in vivo-induced hypoxia, NO, and CO.
Fig. 6.
Induction of the Mtb Dos dormancy regulon by CO. Mtb H37Rv and Mtb ΔdosR cells were independently treated with or without 50 μM CO (dissolved in PBS buffer) for 3 h in 7H9 media. Mycobacterial cells were harvested and RNA was isolated. Real-time PCR was used to analyze the expression of dosR (Rv3133c), hspX (Rv2031c), and fdxA (Rv2007c) in either WT Mtb or Mtb ΔdosR cells.
Discussion
The Mtb DosR/S/T two-component system has been implicated in inducing Mtb to acquire the latent state in response to hypoxia and NO (3–5). Before this work, the ligand(s) and biochemical mechanism of DosS and DosT signal sensing and relay were unknown to our knowledge. We have shown that O2, NO, and CO are modulatory ligands of the heme sensor kinases DosT and DosS. We discovered that Mtb DosS functions as a redox sensor, whereas DosT functions as a hypoxia sensor. Finally, we identified a third potential dormancy signal CO, which is capable of inducing the Mtb Dos regulon. We anticipate that these findings in particular will provide a paradigm for how Mtb initiates the shift down to a persistent state.
Evidence in this work has been obtained by several complementary methodologies and differs significantly from that of previous studies, which either used truncated versions of DosS and DosT (20) or assessed phosphate transfer reactions under aerobic conditions (9). Here, native, full-length DosS and DosT were carefully purified and extensively characterized under both aerobic and anaerobic conditions. Using a combination of established spectroscopic techniques (EPR and UV-visible absorption spectroscopy) reagents and conditions (aerobic and anaerobic) we demonstrate that DosS is in the met (Fe3+) form and that DosT is in the oxy (Fe2+-O2) form. To obtain better insight into the molecular mechanism of how DosS and DosT assist Mtb to enter latency, we sought to identify the physiologically relevant host ligands capable of reacting with these sensor kinases. It is well known that heme-binding sensors such as NifL (21), HemAT (22), and EcDOS (15) are capable of directly sensing O2 through their heme prosthetic groups. We were particularly interested in NO and hypoxia, primarily because several lines of in vitro and in vivo evidence suggest that host-generated NO and hypoxia are associated with the establishment and maintenance of latent TB. For example, Mtb responses to NO and hypoxia were shown to be mediated by DosR/S/T and each induced the same transcription profiles (3–5). Also, Mtb dosR was shown to be essential for full virulence in guinea pigs (23), and iNOS knockout mice were demonstrated to be susceptible to Mtb infection (6). Finally, human Mtb-infected lungs were shown to produce active iNOS (7). These aforementioned studies, coupled with the significant interest that oxygen tension has received in generating a persistent Mtb phenotype in vitro (8), strongly point toward NO and hypoxia as physiologically relevant host ligands.
We tested the hypothesis that O2, NO, and CO are ligands of DosS and DosT. EPR and UV-visible absorption spectroscopy data showed that O2 rapidly oxidizes DosS to generate the met form and that NO and CO bind ferrous DosS. These data demonstrated that NO and CO are ligands of ferrous DosS, and in addition, suggest that DosS functions as a potential redox sensor. In contrast to DosS, we demonstrated that native DosT binds O2.
We identified fundamental differences between DosS and other well studied sensor proteins such as FixL, HemAT, and EcDOS. These sensors use O2 binding as a mechanism to modulate their kinase activity. In contrast, DosS uses the oxidation of its heme iron by O2, as opposed to O2 binding, as a mechanism for altering autokinase activity. Therefore, DosS functions as a redox sensor, which can modulate expression of the Dos regulon. To our knowledge, such a redox-mediated mechanism for O2 sensing has not yet been described and represents a novel mechanism of action. Intriguingly, because ferrous DosS and NO–DosS autokinase activity is similar, it is clear that DosS does not require ligand binding (e.g., NO and CO) for activity. This idea suggests that upon in vivo activation of DosS (via reduction), NO or CO can lock DosS in an active, stable state.
