Abstract
Background
2-Methylthioadenosine 5'-triphosphate (2-MeSATP), formerly regarded as a specific P2Y (metabotropic) purinergic receptor agonist, stimulates Ca2+ influx and evokes catecholamine release from adrenal chromaffin cells. These cells express P2Y and P2X (ionotropic) purinoceptors, with the latter providing an important Ca2+ influx pathway. Using single cell calcium imaging techniques, we have determined whether 2-MeSATP might be a specific P2X receptor agonist in bovine chromaffin cells and assessed the relative role of P2X and P2Y receptors on catecholamine secretion from these cells.
Results
ATP raised the [Ca2+]i in ~50% of the cells. Removing extracellular Ca2+ suppressed the [Ca2+]i-raising ability of 2-MeSATP, observed in ~40% of the ATP-sensitive cells. This indicates that 2-MeSATP behaves as a specific ionotropic purinoceptor agonist in bovine chromaffin cells. The 2-MeSATP-induced [Ca2+]i-rises were suppressed by PPADS. UTP raised the [Ca2+]i in ~40% of the ATP-sensitive cells, indicating that these expressed Ca2+-mobilizing P2Y receptors. UTP-sensitive receptors may not be the only P2Y receptors present, as suggested by the observation that ~20% of the ATP-sensitive pool did not respond to either 2-MeSATP or UTP. The average sizes of the ATP- and 2-MeSATP-evoked [Ca2+]i responses were identical in UTP-insensitive cells. 2-MeSATP stimulated Ca2+ influx and evoked catecholamine release, whereas UTP elicited Ca2+ release from intracellular stores but did not evoke secretion. 2-MeSATP-induced secretion was strongly inhibited by Cd2+ and suppressed by extracellular Ca2+ or Na+ removal. TTX inhibited 2-MeSATP-evoked secretion by ~20%.
Conclusion
2-MeSATP is a specific P2X purinoceptor agonist and a potent secretagogue in bovine chromaffin cells. Activation of 2-MeSATP-sensitive receptors stimulates Ca2+ influx mainly via voltage-sensitive Ca2+ channels. For the most part, these are activated by the depolarization brought about by Na+ influx across P2X receptor pores.
Background
Extracellular ATP plays an important role in catecholamine release from adrenal chromaffin cells, either facilitating cholinergic stimulation via ionotropic (P2X) purinoceptors or inhibiting evoked release through a delayed action on metabotropic (P2Y) purinoceptors (see [1] and references therein). The latter may provide the basis for an auto-inhibitory feedback loop involving both autocrine and paracrine interactions. There are at least seven P2X receptor subunits encoded by distinct genes, which may form homo- or heterotrimeric ionotropic purinoceptors; functional P2X receptors are Ca2+-permeable and provide an important Ca2+ influx pathway, both in neurons and other cell types (for review see [2,3]).
The presence of P2X receptor subtypes in chromaffin cells is species-dependent [4] (see [3] for a review). Thus, rat chromaffin cells have either been reported to lack P2X receptors [4] or to show a variable expression of P2X receptor subtypes (P2X1, P2X2, P2X5 and P2X7) [5,6]; there is also some evidence that these cells express P2X4 receptors in aged animals [7]. Guinea-pig chromaffin cells seem to express P2X6 receptors [8], although functional studies point strongly to the presence of P2X2-like receptors [4]. There are no studies reporting the expression of specific P2X receptor subtypes in bovine chromaffin cells. However, the presence of P2X receptors in these cells has been suggested by functional studies involving mostly cytosolic free Ca2+ measurements [9-11]. ATP-evoked inward currents have been detected in a limited fraction of the cells [12]. The prevailing P2Y receptor subtype in bovine chromaffin cells seems to be an UTP-sensitive, Gi/o-coupled P2Y receptor [1,10,13].
ATP and other purinergic agonists evoke catecholamine release from either whole glands or isolated chromaffin cells [9,10,14]. This action is strictly Ca2+-dependent, suggesting that it might be mediated by either Ca2+ influx through the receptor-associated pores, opening of voltage-sensitive Ca2+ channels or both. Studying P2X receptor-mediated modulation of chromaffin cell function has been made difficult by the lack of specific agonists and antagonists of P2X receptor subtypes. 2-MeSATP, for example, has been classically regarded as a P2Y receptor agonist; more recently, however, several P2Y receptor subtypes have been found to be insensitive to the ATP derivative [2,3]. Moreover, 2-MeSATP is now known to activate P2X receptor subtypes [15]. There is evidence that 2-MeSATP behaves as a P2X agonist in guinea-pig chromaffin cells, as assessed by its ability to evoke inward currents [4,16]. Previous studies provided conflicting evidence regarding the action of 2-MeSATP on cytosolic free Ca2+ concentration ([Ca2+]i) in bovine chromaffin cells, with one study reporting sizeable responses [11] and another feeble and sporadic responses [9]. Whether 2-MeSATP might discriminate between P2X and P2Y receptors in chromaffin cells remains unknown.
