Abstract
Detection of minimal residual disease or micrometastases in rhabdomyosarcoma (RMS) has been an unresolved problem in 70 to 80% of RMS patients. In patients with alveolar type RMS, which harbors chromosomal translocations and produces tumor-specific fusion products, polymerase chain reaction (PCR)-based diagnosis is clear-cut. In the more frequent embryonal RMS, however, no such PCR-based marker has been described. Recently it has been suggested that the PCR-based detection of MyoD1 may be a valuable adjunct in the diagnosis of minimal disease in embryonal RMS. We report here that MyoD1 mRNA is not specific for RMS, but can be amplified from ex vivo samples of many other childhood tumors and some normal tissues. By contrast, simultaneous amplification of α and γ subunit message of the fetal type acetylcholine receptor (AChR), by a novel duplex PCR, and the quantification of both transcripts resulting in a α/γAChR ratio <1 was 100% sensitive in alveolar (n = 8) and embryonal (n = 10) RMS. Moreover, γAChR was not detected in other childhood (n = 27) or adult tumors (n = 12), or normal tissues, except thymus. The high sensitivity and specificity of the method were confirmed by the successful detection of five cases of cytologically or molecularly verified RMS bone marrow micrometastases among 47 bone marrow samples from childhood tumor patients. By contrast, MyoD1 showed no amplification because of its low level of transcription. We conclude that mRNA of the fetal type AChR is a more specific and (about 100 times) more sensitive marker for the molecular detection of RMS than MyoD1, and thus appears to be a promising candidate for the detection of minimal disease in RMS lacking tumor-specific translocations.
Nearly 50% of all pediatric soft tissue sarcomas are rhabdomyosarcomas (RMS). 1, 2 Currently, RMS are classified according to the International Classification of Rhabdomyosarcomas. 3 Because of the recent availability of antibodies against MyoD1 and myogenin, 4, 5, 6 the differential diagnosis of RMS from other childhood neoplasms has become easier due to the high specificity and sensitivity of these immunohistochemical markers even in tumors with a low degree of rhabdomyomatous differentiation. However, the detection of minimal disease (minimal residual tumors or micrometastases) remains a challenge. 7, 8, 9, 10, 11
Molecular biology can improve the diagnosis in some cases; in 60 to 70% of alveolar RMS (20–30% of all RMS patients), the diagnosis has been simplified by polymerase chain reaction (PCR)-based detection of characteristic translocations t (2;13) (q35;q14) and t (1;13) (p36;q14), involving the PAX3 gene on chromosome 2, the PAX7 gene on chromosome 1, and the FKHR gene on chromosome 13. 12, 13, 14, 15, 16, 17, 18, 19 In embryonal RMS, a consistent loss of heterozygosity (LOH) at 11p15 is detectable. 20, 21 However, reliable identification of LOH by PCR requires samples containing more than 80% of tumor cells or enrichment for neoplastic tissue by microdissection of pathological specimens. 22, 23 Therefore, PCR-based detection of LOH is not applicable to the identification of minimal disease, which typically occurs against a high background of normal tissue or cells. Thus, for embryonal RMS and for the alveolar RMS lacking tumor-specific translocations no unequivocal molecular markers based on PCR are available.
The nicotinic AChR of skeletal muscle is a pentameric ion channel, which is composed of four subunits. 24, 25, 26, 27 During development of the neuromuscular junction, a change from the fetal type (α2βγδ) to the adult type (α2βɛδ) occurs, with replacement of the γsubunit by the ɛsubunit. 28, 29 After birth, the fetal type of the AChR is limited to myoid cells in the thymus 30, 31 and some extraocular muscle fibers, 32 but it is re-expressed in normal skeletal muscle after denervation. 33 Because RMS consist of immature and noninnervated neoplastic myoblasts, it is not surprising that the fetal type of the AChR, specifically its γ subunit, is found to be a tumor-specific immunohistochemical marker distinguishing RMS from normal muscle and other childhood tumors. 34 However, the immunohistochemical detection of the γ subunit in RMS has a relatively low sensitivity. 34 This contrasts with the expression of some myogenic factors such as myogenin and MyoD1, which are both specific and highly sensitive immunohistochemical markers. 4, 5, 6 However, we showed recently that the PCR-based detection of myogenin mRNA is not specific for RMS because of significant illegitimate transcription of the myogenin gene in many nonrhabdomyomatous tumors in almost all normal tissues. 36 By contrast, a recent study based on the investigation of tumor cell lines concluded that MyoD1 mRNA may be a sensitive and specific marker for the molecular diagnosis of RMS. 35 In the present study we have looked at MyoD1 transcription in ex vivo RMS biopsies and control tissue and used a novel duplex PCR strategy to examine the transcription of the fetal type AChR. We could not confirm the specificity of MyoD1 for RMS in vivo, but show that mRNA of the fetal type AChR is both a specific and a sensitive marker for the molecular detection of RMS compared to the mRNA expression of MyoD1 in RMS, other childhood and adult tumors, bone marrow samples, normal muscles, and normal tissues, respectively.
Materials and Methods
Materials
Eighteen RMS and 30 other childhood tumors of various types were studied using cryostat sections from snap-frozen tissue obtained on ice within 15 minutes to 4 hours after surgery. RMS were classified according to the International Classification of Rhabdomyosarcomas. 3 Twelve adult nonrhabdomyomatous tumors were obtained for frozen section diagnosis within 15 minutes after biopsy. Eight normal muscles and eight other normal tissues were derived from either autopsy or biopsy. Autopsy material was obtained within 4 hours after death and checked by PCR analysis of glyceraldehyde phosphate dehydrogenase (GAPDH) message (22 cycles) for integrity of RNA. Biopsies were obtained within 15 minutes. The embryonal RMS cell line TE671 37 served as a positive control.
Finally, 47 bone marrow samples blinded for investigation were studied. The samples were retrieved from the files of the cooperative soft tissue sarcoma study (CWS) and the European Ewing’s sarcoma study (EICESS). Clinical and pathological findings of the patients investigated are given in Tables 1 2 3 4 .
Table 1.
RT-PCR and Immunohistochemical (IH) Findings in Snap-Frozen Rhabdomyosarcoma (RMS) Biopsies from the Respective Primary Tumors
| Diagnosis | PAX3/FKHR RT-PCR | α/γAChR* RT-PCR | MyoD1 RT-PCR | γAChR IH† |
|---|---|---|---|---|
| Alveolar RMS | 8 /8 | 8 /8 | 8 /8 | 4 /8 |
| (n = 8) | ||||
| Embryonal RMS | 0 /10 | 10 /10 | 10 /10 | 6 /10 |
| (n = 8) |
α/γAChR ratio 21
Mouse mAb anti-γAChR MIB8 was used for immunohistochemical stainings as described previously. 34
Table 2.