Autokinase experiments also showed that oxy DosT is in the inactive state, whereas deoxy-DosT is in the active state. These findings provide strong evidence that DosT is a hypoxia sensor and that O2 inactivates DosT. Furthermore, DosT activity can also be modulated by changing the O2 ligation state, which allows it to bind NO or CO, which can also lock DosT in the active state. Because we demonstrated that bound ligands or the redox state of the heme iron could either increase (e.g., ferrous DosS, CO–DosT) or decrease (met DosS, oxy DosT) autokinase activity, we strongly anticipate that these findings have important in vivo implications.
To date, only NO and hypoxia are linked to latent TB in vivo. Our biochemical data pointed toward CO as a third possible dormancy signal. Subsequently, our real-time expression analysis confirmed the induction of key members of the Mtb Dos regulon (e.g., hspX, fdxA, and dosR) by CO. Importantly, as is the case for NO and hypoxia, the differential regulation of these genes is mediated via DosR (Fig. 6). CO as a physiologically relevant host signaling molecule has been well described (24) and is produced from the enzymatic degradation of heme by HO-1 to modulate antiinflammatory and antiapoptotic activity. As a result, we conclude that CO is a physiological relevant in vivo signal.
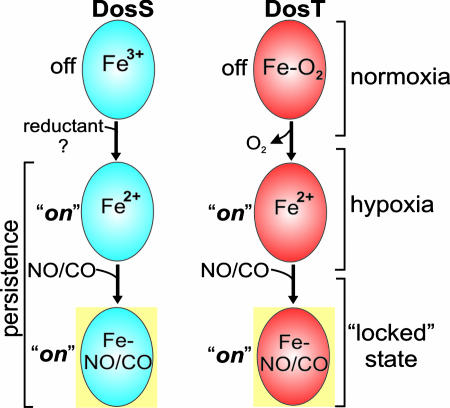
In light of the results generated in this work, several important questions are raised. For example, do hypoxia, NO, and CO function alone or in conjunction with each other to initiate Mtb persistence in the host? Also, why does Mtb have two sensor kinases that sense protective host molecules through related, but distinctly different, mechanisms? Based on our data, we believe that DosS and DosT are fully capable of sensing and responding to O2, NO, and CO. Our “sense-and-lock” working model (Fig. 7) proposes that during aerobic respiration, DosS and DosT sense O2 and are kept primarily in the oxidized (inactive) and oxy (inactive) form, respectively, maintaining the Dos regulon at a basal level. During hypoxia, a reductant converts DosS to the reduced (active) form to induce the Dos regulon. The ferrous form of DosS is also required to react with NO/CO and allows subsequent host-mediated modulation of the Dos regulon. Because NO (3, 5) and CO (Fig. 6) induce the Dos regulon during aerobic respiration in vitro, NO/CO also triggers a factor that reduces DosS, and the latter then induces the Dos regulon. The identification of this unknown reductant or enzyme will lead to a better understanding of the initiation of dormancy. Our model also proposes that oxy DosT keeps the Dos regulon at basal level. During hypoxia, DosT is deoxygenated, which can either induce the Dos regulon or react with NO/CO to allow host-mediated modulation of the Dos regulon. Consequently, because of the stability of the heme–nitrosyl complex (25), NO/CO may further modulate persistence by keeping CO/NO-DosS and CO/NO-DosT in the locked (active) state. Altogether, our model favors an ordered and coordinated, but complex, response to O2, NO, and CO to enter and/or maintain a latent state. For example, DosS first has to be reduced before it can bind NO/CO. Also, DosT first has to be deoxygenated to bind NO. Finally, these three ligands most likely operate in conjunction with each other in vivo.
Fig. 7.
Proposed mechanism for the role of Mtb DosS and DosT in the shift down of tubercle bacilli to the persistent state. The sense-and-lock model proposes that during disease progression hypoxia NO and CO are being sensed, independently or in conjunction, to induce the persistent state. Hypoxia, NO, and CO induce reductases to generate active ferrous DosS, whereas deoxygenation generates active ferrous DosT. Both ferrous DosS and DosT is now capable of binding NO and CO to generate NO–DosS, CO–DosS, NO–DosT, and CO–DosT, which keep the Dos regulon in a locked (active) state. See Discussion for more details.