In this work, we aimed at establishing 2-MeSATP as a specific P2X receptor agonist in bovine chromaffin cells by single-cell calcium imaging. We then investigated the effects of 2-MeSATP and UTP on catecholamine release, aiming at clarifying the relative role of P2X and Ca2+-mobilizing P2Y receptors.
Results
[Ca2+]i rises evoked by purinoceptor agonists
[Ca2+]i changes evoked by ATP receptor agonists were monitored by digital fluorescence imaging of the F340/F380 fura-2 fluorescence ratio (ΔR). Only cells that displayed sizeable [Ca2+]i responses to 10 μM 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP), an established agonist of acetylcholine nicotinic receptors in chromaffin cells, were considered for the study. To this end, cells were perifused at the tail of the experiments with 10 μM DMPP for brief periods of time. Our first basic protocol was to stimulate chromaffin cells with ATP, UTP and 2-MeSATP in order to investigate whether UTP-sensitive cells might also be sensitive to 2-MeSATP. Cells were typically subjected to ~60 s pulses of solutions containing each purinoceptor agonist and washed extensively for at least 10 min to minimize receptor desensitization at the time of the next challenge.
Typical pseudocolor fura-2 fluorescence images are depicted in Fig. 1(A–D). Representative time courses of [Ca2+]i changes for selected chromaffin cells are depicted in E. Resting [Ca2+]i was low prior to stimulation (A, blue). Challenging cells with 100 μM ATP elicited sizeable, albeit variable peak [Ca2+]i responses from a large pool of chromaffin cells (B and E; green, yellow or red). It is noteworthy that some cells did not respond to ATP (e.g. cell 4). Several cells (e.g. cell 1) exhibited pronounced [Ca2+]i rises in response to ATP but not 100 μM UTP. Exposure to 100 μM 2-MeSATP invariably increased the [Ca2+]i in these cells. In contrast, several cells (e.g. cell 2) responded to both ATP and UTP. For the most part these cells did not respond at all to 2-MeSATP. A fraction of the ATP-sensitive cells responded both to UTP and 2-MeSATP (e.g. cell 3).
Figure 1.
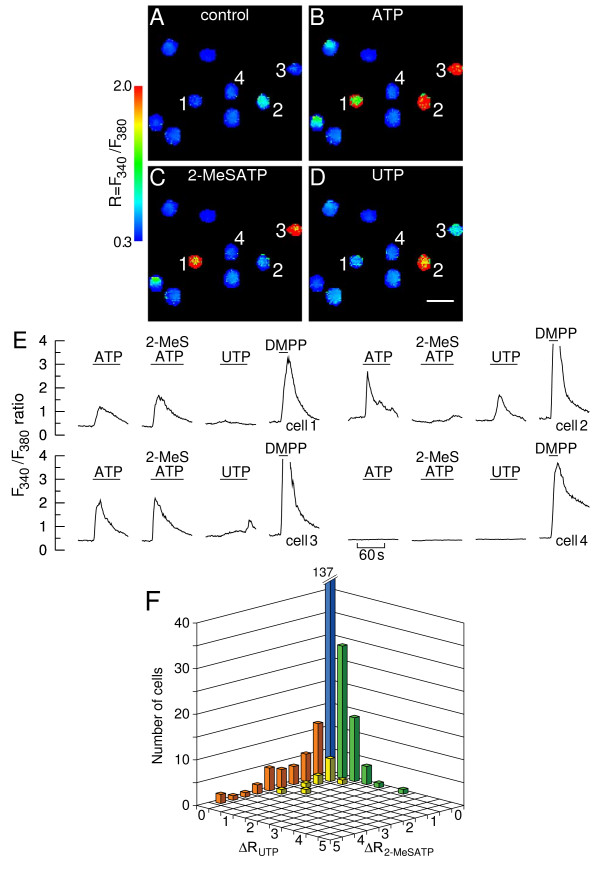
Calcium responses to ATP, UTP and 2-MeSATP. A-D. Calcium images showing a group of chromaffin cells before (A, "control") and during stimulation with 100 μM ATP (B), 100 μM 2-MeSATP (C) and 100 μM UTP (D). At the end of each experiment cells were stimulated with 10 μM DMPP. Cells were allowed to rest for ~10 min between consecutive stimulations. The fura-2 fluorescence ratio F340/F380 was determined for each cell in a field on a pixel-by-pixel basis. Images were coded in pseudocolor to show differences in the F340/F380 ratio. The images corresponding to agonist stimulation (B-D) were captured at the response peaks. Calibration bar: 50 μm; E. Time courses of changes in F340/F380 fluorescence ratio for a 2-MeSATP-sensitive/UTP-insensitive cell (cell 1), an UTP-sensitive/2-MeSATP-insensitive cell (cell 2), a cell displaying a mixed response (cell 3) and an ATP-insensitive cell (cell 4). The lines denote superfusions with DMPP or purinergic agonists. Some of the peak responses to DMPP were truncated for scaling reasons; F. Frequency distribution histogram of calcium responses. Changes in ΔR = F340/F380 were determined from the experiment depicted in A-D and 2 similar experiments (n = 234 cells). The column at the origin represents cells that did not respond to either UTP or 2-MeSATP (ΔR < 0.5, n = 137 cells). This column was truncated for scaling reasons. Columns in orange: cells responding to 2-MeSATP only; columns in green: cells responding to UTP only; columns in yellow: cells exhibiting mixed responses.