Pathological, Clinical, and RT-PCR Findings in Patients with Different Childhood Tumors Other than RMS
| Case | Diagnosis | Sex/Age | α/γAChR RT-PCR | MyoD1 RT-PCR |
|---|---|---|---|---|
| 22064/90 | NB | F/3 | − | + |
| 16729/91 | NB | F/3m | − | − |
| 5964/92 | NB | M/2 | − | − |
| 17600/93 | NB | M/5m | − | + |
| 10796/97 | NB | M/8 | − | − |
| 11633/96-2 | ES | F/41 | +* | + |
| 11633/96-5 | ES | F/41 | − | + |
| 18191/92 | ES | F/1 | − | − |
| 21591/93 | ES | M/10 | − | − |
| 24483/95 | ES | M/18 | − | − |
| 8053/93 | SS | F/11 | − | + |
| 20897/96 | OS | F/17 | − | − |
| 871/90 | CS | M/11 | − | − |
| 14744/89 | WT/RD | F/4 | +* | + |
| 9496/94 | WT | F/1 | − | − |
| 7454/94 | WT | F/5 | − | − |
| 19002/93 | WT | M/5 | − | − |
| 3839/96 | WT | F/8 | − | − |
| 4841/90 | WT/RD | M/9 | +* | + |
| 13126/93 | GS | M/1 | − | − |
| 19217/93 | TE | M/1 | − | − |
| 11037/96 | SE | M/15 | − | − |
| 22046/96 | MG | M/21 | − | − |
| 12531/93 | ME | M/3 | − | − |
| 27262/94 | GN | F/5 | − | − |
| 22946/95 | SCHW | F/8 | − | − |
| 7400/96 | CAC | M/2 | − | − |
| 883/97 | ACC | M /13 | − | − |
| 26777/98 | IH | F/3 | −† | + |
| 3551/97 | LBL | M/16 | − | − |
| 5171/96 | LBL | M/4 | − | − |
| 1007/99 | SCLC/CM | M/73 | −† | − |
NB, neuroblastoma; ES, Ewing’s sarcoma; SS, synovial sarcoma; OS, osteosarcoma; CS, chondrosarcoma; WT, Wilms’ tumor; GS, germinal stroma tumor; TE, teratoma; SE, seminoma; MG, malignant germinoma; ME, meningioma; GN, ganglioneuroma; SCH, schwannoma; CAC, carcinoma of adrenal cortex; ACC, adenoid cystic carcinoma; IH, intramuscular hemangioma; LBL, lymphoblastic lymphoma; SCLC/CM, small cell lung cancer with contaminating muscle; NA, not available; WT/RD, Wilms’ tumor with rhabdomyomatous differentiation.
α/γAChR ratio <1
α/γAChR ratio ≫1
Table 3.
RT-PCR Results for MyoD1 and α/γAChR in Adult Nonrhabdomyomatous Tumors, Normal Tissues, and Normal Muscles
| Diagnosis | α/γAChR RT-PCR | MyoD1 RT-PCR |
|---|---|---|
| Adult tumors* | 0 /18‡ | 4 /18§ |
| (n = 18) | ||
| Normal tissues† | 0 /8‡ | 3 /8§ |
| (n = 8) | ||
| Normal muscles | 8 /8‡ | 8 /8 |
| (n = 8) |
Three carcinomas of the stomach, three of the kidney, three of the breast, three of the ovary, three of the lung, and three leiomyosarcomas.
Brain, heart, liver, lung, lymph node, kidney, stomach, and tonsil.
α/γAChR ratio ≫1
Detailed in Figures 3 and 4
Table 4.
RT-PCR Findings in Bone Marrow Samples Infiltrated by Different Childhood Tumors including RMS, Acute Lymphatic Leukemia, Neuroblastoma, and Ewing’s Sarcoma
| Cases | PAX3/FKHR | α/γAChR | Myogenin | MyoD1 |
|---|---|---|---|---|
| Bone Marrow | 0 /26 | 0 /26* | 4 /26 | 0 /26 |
| BM/RMS (n = 5) | 5 /5 | 5 /5† | 5 /5 | 0 /5 |
| BM/ALL (n = 4) | 0 /4 | 0 /4* | 2 /2 | 0 /4 |
| BM/NB (n = 8) | 0 /8 | 0 /8* | 2 /4 | 0 /8 |
| BM/Ewing’s (n = 4) | 0 /4 | 0 /4* | 2 /4 | 0 /4 |
Bone marrow was from alveolar RMS patients, free of alveolar RMS, confirmed by Pax3/FKHR RT-PCR.
BM/RMS, bone marrow infiltrated by alveolar RMS; BM/ALL, bone marrow infiltrated by acute lymphatic leukemia; BM/NB: bone marrow infiltrated by neuroblastoma; BM/Ewing’s: bone marrow infiltrated by Ewing’s sarcoma.
α/γAChR ratio ≫1
α/γAChR ratio <1
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared from 100 mg of snap-frozen tissue cut into 10-μm sections on a cryostat or from 106 cells using the GTC method 38 . After cDNA synthesis with oligo-dT primers and MMLV reverse transciptase (Gibco, Eggenstein, Germany), 1/20 of the reaction was amplified using Taq polymerase (Amersham, Braunschweig, Germany) and sequence-specific primers. The oligonucleotide primers for the acetylcholine receptor α and γ subunit (henceforth called αAChR and γAChR, respectively), 39, 40, 41, 42 myogenin and MyoD1, 35, 43 and PAX3/FKHR 19 were as follows: FMyoD1, 5′AGCACTACAGCGGCGACT3′; RMyoD1, 5′GCGACTCAGAAGGCACGTC3′. 35 Forward (F) αAChR, 5′AAGCTACTGTGAGATCATCGTCAC3′, reverse (R) αAChR, 5′TGACGAAGTGGTAGGTGATGTCCA3′; FγAChR, 5′ATCTCAGTCACCTACTTCCCC3′; RγAChR, 5′TACTTGCTGATGAGTGGCACC3′; Fmyogenin, 5′TAAGGTGTGTAAGAGGAAGTC3′; Rmyogenin, 5′TACATGGATGAGGAAGGGGAT3′; FPAX3/FKHR, 5′AGCTCACCGAGGCCCGAGT3′; RPAX3/FKHR, 5′ AACTGTGATCCAGGGCTGTC3′. 19
Amplifications were carried out at 65°C for αAChR and γAChR primers, using 2 U Taq polymerase for the simultaneous amplification of the α and γ subunit of the AChR, at 64°C for myogenin primers, and at 60°C for MyoD1 primers and for PAX3/FKHR primers, 35 cycles each. Primer pairs for GAPDH were used as a control (60°C, 22 cycles). 44