In summary, we have shown that under aerobic conditions Mtb DosS is in the met form and DosT in the oxy form. We have demonstrated that O2, NO, and CO are modulatory ligands of DosS and DosT whose binding modulates autokinase activity. Importantly, we have shown that DosS functions as a redox sensor, whereas DosT functions as a hypoxia sensor. Finally, we have linked a third signal, CO, to Mtb persistence. We conclude that DosS, DosT, and DosR are part of the precise redox and hypoxia-mediated mechanisms that Mtb has evolved to sense the protective host immune response and induce a state of bacterial persistence.
Materials and Methods
Cloning, Expression, and Purification of DosS and DosT.
For standard cloning and purification experiments see SI Text.
Electronic Absorption Spectroscopy.
The absorption spectra of purified DosS or DosT (3 μM each) were analyzed at 23°C in stoppered quartz cuvettes with a DU800 spectrophotometer (Beckman Coulter, Fullerton, CA) unless specified otherwise. When necessary, protein samples were transferred to an anaerobic glove box (Plas Labs, Lansing, MI) and treated with 30 μM DTH before sealing the cuvettes and recording the spectra. For air-exposed samples, aliquots of protein were exposed to air by pipetting for 30–120 s into the cuvettes. In all experiments, DTH was removed by using D-Salt Polyacrylamide Desalting Columns (6K; Pierce, Rockford, IL).
EPR Spectroscopy.
Protein samples were transferred to SQ EPR tubes (Wilmad Glass, Buena, NJ) immediately before freezing the tubes in liquid nitrogen. EPR spectra were recorded with an Elexsys/Oxford Cryostat EPR instrument (Bruker, Billerica, MA). For analyzing the redox status of DosS or DosT, EPR was performed at 5.6-G modulation amplitude, 0.2-mW microwave power, 9.63-GHz microwave frequency, and 10 K of helium temperature. When necessary, EPR tubes were sealed inside the anaerobic glove box. To analyze nitrosylation of DosS or DosT, EPR spectroscopy was performed at a temperature 8 K of helium temperature, with a microwave frequency at 9.63 GHz and microwave power of 1 mW. Hemoglobin exposed to proline NONOate was used as a control in the spectroscopy analysis.
In Vitro Phosphorylation Assay.
In vitro phosphorylation assays were performed as described (9), analyzed on 4–20% Tris·HCl gradient PAGE gels, dried, and exposed to x-ray film overnight at −80°C. Formation of a heme-nitrosyl/carbonyl complex was first confirmed by absorption spectroscopy before analyzing samples for autokinase activity. As an additional control, air-exposed samples were also treated with proline NONOate. The effect of CO on autokinase activity was measured as described for NO, except that protein samples were flushed with 100% CO for 30 s. All autokinase reactions were performed inside the glove box.
Expression Analysis of Dormancy Regulon in Response to CO.
Real-time PCR expression analysis was performed on WT Mtb or Mtb ΔdosR cells treated with 50 μM CO. See SI Text for complete details.
Supplementary Material
Acknowledgments
We thank members of A.J.C.S.'s laboratory and Mary Hondalus for critical review of this manuscript and David Sherman (University of Washington, Seattle, WA) for providing the Mtb dosR mutant strain. This work was supported by the University of Alabama Center for AIDS Research (A.J.C.S.) and National Institutes of Health Grants AI058131 (to A.J.C.S.) and HL074391 and HL71189 (to J.R.L.).
Abbreviations
- Mtb
Mycobacterium tuberculosis
- TB
tuberculosis
- iNOS
inducible NO synthase
- DTH
dithionite
- EcDOS
Escherichia coli direct oxygen sensor.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705054104/DC1.