The statistical assessment of the [Ca2+]i responses of chromaffin cells to UTP and 2-MeSATP is presented in Fig. 1F in the form of a 3D histogram. The column at the origin of the histogram (blue) depicts cells that did not respond both to UTP and 2-MeSATP (137 in 234 cells, i.e. 59%); ATP evoked [Ca2+]i rises in 27 of these cells. Cells displaying a variable response to 2-MeSATP and no UTP response (orange) accounted for approximately 30% of the ATP-sensitive pool; the mean size of the 2-MeSATP-evoked [Ca2+]i responses was not significantly different from the ATP responses in these cells (1.53 ± 1.22 vs. 1.55 ± 1.18, respectively; n = 37 cells, p = 0.8). UTP-sensitive cells (green) were for the most part (approximately 40% of the ATP-sensitive pool) insensitive to 2-MeSATP, although a minority (ca. 8%, yellow) displayed a mixed sensitivity. The mean sizes of the UTP and ATP responses in UTP-sensitive/2-MeSATP-insensitive cells were 0.71 ± 0.61 and 0.92 ± 0.73, respectively (n = 49 cells; statistically different, p < 0.0001).
None of the cells responding to 2-MeSATP in presence of Ca2+ responded in the virtual absence of extracellular Ca2+ (Fig. 2A,B,E), suggesting that the ATP derivative is a specific P2X receptor agonist in bovine chromaffin cells. We have previously reported that suramin blocked the ATP-evoked [Ca2+]i responses in cells lacking ATP responses in the virtual absence of extracellular Ca2+; suramin did not affect the UTP responses, suggesting that it behaves as a specific P2X receptor antagonist [10]. We have now extended this conclusion to pyridoxalphosphate-6-azophenyl- 2',4'-disulphonic acid (PPADS, 50 μM). Indeed, the drug blocked the 2-MeSATP (20 μM)-evoked [Ca2+]i rises in all cells tested (n = 9 cells; Fig. 2C,D,E). Moreover, in parallel experiments, PPADS did not affect the UTP-evoked [Ca2+]i rises in cells displaying no response to 2-MeSATP (0.69 ± 0.33 vs. 0.73 ± 0.32 in absence of PPADS; n = 11 cells, p = 0.1).
Figure 2.
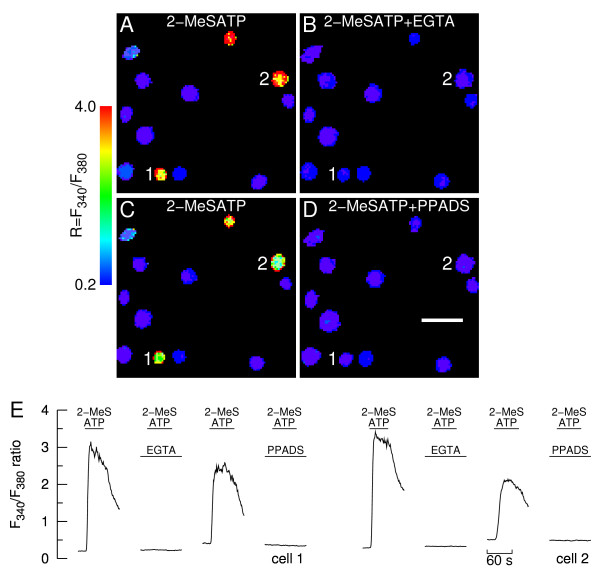
Effects of PPADS and extracellular Ca2+ removal on calcium responses to 2-MeSATP. A-B. Calcium images showing a group of chromaffin cells during stimulation with 20 μM 2-MeSATP in presence of extracellular calcium (A) and during stimulation in the virtual absence of extracellular calcium ("2-MeSATP +EGTA", B); C-D. The cells were subsequently superfused with a Ca2+-containing solution and challenged with 2-MeSATP, in the absence (C) or presence of 50 μM PPADS ("2-MeSATP +PPADS", D). Calibration bar: 50 μm. More in the legend to Fig. 1(A-D); E. Time courses of changes in F340/F380 fluorescence ratio for two representative cells (cells 1 and 2, also depicted in A-D). The lines denote superfusions with 2-MeSATP, PPADS or EGTA-containing solution. The responses are representative of data from 3 experiments.