Semiquantitative RT-PCR
RNA integrity was confirmed in all samples by the detection of a 920-bp GAPDH product in ethidium bromide-stained gels. A semiquantitative PCR was established by adjusting all cDNAs to equal amounts of GAPDH transcripts. Ethidium bromide staining of the MyoD1, αAChR, γAChR, myogenin, and PAX/FKHR amplification products revealed bands of the expected molecular size, and subsequent sequencing of the PCR products in all cases confirmed that the cDNA fragments were identical to published MyoD1, AChR subunits, myogenin, and PAX3/FKHR gene fusion product sequences. 19, 39, 40, 41, 42, 43
Quantification of αAChR and γAChR Transcripts in Normal Muscle and Rhabdomyosarcomas by Duplex RT-PCR
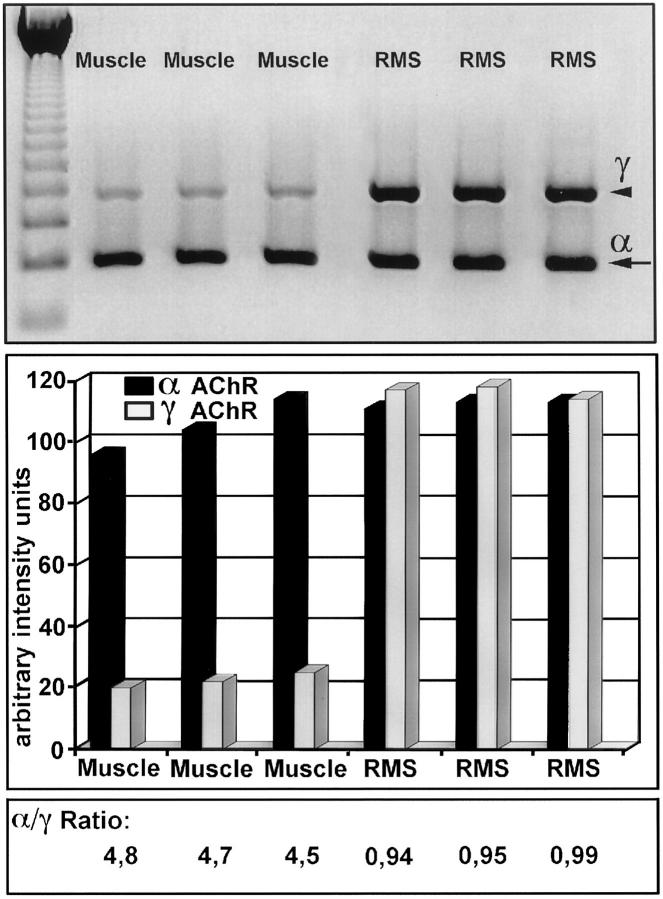
The simultaneous amplification of the αAChR and γAChR genes under identical conditions in one tube allows the quantification of the transcripts and their relation within one case. Therefore, we scanned the ethidium bromide-stained gel photography (Agfa scanner) of RT-PCR products and measured the intensity using the National Institutes of Health (MacIntosh) software (Figure 1) .The ratio of the absolute intensity for the αAChR and γAChR transcripts, henceforth called α/γ ratio, was ≫1 in all normal muscles and <1 in all RMS. For any given sample these ratios were highly reproducible with standard deviation <5%. Therefore, the determination of the α/γ ratio allows a differentiation between normal innervated muscle and RMS.
Figure 1.
Semiquantitative determination of αAChR and γAChR RT-PCR products from normal muscles and RMS (α/γ ratio). Intensities of αAChR (α, arrow) and γAChR (γ, arrowhead) were measured by scanning densitometry using an Agfa Scanner ARCUS II in normal muscles (Lanes 1–3) and RMS (Lanes 4–6). Intensities are given as arbitrary intensity units applying the NIH MacIntosh software. Using these intensity values to calculate α/γ ratios, it was revealed that α/γ ratios in normal muscles are ≫1 and 21 in all RMS.
Cloning and Sequencing of the PCR Products
For sequencing of the PCR products, bands were cut from agarose gels and DNA was extracted with jet-sorb (Genomed, Bad Oeynhausen, Germany). Eluted DNA was cloned into the pGEM-T-vector (Promega, Heidelberg, Germany) and the ligation mixture was transformed in JM 109-competent cells. DNA of recombinant colonies was isolated by minipreparation 45 and sequenced by the cycle sequencing method using dye terminators and the ABI 373A sequencer, following instructions of the manufacturer (Applied Biosystems, Weiterstadt, Germany).
Southern Blot Analysis
Ten microliters of each PCR product were run on a 1.5% agarose gel containing ethidium bromide. For hybridization, probes specific for the γ subunit of the AChR, myogenin, and MyoD1 were labeled with (α-32P) dATP from DuPont (Bad Homburg, Germany) using terminal deoxynucleotidyl transferase (GIBCO). The sequences of the probes are γAChR (611–635) 5′TTGTGGCCAAGAAGGTGCCTGAAAC3′; MyoD (511–535) 5′ AACTGCTACGAAGGC CGCCTACTACA3′.
Duplicate samples were tested by RT-PCR and Southern blot hybridization and the assays were repeated twice. PCR products were transferred onto a positively charged nylon membrane (Hybond N+, Amersham) by overnight alkaline-capillary blotting, hybridized, and washed under standard conditions. 45 The film was exposed for 6 hours at −70°C.
Results
MyoD1 and γ AChR mRNA Are Strongly Expressed in RMS and Tumors with Rhabdomyomatous Differentiation
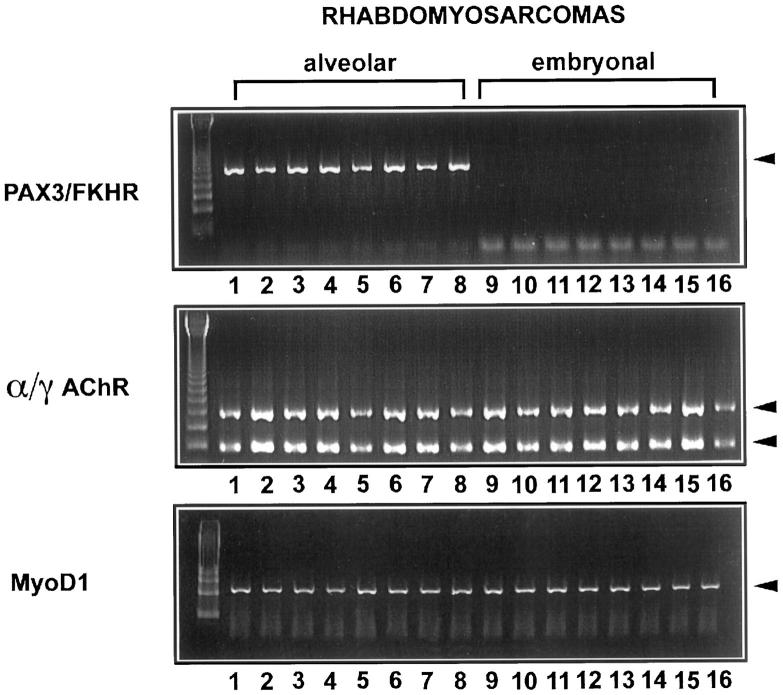
Eighteen RMS were examined applying RT-PCR for MyoD1 and α/γAChR subunits. In addition, RT-PCR with PAX3/FKHR-specific primers was applied to unequivocally identify translocation-positive alveolar RMS among the RMS cases studied. In all RMS, MyoD1 was also easily detected and transcripts from α/γAChR could be detected in ratios <1 in all RMS cases (Figure 2) .The PAX3/FKHR fusion product could be amplified in all alveolar RMS but in none of the embryonal RMS. In two Wilms’ tumors with a rhabdomyomatous differentiation (case 14744/89 and case 4841/90) (Figure 3and Table 2 ) transcripts from MyoD1 as well as an α/γAChR ratio <1 could be detected, similar to the results shown in RMS.