References
- 1.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. J Am Med Assoc. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Nathan C, Shiloh MU. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, Jacobs WR, Jr, Chan J. Cell Microbiol. 2003;5:637–648. doi: 10.1046/j.1462-5822.2003.00307.x. [DOI] [PubMed] [Google Scholar]
- 4.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. Mol Microbiol. 2003;48:833–843. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. J Exp Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, Nathan CF. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberg JB. Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wayne LG, Sohaskey CD. Annu Rev Microbiol. 2001;55:139–163. doi: 10.1146/annurev.micro.55.1.139. [DOI] [PubMed] [Google Scholar]
- 9.Roberts DM, Liao RP, Wisedchaisri G, Hol WG, Sherman DR. J Biol Chem. 2004;279:23082–23087. doi: 10.1074/jbc.M401230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinger AK, Tyagi JS. Gene. 1993;131:113–117. doi: 10.1016/0378-1119(93)90678-v. [DOI] [PubMed] [Google Scholar]
- 11.Dasgupta N, Kapur V, Singh KK, Das TK, Sachdeva S, Jyothisri K, Tyagi JS. Tuber Lung Dis. 2000;80:141–159. doi: 10.1054/tuld.2000.0240. [DOI] [PubMed] [Google Scholar]
- 12.Sardiwal S, Kendall SL, Movahedzadeh F, Rison SC, Stoker NG, Djordjevic S. J Mol Biol. 2005;353:929–936. doi: 10.1016/j.jmb.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Gilles-Gonzalez MA, Gonzalez G. J Inorg Biochem. 2005;99:1–22. doi: 10.1016/j.jinorgbio.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Gilles-Gonzalez MA, Gonzalez G, Perutz MF, Kiger L, Marden MC, Poyart C. Biochemistry. 1994;33:8067–8073. doi: 10.1021/bi00192a011. [DOI] [PubMed] [Google Scholar]
- 15.Delgado-Nixon VM, Gonzalez G, Gilles-Gonzalez MA. Biochemistry. 2000;39:2685–2691. doi: 10.1021/bi991911s. [DOI] [PubMed] [Google Scholar]
- 16.Martin E, Berka V, Bogatenkova E, Murad F, Tsai AL. J Biol Chem. 2006;281:27836–27845. doi: 10.1074/jbc.M601078200. [DOI] [PubMed] [Google Scholar]
- 17.Romberg RW, Kassner RJ. Biochemistry. 1979;18:5387–5392. doi: 10.1021/bi00591a020. [DOI] [PubMed] [Google Scholar]
- 18.Aono S, Nakajima H, Saito K, Okada M. Biochem Biophys Res Commun. 1996;228:752–756. doi: 10.1006/bbrc.1996.1727. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly LE, Barnes PJ. Am J Respir Cell Mol Biol. 2001;24:295–303. doi: 10.1165/ajrcmb.24.3.4001. [DOI] [PubMed] [Google Scholar]
- 20.Saini DK, Malhotra V, Tyagi JS. FEBS Lett. 2004;565:75–80. doi: 10.1016/j.febslet.2004.02.092. [DOI] [PubMed] [Google Scholar]
- 21.Gilles-Gonzalez MA, Ditta GS, Helinski DR. Nature. 1991;350:170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- 22.Hou S, Larsen RW, Boudko D, Riley CW, Karatan E, Zimmer M, Ordal GW, Alam M. Nature. 2000;403:540–544. doi: 10.1038/35000570. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra V, Sharma D, Ramanathan VD, Shakila H, Saini DK, Chakravorty S, Das TK, Li Q, Silver RF, Narayanan PR, Tyagi JS. FEMS Microbiol Lett. 2004;231:237–245. doi: 10.1016/S0378-1097(04)00002-3. [DOI] [PubMed] [Google Scholar]
- 24.Kim HP, Ryter SW, Choi AM. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 25.Brandish PE, Buechler W, Marletta MA. Biochemistry. 1998;37:16898–16907. doi: 10.1021/bi9814989. [DOI] [PubMed] [Google Scholar]
- 26.Singh A, Guidry L, Narasimhulu KV, Mai D, Trombley J, Redding KE, Giles GI, Lancaster JR, Jr, Steyn AJC. Proc Natl Acad Sci USA. 2007;104:11562–15567. doi: 10.1073/pnas.0700490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.