Effects of purinergic agonists on catecholamine secretion
Both ATP and 2-MeSATP evoked catecholamine secretion in a dose-dependent way, as assessed amperometrically from cell batches (Fig. 3A). In these experiments cells were challenged with brief pulses of either agonist at increasing concentrations, being allowed to rest for ~10 min between successive applications. The concentration-response curves provided in Fig. 3B suggest that 2-MeSATP might be a more potent agonist for purinergic receptors (EC50 = 3.1 μM vs. 7.7 μM for ATP; but see the Discussion section for a critical assessment of this hypothesis). (Concentrations of test agents are henceforth given as nominal concentrations. Actual concentrations at the cell bed differ from nominal concentrations by a factor of 1.64, see Methods and legend to Fig. 3.) UTP (100 μM) did not evoke catecholamine secretion. PPADS (50 μM) abolished 2-MeSATP (10 μM)-evoked secretion (Fig. 3B).
Figure 3.
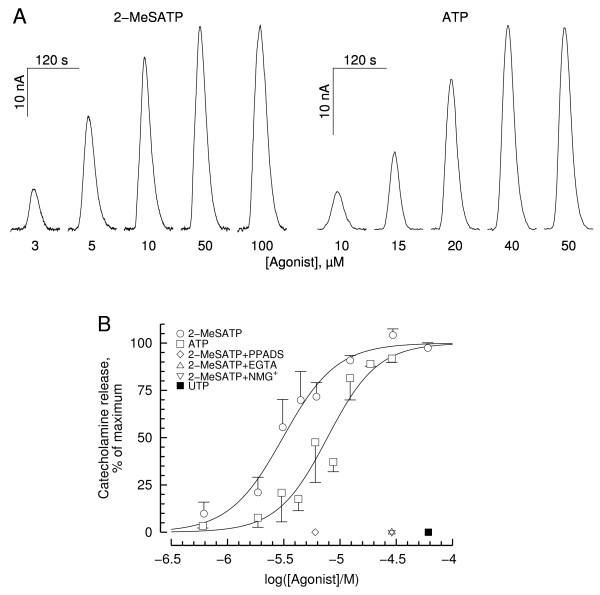
Effects of 2-MeSATP and ATP on catecholamine release. A. Amperometric currents recorded from chromaffin cell batches in response to brief pulses of increasing 2-MeSATP and ATP concentrations. Cells were allowed to rest for ~10 min between successive applications. Nominal agonist concentrations (in μM) are indicated beneath each trace. Nominal concentrations of test agents are used throughout this legend. Actual concentrations at the cell bed differ from nominal concentrations by a factor of 1.64 (see Methods). Concentrations along the X axis in Fig. 3B are actual concentrations at the cell bed; B. Dose-response curves of catecholamine release evoked by 2-MeSATP and ATP (circles and squares, respectively). Data from the experiment depicted in A and two similar experiments. Also depicted are data for UTP (100 μM; filled square) as well as for 2-MeSATP stimulations in presence of 50 μM PPADS (diamond), in the virtual absence of extracellular Ca2+ (EGTA-containing solution, inverted triangle) and in absence of extracellular Na+ (Na+ replacement for NMG+, triangle) (n = 3 experiments for each condition).