Figure 2.
RT-PCR analysis of RMS biopsies with primers specific for the PAX3/FKHR gene fusion product, MyoD1 and α/γAChR. Lane 1 = 10162/97,AR; lane 2 = 15378/89,AR; lane 3 = 17940/94,AR; lane 4 = 14097/90,AR; lane 5 = 5750/93,AR; lane 6 = 11421/89,AR; lane 7 = 32931/88,AR; lane 8 = 18471/96,AR; lane 9 = 7547/90,ER; lane 10 = 18325/95,ER; lane 11 = 25923/95,ER; lane 12 = 11563/92,ER; lane 13 = 17285/90,ER; lane 14 = 11279/87,ER; lane 15 = 7863/95,ER; lane 16 = 3207/93,ER. AR, alveolar RMS; ER, embryonal RMS.
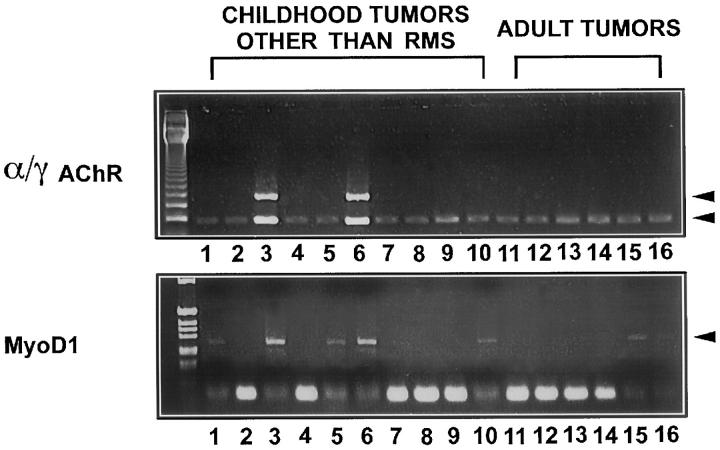
γAChR mRNA but Not MyoD1 mRNA Is Absent from Tumors without Rhabdomyomatous Differentiation
The expression of MyoD1 and α/γAChR transcripts was then tested in 28 childhood and 12 adult non-rhabdomyomatous tumors. In almost all tumors, transcripts for the αAChR gene could be amplified, as shown in Figure 3 and Table 2 . MyoD1 transcripts could be detected in two neuroblastomas, two biopsies of one Ewing’s sarcoma (cases 11633/96-2 and 11633/96-5), and one synovial sarcoma, and in a muscle infiltrated by a Ewing’s sarcoma. In adult tumors MyoD1 was found in two prostate and two renal cell carcinomas. By contrast, the γAChR mRNA was detected only in a Ewing’s sarcoma biopsy (case 11633/96-2) containing denervated muscle as shown previously. 34, 36 Interestingly, another Ewing’s sarcoma biopsy from the latter patient, derived from the vertebral canal and devoid of tumor-infiltrated muscle (case 11633/96-5), showed no amplification of γAChR mRNA (Figure 3) .
Figure 3.
Investigation of various childhood and adult tumors other than RMS with RT-PCR and primers specific for MyoD1 and α/γAChR. Transcripts for MyoD1 could be amplified in two neuroblastomas (one shown in Lane 1, case 22064/90), two biopsies of one Ewing’s sarcoma (Lane 5, case 11633/96-5 and Lane 6, case 11633/96-2) and in two Wilms’ tumors with a rhabdomyomatous differentiation (one shown in Lane 3, case 14744/89). In adult tumors MyoD1 was found in one breast (Lane 10, two prostate, and two renal cell carcinomas (one of each shown in Lane 15 and Lane 16). The γAChR mRNA was detected only in two Wilms’ tumors wih a rhabdomyomatous differentiation (one shown in Lane 3, case 14744/89) and in denervated muscle infiltrated by a Ewing’s sarcoma (Lane 6, case 11633/96-2). Another Ewing’s sarcoma biopsy from the latter patient, derived from the vertebral canal and devoid of tumor-infiltrated muscle (Lane 5, case 11633/96–5), showed no amplification of γAChR mRNA. Most investigated tumors showed amplification of αAChR.
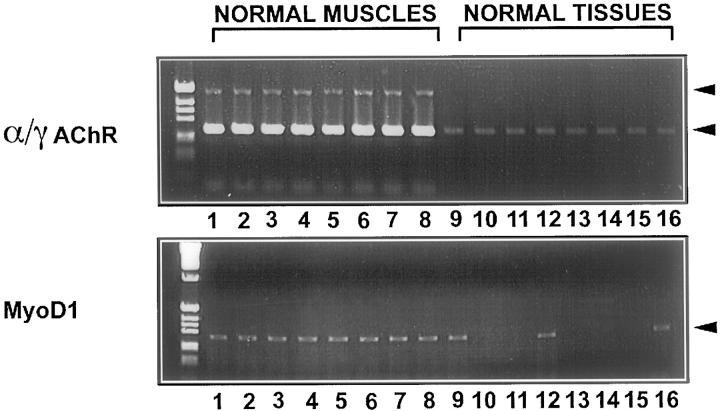
MyoD1 and γAChR mRNA Are Differentially Transcribed in Normal Muscles and Normal Tissues
Next we tested eight normal muscles and eight normal tissues for the transcription of MyoD1 and α/γAChR subunits. Transcripts of αAChR could be detected in all normal muscles (Figure 4) ,whereas very few transcripts of MyoD1 were found in normal muscles. In some normal tissues, MyoD1 transcripts could be amplified, as shown in Figure 4 and Table 3 . By contrast, very few transcripts of the γAChR were amplified in normal muscles, and they were totally absent from other normal tissues.
Figure 4.
RT-PCR analysis of MyoD1 and α/γAChR subunit gene transcription in normal muscles and normal tissues. Only a few transcripts of MyoD1 and even less of αAChR were found in all normal muscles. In other normal tissues, MyoD1 transcripts were detected in heart (Lane 9), liver (Lane 12), and prostate (Lane 16). By contrast, transcripts of the γAChR were totally absent from other normal tissues, whereas strong transcription of αAChR could be detected in all normal muscles and very little αAChR mRNA was detected in all normal tissues.