Challenging the cells with 50 μM 2-MeSATP in the virtual absence of extracellular calcium failed to elicit secretion (Fig. 3B), indicating that the stimulatory action of the purinoceptor agonist is strictly calcium-dependent. This action may be mediated mostly by activation of voltage-sensitive Ca2+ channels, since 0.5 mM Cd2+ (a blocker of these channels) reduced 2-MeSATP-evoked secretion by 73.0 ± 1.5% (n = 3 experiments); Cd2+ inhibited high K+ (50 mM)-evoked catecholamine secretion by ~98% (n = 3 experiments). Replacing extracellular Na+ for N-methyl-D-glucamine (NMG+) abolished 2-MeSATP-evoked secretion (Fig. 3B); tetrodotoxin (TTX, 1 μM) had a modest (18.5 ± 3.0%, n = 3 experiments) inhibitory effect on evoked secretion, suggesting that Na+ influx through TTX-sensitive, voltage-dependent Na+ channels played a minimal role.
Discussion
We found that ~50% of the cells examined by fluorescence imaging exhibited [Ca2+]i responses to ATP and that, among these, ~40% yielded positive responses to 2-MeSATP. None of the 2-MeSATP-sensitive cells responded in the virtual absence of extracellular Ca2+. Since cell exposure to EGTA-containing solutions caused minimal depletion of intracellular Ca2+ stores [1,10], this implies that 2-MeSATP elicits [Ca2+]i rises by stimulating Ca2+ influx. Hence, 2-MeSATP behaves as a specific P2X receptor agonist in bovine chromaffin cells.
The 2-MeSATP-sensitive P2X receptors in these cells are blocked by low concentrations of suramin [10] and PPADS (this work). In agreement with a previous study [9], we show here that 2-MeSATP is a stronger secretagogue than ATP. This might suggest that ATP is a weaker agonist of the P2X receptor, in apparent disagreement with the information provided by our former [Ca2+]i study [11] where the order of potency for purinergic agonists was ATP > 2-MeSATP >> αβ-MeATP, ADP [βS], AMP. We note, however, that activation of a P2Y receptor coupled to Gi/o inhibits exocytotic release of catecholamines from rat chromaffin cells downstream voltage-sensitive Ca2+ channels and cytosolic Ca2+ elevation [17]. Moreover, activation of P2Y receptors inhibits voltage-sensitive Ca2+ channels via Gi/Go proteins and, thus, depresses Ca2+-dependent exocytosis [12,13,18-22]. Subclassification of P2X receptors in bovine chromaffin cells was clearly outside the objectives of the present functional study. Nonetheless, taking into account the sensitivity to inhibitors, as well as the above order of potency for purinergic agonists, P2X receptors expressed in bovine chromaffin cells appear to have the pharmacological profile of P2X2 or P2X5receptors [2,4,15]. As a note of caution we emphasize that P2X receptors in situ most probably are composed of different unit subtypes with a pharmacology distinct from that of the homomeric receptors cloned and transfected to cells, which difficults any attempt at identifying P2X receptor subtypes on the basis of functional studies.
We have also found that ~50% of the ATP-sensitive cells yielded positive responses to UTP. Thus, since UTP is a specific agonist for P2Y receptors in bovine chromaffin cells [1], approximately half of the ATP-sensitive cell population expressed functional Ca2+-mobilizing P2Y receptors. This agrees with the finding by Ennion et al. that these cells express an as yet unidentified UTP-sensitive, Gi/o-coupled P2Y receptor [13]. It is noteworthy that the authors also provided evidence for the presence of a Gi/o-linked, adenine nucleotide-specific P2Y12 receptor and detected transcripts for P2Y1 receptors by RT-PCR analysis. Accordingly, our results suggest that bovine chromaffin cells express a Ca2+-mobilizing, UTP-insensitive P2Y receptor. Indeed, ~20% of the ATP-sensitive pool did not respond to either 2-MeSATP or UTP. Taking into account that P2X receptors are fully sensitive to 2-MeSATP in bovine chromaffin cells, the most likely explanation for this finding is that, indeed, some cells express a Ca2+-mobilizing, UTP-insensitive P2Y receptor.
Chromaffin cells exist in the form of two major phenotypes, epinephrine-secreting (adrenergic) and norepinephrine-secreting (noradrenergic) cells [23]. Our previous work [1] showed that P2X receptors and UTP-sensitive P2Y receptors are asymmetrically distributed among two distinct cell pools (noradrenergic and adrenergic cells). The present data are fully compatible with this finding. Indeed, approximately 30% of the ATP-sensitive cells yielded positive responses to 2-MeSATP but not to UTP, suggesting that they express P2X receptors preferentially. Moreover, approximately 40% of the ATP-sensitive cells yielded positive responses to UTP but not to 2-MeSATP, suggesting that they express Ca2+-mobilizing P2Y receptors only. A small fraction of the ATP-sensitive cells examined in this study responded both to UTP and 2-MeSATP, suggesting that they co-express P2X and P2Y receptors (see also [10]).
There is evidence that stimulated Ca2+ influx is strongly coupled to catecholamine release; in contrast, Ca2+ release from intracellular stores appears to be loosely coupled to secretion [10,24]. The present data support this concept, inasmuch as P2X receptor activation by 2-MeSATP stimulated Ca2+ influx and evoked catecholamine release, whereas activation of P2Y receptors by UTP elicited Ca2+ release from intracellular stores but did not evoke secretion. We found that, while 2-MeSATP-induced secretion was strictly Na+-dependent, the voltage-sensitive Na+ channel blocker TTX [25,26] inhibited evoked release by a modest 20%. This suggests that voltage-sensitive Ca2+ channels were mostly activated by the depolarization brought about by Na+ influx across P2X receptor pores. That voltage-sensitive Ca2+ channels are involved in 2-MeSATP-induced secretion is suggested by the strong inhibitory effect of Cd2+. Indeed, Cd2+ blocks Ca2+ influx across these channels without affecting P2X receptors [27,28].
Conclusion
2-MeSATP is a specific P2X purinoceptor agonist and a potent secretagogue in bovine chromaffin cells. Activation of P2X receptors stimulates Ca2+ influx mainly via voltage-sensitive Ca2+ channels. For the most part, these are activated by the depolarization brought about by Na+ influx across P2X receptor pores.
Methods
Cell culture
Bovine adrenal glands were obtained from the local slaughterhouse and kept on ice during transportation. Adrenal medulla cells were isolated by collagenase digestion of the glands and purified on a Percoll density gradient essentially as described previously [29,30]. The purified cell fraction thus obtained is enriched in chromaffin cells. Cells were cultured according to established procedures [1]. For the fluorescence imaging experiments, the cells were plated on round (16 mm diameter) glass coverslips coated with poly-L-lysine. For the secretion experiments, the cells were cultured in 60 mm diameter plastic Petri-dishes. Cells were typically used between days 2 and 5 after plating.
Solutions
The Ca2+-containing salt solution used in the imaging and secretion experiments had the following composition (mM): 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 mM HEPES and 10 glucose (pH 7.4). In some experiments extracellular free [Ca2+] was buffered at 100 nM by mixing appropriate amounts of Ca2+ and EGTA, as described elsewhere [31].
[Ca2+]i imaging
The coverslips containing the cells were washed in physiological saline supplemented with 1% bovine serum albumin (BSA). The cells were then loaded with 2.5 μM fura-2/AM (the acetoxymethyl ester of fura-2 [32]) for 45 min at 37°C in this medium, under a 95% O2/5% CO2 atmosphere. Cell handling after loading was carried out following established procedures. Specifically, cells were continuously perifused (approximately 1.5 ml/min) with physiological saline at room temperature. Solution exchange was provided by a four-way stopcock valve located near the recording chamber [1,10,11]. Fluorescence changes were recorded using a multiple excitation MagiCal imaging system (Applied Imaging, U.K.), essentially as described [1,33].
Catecholamine secretion
Catecholamine secretion from chromaffin cells was measured on-line using a perifusion system similar to that described previously [34]. Approximately 106 cells were placed in a 0.45 μm flow filter and perifused with the HEPES-containing solution with the aid of a peristaltic pump (Gilson Miniplus 3) at a rate of 1 ml/min. Test drugs were added as brief pulses through a 500-μl loop injector. Taking into account the dead volume of the filter chamber and the flow rate, the actual concentration of a given drug inside the chamber (cell bed) was reduced by a factor of 1.64. Thus, while drug concentrations are given as nominal concentrations throughout the main text and legend to Fig. 3, the concentrations along the X axis in Fig. 3B are corrected for the dilution factor (i.e. they refer to the actual concentrations acting on cells). The effluent solution exiting the filter was driven into an electrochemical detector (set at +500 mV, Omni 90 Potentiostat, Cypress Systems, Lawrence, KS) for direct measurement of catecholamine oxidation current, which was monitored on a chart recorder.
Data analysis
Data are presented as mean ± S.D. Statistical significance of differences was assessed by paired (within the same experiment) or unpaired (between experiments) Student's t-test; differences were considered significant at the 95% confidence level (P < 0.05).
Other materials
Fura-2/AM was from Molecular Probes (Eugene, Ore., USA). ATP was from Boehringer (Mannheim, Germany). Unless otherwise specified, all other chemicals were from Sigma Chemical Co. (St. Louis, Mo., USA).