Sensitivity of RT-PCR in Detecting MyoD1 and AChR mRNA Expression
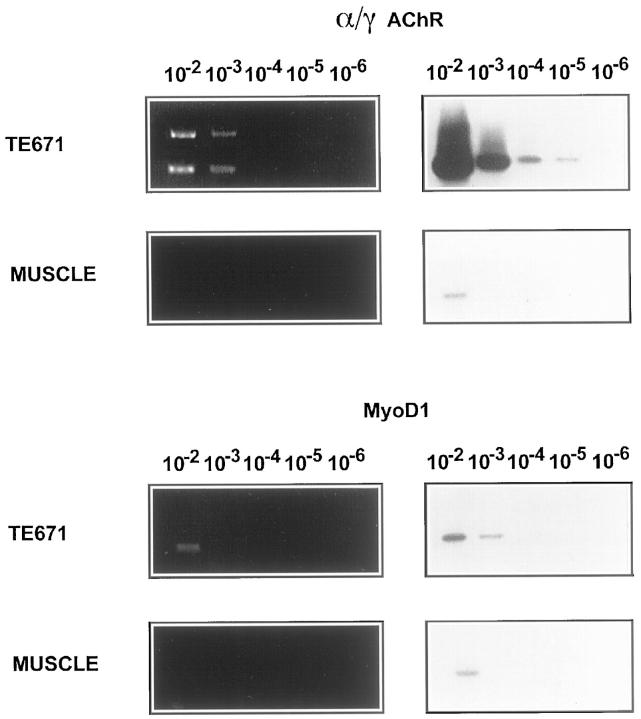
The usefulness of the RT-PCR technique for the detection of minimal residual disease or for cytology also depends on its sensitivity. To determine this, we performed serial dilutions of TE671 RMS cells with Raji cells to obtain mixtures of 104, 103, 102, and 1 RMS cell(s) in 106 Raji cells for RNA extraction. In addition, RNAs extracted from normal muscle biopsies and from Raji cells were adjusted to identical mRNA contents according to GAPDH expression and mixed to make dilutions of 10−2 to 10−6 muscle RNA in Raji cell RNA. Duplicate samples were tested by RT-PCR and Southern hybridization (Figure 5) .
Figure 5.
Sensitivity of RT-PCR for detection of MyoD1 and γAChR. RNA from TE671 cells were mixed with 106 Raji cells and RNAs extracted from normal muscle biopsies and from Raji cells were adjusted to identical mRNA contents according to GAPDH expression and mixed to make dilutions of 10−2 to 10−6 muscle RNA in Raji cell RNA. PCR products were stained with ethidium bromide (left) and detected using Southern blot hybridization (right). The mRNA of γAChR was detected at concentrations as low as 10 TE671 cells in 106 Raji cells, whereas MyoD1 transcripts were detected at a concentration of 1000 TE671 cells in 106 Raji cells, respectively. In normal muscle, the γAChR and MyoD1 mRNA were detected at a concentration of 10,000 cells mixed with 106 Raji cells.
Transcripts of MyoD1 were detected in TE671 cells at concentrations equivalent to 1000 cells in 106 Raji cells. In normal muscle, MyoD1 mRNA was detected only at concentrations equivalent to 10,000 cells mixed with 106 Raji cells.
By contrast, mRNA of γAChR was detected in TE671 cells at concentrations as low as 10 cells mixed with 106 Raji cells. In normal muscle the γAChR mRNA was detected only at concentrations equivalent to or higher than 10,000 cells in 106 Raji cells. Therefore, MyoD1 RT-PCR is about 100-fold less sensitive than RT-PCR for the γ subunit of the AChR.
Investigation of Bone Marrow Samples Infiltrated by Various Childhood Tumors
As a prototypical model of minimal disease we investigated retrospectively 47 bone marrow aspiration biopsies that were either free of tumor (n = 26) or infiltrated by neuroblastomas (n = 8), Ewing’s sarcomas (n = 4), alveolar RMS (n = 5), or acute lymphoblastic leukemia (n = 4). The infiltration by alveolar RMS was verified in 3 cases cytologically and by PAX3/FKHR PCR, and in 2 cases only by the PAX3/FKHR PCR as no tumor cells could be seen cytologically, indicating a submicroscopic infiltration. Remarkably, no transcripts of MyoD1 could be detected in any of the bone marrow samples, including all those infiltrated by alveolar RMS (Figure 6 and Table 4 ). By contrast, we found α/γAChR ratios <1 in all 5 bone marrow biopsies that were infiltrated by alveolar RMS. When serial dilutions (1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256 in Raji cells) of RNA from RMS-positive bone marrow aspirates were studied by RT-PCR for fetal AChR and PAX3/FKHR transcription, both techniques had a similar sensitivity for the detection of alveolar RMS (legend to Figure 6 ).In nonrhabdomyomatous tumor samples, none or very few transcripts (α/γAChR ratio ≫1) were detected, suggesting contamination of bone marrow samples with normal muscle (Figure 6 and Table 4 ). In this part of the study we also investigated the transcription of the myogenin gene in the 47 bone marrow aspirates, because myogenin mRNA had not been checked before for its usefulness as a diagnostic molecular marker for bone marrow micrometastases. However, myogenin mRNA could be amplified in bone marrow samples infiltrated by various childhood tumors other than RMS including 2 neuroblastomas, 2 Ewing’s sarcomas, and 2 acute lymphatic leucemias, as well as in 4 probes free of tumor.
Figure 6.
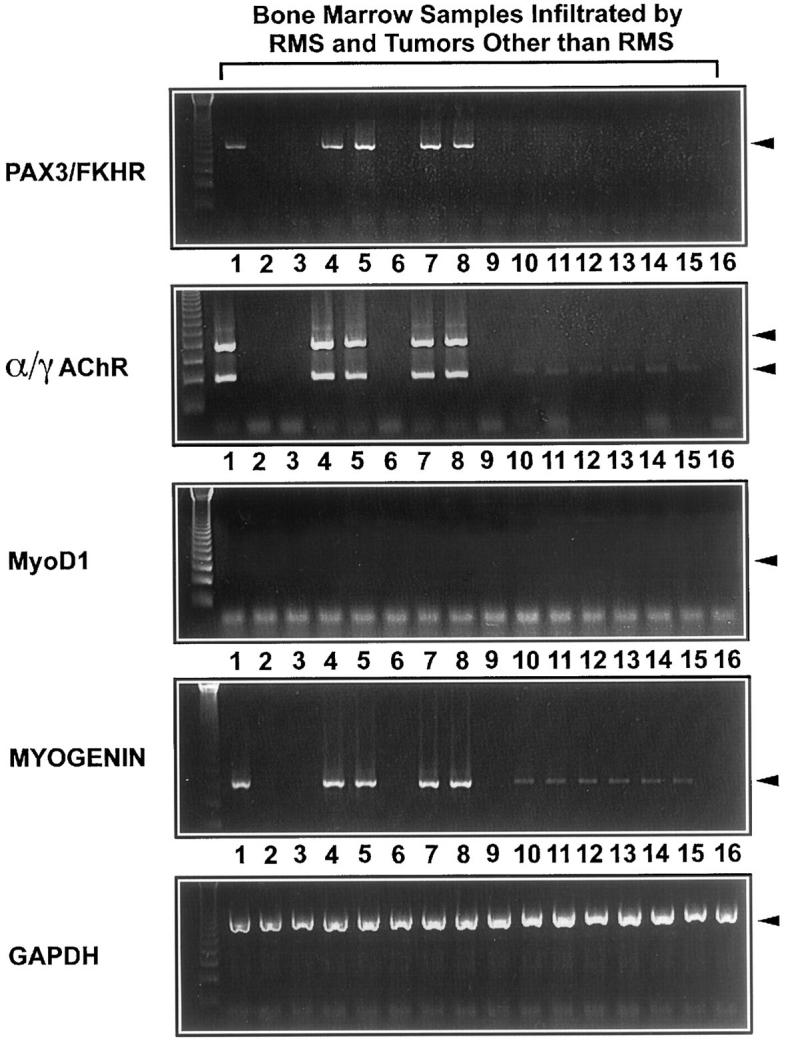
RT-PCR analysis of pediatric bone marrow aspiration biopsies with primers specific for the PAX3/FKHR fusion gene product, α/γAChR, MyoD1, and myogenin. In all bone marrow samples infiltrated by alveolar RMS (Lanes 1,* 4, 5, 7 and 8), transcripts of PAX3/FKHR, α/γAChR, and myogenin were detected in similar quantities. In contrast to PAX3/FKHR and γAChR, myogenin and αAChR transcripts were found in, respectively, two bone marrow samples infiltrated by neuroblastomas (Lanes 10 and 11), Ewing’s sarcomas (one shown in Lane 12) and two acute lymphatic leukemias (one shown in Lane 13), as well as in four probes free of tumor (two shown in Lanes 14 and 15). No transcripts of MyoD1 were detected in any of the bone marrow samples including all probes infiltrated by alveolar RMS. *Lane 1: This bone marrow sample infiltrated by alveolar RMS was used for dilution experiments and following RT-PCR with primers specific for PAX/FKHR and α/γAChR. Both techniques had a similar sensitivity for the detection of alveolar RMS.
Sensitivity and Specificity of the MyoD1 and α/γAChR RT-PCR in the Presence of Muscle Contamination
Inadvertent contamination of fine-needle biopsies or marrow trephines with normal muscle adjacent to the site of interest is a very common finding 46 that may impair the specificity of PCR-based diagnostic approaches.
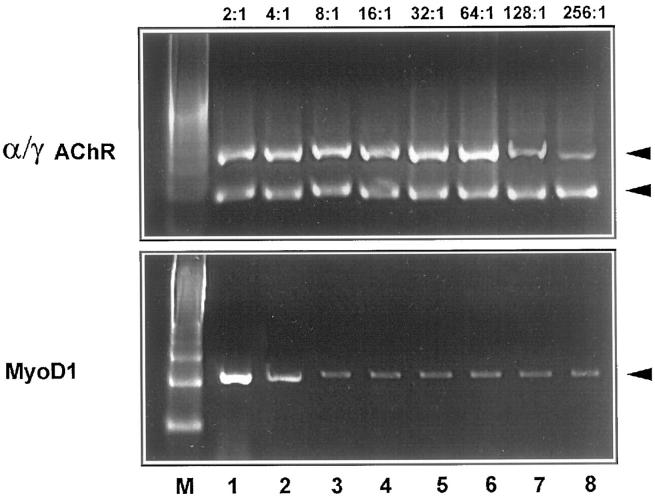
To investigate how normal muscle contaminating a RMS tumor sample can influence the MyoD1 signal and the α/γ ratio, we tried to mimic in vivo contamination by mixing RNA from normal muscle and RMS biopsies in ratios of 2:1, 4:1, 8:1, 16:1, 32:1, 64:1, 128:1, and 256:1. Hyperexpression of MyoD1 was already obscured when RMS RNA was mixed with normal muscle RNA at dilutions beyond 4:1 (Figure 7) .By contrast, the α/γ ratio 21 is detectable even when RMS RNA is diluted with a 128:1 excess of normal muscle RNA (Figure 7) precluding a false negative diagnosis of normal muscle. This conclusion could be verified in an ex vivo embryonal RMS biopsy heavily contaminated by skeletal muscle (Figure 8A) .Due to the high constitutive RNA expression of myogenin in normal muscle, 36 no RMS-specific hyerexpression of myogenin RNA was detectable at any dilution of RMS RNA mixed with normal muscle RNA (not shown).
Figure 7.
Determination of the sensitivity of the MyoD1 RT-PCR compared to the α/γAChR duplex RT-PCR for detection of RMS in the presence of muscle contamination. Normal muscle RNA was mixed in dilutions of 2:1, 4:1, 8:1, 16:1, 32:1, 64:1, 128:1, and 256:1 with RMS RNA (case 17940/94). Hyperexpression of MyoD1 was already obscured when RMS RNA was mixed with normal muscle RNA at dilutions weaker than 4:1. By contrast, an α/γAChR ratio <1 was still detectable in the 128:1 dilution, indicating that RMS cells can be detected by the α/γAChR RT-PCR even when 99% of the RMS-containing biopsy consists of normal muscle.
Figure 8.
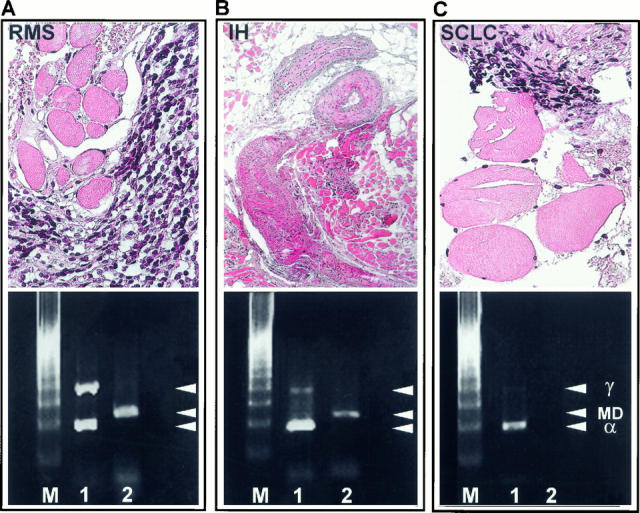
Determination of the specificity of the MyoD1 RT-PCR compared to the α/γAChR duplex RT-PCR. Ex vivo embryonal RMS biopsy (case 25923/95) contaminated by skeletal muscle (A) showed strong transcription of MyoD1 and a RMS specific α/γ ratio of 0.93 (A, Lane 1), indicating a dramatic overexpression of the γAChR in RMS. In an intramuscular hemangioma (IH, case 26777/98) (B> the α/γ ratio was 6.2 (B, Lane 1) and in a biopsy derived from a small-cell lung cancer (SCLC, case 1007/99) contaminated by normal muscle (C>, the α/γ ratio was 7.3 (C, Lane 1>. By contrast, MyoD1 was detected in the intramuscular hemangioma (B, Lane 2> in quantities similar to those shown in RMS (A>, but MyoD1 mRNA was not found in the biopsy with small amounts of contaminating muscle due to its low sensitivity (C, Lane 2>. M, marker; Lane 1 = α/γAChR RT-PCR; Lane 2 = MyoD1 RT-PCR; γ = γAChR; MD = MyoD1; α = αAChR.