Authors' contributions
ART carried out the experiments, performed the statistical analysis, prepared the figures, participated in conceiving and designing the study, and helped to draft the manuscript; EC helped in carrying out the experiments and participated in conceiving and designing the study; RMS helped to draft the manuscript; LMR participated in conceiving and coordinating the study and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was partially financed by a grant from FCT (Fundação para a Ciência e a Tecnologia, Portugal). Dr. E. Castro was supported by a FEBS fellowship. The facilities provided by the Faculty of Medicine (University of Coimbra) are gratefully acknowledged.
Contributor Information
Ângelo R Tomé, Email: atome@ci.uc.pt.
Enrique Castro, Email: ecastro@dbbf.ulpgc.es.
Rosa M Santos, Email: rmsantos@ci.uc.pt.
Luís M Rosário, Email: lrosario@ci.uc.pt.
References
- Tomé AR, Castro E, Santos RM, Rosário LM. Functional distribution of Ca2+-coupled P2 purinergic receptors among adrenergic and noradrenergic bovine adrenal chromaffin cells. BMC Neurosci. 2007;8:39. doi: 10.1186/1471-2202-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/S0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Liu M, Dunn PM, King BF, Burnstock G. Rat chromaffin cells lack P2X receptors while those of the guinea-pig express a P2X receptor with novel pharmacology. Br J Pharmacol. 1999;128:61–68. doi: 10.1038/sj.bjp.0702790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afework M, Burnstock G. Distribution of P2X receptors in the rat adrenal gland. Cell Tissue Res. 1999;298:449–456. doi: 10.1007/s004410050067. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc Natl Acad Sci USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afework M, Burnstock G. Age-related changes in the localization of P2X (nucleotide) receptors in the rat adrenal gland. Int J Dev Neurosci. 2000;18:515–520. doi: 10.1016/S0736-5748(00)00023-X. [DOI] [PubMed] [Google Scholar]
- Afework M, Burnstock G. Localization of P2X receptors in the guinea pig adrenal gland. Cells Tissues Organs. 2000;167:297–302. doi: 10.1159/000016793. [DOI] [PubMed] [Google Scholar]
- Reichsman F, Santos S, Westhead EW. Two distinct ATP receptors activate calcium entry and internal calcium release in bovine chromaffin cells. J Neurochem. 1995;65:2080–2086. doi: 10.1046/j.1471-4159.1995.65052080.x. [DOI] [PubMed] [Google Scholar]
- Castro E, Mateo J, Tome AR, Barbosa RM, Miras-Portugal MT, Rosario LM. Cell-specific purinergic receptors coupled to Ca2+ entry and Ca2+ release from internal stores in adrenal chromaffin cells. Differential sensitivity to UTP and suramin. J Biol Chem. 1995;270:5098–5106. doi: 10.1074/jbc.270.10.5098. [DOI] [PubMed] [Google Scholar]
- Castro E, Tome AR, Miras-Portugal MT, Rosario LM. Single-cell fura-2 microfluorometry reveals different purinoceptor subtypes coupled to Ca2+ influx and intracellular Ca2+ release in bovine adrenal chromaffin and endothelial cells. Pflugers Arch. 1994;426:524–533. doi: 10.1007/BF00378530. [DOI] [PubMed] [Google Scholar]
- Diverse-Pierluissi M, Dunlap K, Westhead EW. Multiple actions of extracellular ATP on calcium currents in cultured bovine chromaffin cells. Proc Natl Acad Sci USA. 1991;88:1261–1265. doi: 10.1073/pnas.88.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennion SJ, Powell AD, Seward EP. Identification of the P2Y(12) receptor in nucleotide inhibition of exocytosis from bovine chromaffin cells. Mol Pharmacol. 2004;66:601–611. doi: 10.1124/mol.104.000224. [DOI] [PubMed] [Google Scholar]
- Lin LF, Bott MC, Kao LS, Westhead EW. ATP stimulated catecholamine secretion: response in perfused adrenal glands and a subpopulation of cultured chromaffin cells. Neurosci Lett. 1995;183:147–150. doi: 10.1016/0304-3940(94)11136-7. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Historical review: ATP as a neurotransmitter. Trends Pharmacol Sci. 2006;27:166–176. doi: 10.1016/j.tips.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Otsuguro K, Asano T, Ohta T, Ito S, Nakazato Y. ATP-evoked membrane current in guinea pig adrenal chromaffin cells. Neurosci Lett. 1995;187:145–148. doi: 10.1016/0304-3940(95)11359-5. [DOI] [PubMed] [Google Scholar]