On the other hand, normal muscle contaminating a biopsy derived from a small cell carcinoma of the lung (Figure 8B) or an intramuscular hemangioma (Figure 8C) results in an α/γ ratio ≫1, precluding a false positive diagnosis of RMS. By contrast, MyoD1 transcripts were detected in the intramuscular hemangioma (Figure 8C) in quantities similar to those shown in RMS (Figure 8A) , whereas MyoD1 mRNA was not found in the biopsy with small amounts of contaminating muscle (Figure 8B) , due to the low sensitivity of MyoD RT-PCR.
Discussion
Among RMS, only the alveolar RMS exhibit a characteristic translocation, t(2;13)(q35;q14), that results in the gene fusion product PAX3/FKHR and can be detected by PCR in almost all cases. 12, 13, 14, 15, 16, 17, 18, 19 Unfortunately such an unequivocal, PCR-based marker has not been available for the rare alveolar RMS lacking these translocations, and for the majority of RMS that belong to the embryonal subtype.
In this study we applied a novel duplex PCR-based method to the simultaneous detection of AChR α and γ subunit message in embryonal and alveolar RMS compared to other childhood and adult tumors. This one-tube amplification of two different gene products under identical conditions enables one to compare the amount of each of the two mRNAs, and we could show that the α/γAChR ratio was <1 in all RMS, whereas in normal muscle the α/γAChR PCR revealed a ratio ≫1. In all investigated childhood tumors other than RMS and free of contaminating normal or neoplastic muscle, no transcripts of the γAChR could be detected. Applying this highly specific technique, we compared the transcription of α/γAChR with the transcription of MyoD1 to evaluate which RNA might be the best molecular marker for the diagnosis of minimal disease in the majority of RMS that lack specific translocations. MyoD1 was chosen for comparison with α/γAChR because it is expressed on the protein level in almost all RMS, 6, 7, 8 and because a recent study based on tumor cell lines suggested that MyoD1 might be a promising molecular marker for the diagnosis of translocation-negative RMS. 35
Surprisingly, MyoD1 transcripts were detected not only in all RMS tested, but also in various nonrhabdomyomatous ex vivo tumor biopsies and in some normal tissues. This low specificity of MyoD1 mRNA expression is only somewhat better than the even lower specificity of myogenin, as shown previously. 36 In addition to this low specificity, MyoD1 is a much less sensitive marker for RMS cells than γAChR. This was particularly obvious when we tested 47 bone marrow aspiration biopsies infiltrated by various childhood tumors, including five alveolar RMS. Three among these five samples were diagnosed cytologically and by PAX3/FKHR PCR, whereas the other two were only detected by PAX3/FKHR PCR, but not cytologically, indicating a submicroscopic infiltration. In all tissue known by PAX3/FKHR PCR to be infiltrated by an alveolar RMS, the α/γAChR duplex PCR showed an α/γAChR ratio 21. MyoD1, however, could be amplified neither from the PAX3/FKHR PCR-verified samples nor from nonrhabdomyomatous tumor-infiltrated bone marrow samples. This indicates a low transcription of the MyoD1 gene and is in agreement with our finding that the sensitivity of the MyoD1-RT-PCR is about 100-fold less sensitive than the RT-PCR for the γ subunit of the AChR (Figure 6) . By contrast, myogenin could be found not only in all RMS-infiltrated bone marrow samples, but also in the majority of other nonrhabdomyomatous tumor-infiltrated bone marrow biopsies and in some samples free of tumor (Figures 3 and 4) . This indicates that myogenin mRNA is not a useful target for the molecular detection of minimal disease. As a further advantage of the α/γAChR RT-PCR compared to MyoD1 RT-PCR, we found that only the α/γAChR ratio can distinguish RMS from a contamination with normal muscle (Figures 7 and 8A) , because even in the presence of a 128-fold excess of normal muscle RNA over RMS RNA, the α/γAChR ratio was 21. Furthermore, contaminating normal muscle in the absence of rhabdomyosarcomatous tumor cells showed a α/γAChR ratio ≫1 (Figure 8B and 8C) excluding a false positive diagnosis of RMS. This contrasts to the finding that normal muscle as tumor-infiltrated tissue may lead to positive PCR results for MyoD1 (Figure 8B) , mimicking a RMS.
In summary, the simultaneous detection of the α/γAChR message by duplex PCR is not only useful in the accurate diagnosis of difficult primary tumors, but also appears to warrant further testing for the detection of micrometastases and minimal residual disease in PAX/FKHR-negative RMS. Indeed, since the α/γAChR and PAX/FKHR RT-PCR have a similar sensitivity, the α/γAChR duplex RT-PCR does not challenge the PAX/FKHR RT-PCR for the diagnosis of translocation positive (PAX/FKHR+) alveolar RMS, given that only the PAX/FKHR approach is absolutely specific for PAX/FKHR+ RMS. In contrast to recently published results, 35 MyoD1, a highly specific marker for RMS at the protein level, does not appear to offer any advantage in the PCR-based diagnosis of RMS in vivo; it is transcribed in nonrhabdomyomatous tumors and in some normal tissues, reveals a sensitivity too low for the detection of minimal residual disease, and cannot distinguish between rhabdomyomatous cells and normal muscle. We conclude from our findings, therefore, that future studies should compare the duplex α/γAChR PCR described here with a MyoD1-directed nested PCR approach, which may combine the higher specificity of the former with the possibly higher sensitivity of the latter. Finally, the perspective of the duplex α/γAChR RT-PCR described here to apply it to the diagnosis of translocation-negative alveolar RMS is obvious but requires future study for confirmation.
Acknowledgments
We thank Mrs. Elke Oswald, Mrs. Christl Kohaut, and Mr. Erwin Schmitt for expert technical assistance and Dr. Ewa Koscelniak (CWS) and Prof. Dr. Herbert Jürgens (EICESS) for contributing the bone marrow samples.
Address reprint requests to Dr. Stefan Gattenloehner, Institute of Pathology, University of Würzburg, Josef-Schneider-Strasse 2, D-97080 Würzburg, Germany. E-mail: gattenloehner@hotmail.com.