- Chen XK, Wang LC, Zhou Y, Cai Q, Prakriya M, Duan KL, Sheng ZH, Lingle C, Zhou Z. Activation of GPCRs modulates quantal size in chromaffin cells through G(betagamma) and PKC. Nat Neurosci. 2005;8:1160–1168. doi: 10.1038/nn1529. [DOI] [PubMed] [Google Scholar]
- Lim W, Kim SJ, Yan HD, Kim J. Ca2+-channel-dependent and -independent inhibition of exocytosis by extracellular ATP in voltage-clamped rat adrenal chromaffin cells. Pflugers Arch. 1997;435:34–42. doi: 10.1007/s004240050481. [DOI] [PubMed] [Google Scholar]
- Powell AD, Teschemacher AG, Seward EP. P2Y purinoceptors inhibit exocytosis in adrenal chromaffin cells via modulation of voltage-operated calcium channels. J Neurosci. 2000;20:606–616. doi: 10.1523/JNEUROSCI.20-02-00606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Guijo JM, Gandia L, Cuchillo-Ibanez I, Albillos A, Novalbos J, Gilsanz F, Larranaga E, de PR, Abad F, Garcia AG. Altered regulation of calcium channels and exocytosis in single human pheochromocytoma cells. Pflugers Arch. 2000;440:253–263. doi: 10.1007/s004240000272. [DOI] [PubMed] [Google Scholar]
- Harkins AB, Fox AP. Activation of purinergic receptors by ATP inhibits secretion in bovine adrenal chromaffin cells. Brain Res. 2000;885:231–239. doi: 10.1016/S0006-8993(00)02952-8. [DOI] [PubMed] [Google Scholar]
- Ulate G, Scott SR, Gonzalez J, Gilabert JA, Artalejo AR. Extracellular ATP regulates exocytosis in inhibiting multiple Ca(2+) channel types in bovine chromaffin cells. Pflugers Arch. 2000;439:304–314. doi: 10.1007/s004240050944. [DOI] [PubMed] [Google Scholar]
- Moro MA, Lopez MG, Gandia L, Michelena P, Garcia AG. Separation and culture of living adrenaline- and noradrenaline-containing cells from bovine adrenal medullae. Anal Biochem. 1990;185:243–248. doi: 10.1016/0003-2697(90)90287-J. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Control of exocytosis in adrenal chromaffin cells. Biochim Biophys Acta. 1991;1071:174–202. doi: 10.1016/0304-4157(91)90024-q. [DOI] [PubMed] [Google Scholar]
- Brandt BL, Hagiwara S, Kidokoro Y, Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976;263:417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick EM, Marty A, Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K. Roles of Ca2+ influx through ATP-activated channels in catecholamine release from pheochromocytoma PC12 cells. J Neurophysiol. 1992;68:2026–2032. doi: 10.1152/jn.1992.68.6.2026. [DOI] [PubMed] [Google Scholar]
- Boehm S. ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors. J Neurosci. 1999;19:737–746. doi: 10.1523/JNEUROSCI.19-02-00737.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario LM, Soria B, Feuerstein G, Pollard HB. Voltage-sensitive calcium flux into bovine chromaffin cells occurs through dihydropyridine-sensitive and dihydropyridine- and omega-conotoxin-insensitive pathways. Neuroscience. 1989;29:735–747. doi: 10.1016/0306-4522(89)90145-0. [DOI] [PubMed] [Google Scholar]
- Rosario LM, Stutzin A, Cragoe EJ, Jr, Pollard HB. Modulation of intracellular pH by secretagogues and the Na+/H+ antiporter in cultured bovine chromaffin cells. Neuroscience. 1991;41:269–276. doi: 10.1016/0306-4522(91)90215-A. [DOI] [PubMed] [Google Scholar]
- Marks PW, Maxfield FR. Preparation of solutions with free calcium concentration in the nanomolar range using 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid. Anal Biochem. 1991;193:61–71. doi: 10.1016/0003-2697(91)90044-T. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Oset-Gasque MJ, Vicente S, Gonzalez MP, Rosario LM, Castro E. Segregation of nitric oxide synthase expression and calcium response to nitric oxide in adrenergic and noradrenergic bovine chromaffin cells. Neuroscience. 1998;83:271–280. doi: 10.1016/S0306-4522(97)00377-1. [DOI] [PubMed] [Google Scholar]
- Castro E, Torres M, Miras-Portugal MT, Gonzalez MP. Effect of diadenosine polyphosphates on catecholamine secretion from isolated chromaffin cells. Br J Pharmacol. 1990;100:360–364. doi: 10.1111/j.1476-5381.1990.tb15809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