Footnotes
Supported by the DFG IZKF Project C-5 (01-KS-9603)
References
- 1.Triche TJ: Pathology of pediatric malignancies. Pizzo PA Poplack PG eds. Principles and Practice of Pediatric Oncology. 1993, :pp 115-152 JB Lippincott, Philadelphia [Google Scholar]
- 2.Diller L: Rhabdomyosarcoma and other soft tissue sarcomas of childhood. Curr Opin Oncol 1992, 4:689-695 [DOI] [PubMed] [Google Scholar]
- 3.Newton WA, Jr.,, Gehan EA, Webber BL, Marsden HB, van Unnik AJM, Hamoudi AB, Tsokos M, Shimada H, Harms D, Schmidt D, Ninfo V, Cavanazza A, Gonzalez-Crussi F, Parham DM, Reiman HM, Asmar L, Beltangady MS, Sachs N, Triche TJ, Maurer HM: Classification of rhabdomyosarcomas and related sarcomas: pathologic aspects and proposal for a new classification. An Intergroup Rhabdomyosarcoma study. Cancer 1995, 76:1073-1085 [DOI] [PubMed] [Google Scholar]
- 4.Dias P, Parham DM, Shapiro DN, Webber BL, Houghton PJ: Myogenic regulatory protein (MyoD1) expression in childhood solid tumors: diagnostic utility in rhabdomyosarcoma. Am J Pathol 1990, 137:1283-1291 [PMC free article] [PubMed] [Google Scholar]
- 5.Rosai J, Dias P, Parham DM, Shapiro DN, Houghton P: MyoD1 protein expression in alveolar soft part sarcoma as confirmatory evidence of its skeletal muscle nature. Am J Surg Pathol 1991, 15:974-981 [DOI] [PubMed] [Google Scholar]
- 6.Wang NP, Marx J, McNutt MA, Rutledge JC, Gown AM: Expression of myogenic regulatory proteins (myogenin and MyoD1) in small blue round cell tumors of childhood. Am J Pathol 1995, 147:1799-1810 [PMC free article] [PubMed] [Google Scholar]
- 7.Andrassy RJ, Corpron CA, Hays D, Raney RB, Wiener ES, Lawrence W, Jr.,, Lobe TE, Bagwell C, Maurer HM: Extremity sarcomas: an analysis of prognostic factors from the Intergroup Rhabdomyosarcoma Study III. J Pediatr Surg 1996, 31:191-196 [DOI] [PubMed] [Google Scholar]
- 8.Hays DM, Lawrence W, Jr.,, Crist WM, Wiener E, Raney RB, Ragab A, Tefft M, Webber B, Johnston J, Maurer HM: Partial cystectomy in the management of rhabdomyosarcoma of the bladder: a report from the Intergroup Rhabdomyosarcoma Study. J Pediatr Surg. 1990, 25:719-723 [DOI] [PubMed] [Google Scholar]
- 9.Hays DM, Lawrence W, Jr.,, Wharam M, Newton W, Jr.,, Ruymann FB, Beltangady M, Maurer HM: Primary reexcision for patients with “microscopic residual” tumor following initial excision of sarcomas of trunk and extremity sites. J Pediatr Surg 1989, 24:5-10 [DOI] [PubMed] [Google Scholar]
- 10.Maurer HM: Current state of the art and the future challenges in childhood rhabdomyosarcoma. 3rd International Congress on Soft Tissue Sarcoma in Children and Adolescents, Stuttgart, Germany, 1997. Book of Abstracts, pp 3–4
- 11.Qualman SJ, Triche T, Barr F, Crist W, Sachs N: Modern diagnostics in soft tissue sarcomas. 3rd International Congress on Soft Tissue Sarcoma in Children and Adolescents, Stuttgart, Germany, 1997. Book of Abstracts, pp 96–97
- 12.Sainati L, Stella M, Montaldi A, Bolcato S, Guercini N, Bonan F, Ninfo V, Zanesco L, Basso G: Value of cytogenetics in the differential diagnosis of the small round cell tumors of childhood. Med Pediatr Oncol 1992, 20:130-135 [DOI] [PubMed] [Google Scholar]
- 13.Biegel JA, Meek RS, Parmiter AH, Conrad K, Emanuel BS: Chromosomal translocation t (1;13) (q35;q14) in a case of rhabdomyosarcoma. Genes Chromosomes Cancer 1991, 3:483-484 [DOI] [PubMed] [Google Scholar]
- 14.Douglass EC, Valentine M, Etcubanas E, Parham D, Webber BL, Houghton PJ, Houghton JA, Green AA: A specific chromosomal abnormality in rhabdomyosarcoma. Cytogenet Cell Genet 1987, 45:148-155 [DOI] [PubMed] [Google Scholar]
- 15.Douglass EC, Shapiro DN, Valentine M, Rowe ST, Caroll AJ, Raney RB, Ragab AH, Abella SM, Parham DM: Alveolar rhabdomyosarcoma with t (2;13): cytogenetic findings and clinicopathological correlations. Med Pediatr Oncol 1993, 21:83-87 [DOI] [PubMed] [Google Scholar]
- 16.Douglass EC, Rowe ST, Valentine M, Parham DM, Berkow R, Bowman WP, Maurer HM: Variant translocations of chromosome 13 in alveolar rhabdomyosarcomas. Genes Chromosomes Cancer 1991, 3:480-482 [DOI] [PubMed] [Google Scholar]
- 17.Trent J, Casper J, Meltzer P, Thompson F, Fogh J: Nonrandom chromosome alterations in rhabdomyosarcoma. Cancer Genet Cytogenet 1985, 16:189-197 [DOI] [PubMed] [Google Scholar]
- 18.Turc-Carel C, Lizard-Nacol S, Justrabo E, Fvrot M, Philip T, Tabone E: Consistent chromosomal translocation in alveolar rhabdomyosarcoma. Cancer Genet Cytogenet 1986, 19:361-362 [DOI] [PubMed] [Google Scholar]
- 19.Galili N, Davis RJ, Fredericks WJ, Mukhopadhyay S, Rauscher FJ, Emanuel BS, Rovera G, Barr FG: Fusion of a forkhead domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet 1993, 5:230-235 [DOI] [PubMed] [Google Scholar]
- 20.Chang WY, Yuan L, Feng L, Hensle T, Tycko B: Chromosome 11p15.5 regional imprinting: comparative analysis of KIP2 and H19 in human tissues and Wilms’ tumors. Hum Mol Genet 1996, 5:1101-1108 [DOI] [PubMed] [Google Scholar]
- 21.Besnard-Guerin C, Newsham I, Winquist R, Cavenee WK: A common region of loss of heterozygosity in Wilms’ tumor and embryonal rhabdomyosarcoma distal to the D11S988 locus on chromosome 11p15.5. Hum Genet 1996, 97:163-170 [DOI] [PubMed] [Google Scholar]
- 22.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Bart RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S: A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. [PubMed]
- 23.Zhuang Z, Vortmeyer AO: Applications of tissue microdissection in cancer genetics. Cell Vis 1998, 5:43-48 [PubMed] [Google Scholar]
- 24.Changeux JP: Functional organization of the nicotinic acetylcholine receptor. C R Acad Sci III 1992, 314 (suppl):89-94 [PubMed] [Google Scholar]
- 25.Tzartos SJ: Myasthenia gravis studied by monoclonal antibodies to the acetylcholine receptor. In Vivo 1988, 2:105-110 [PubMed] [Google Scholar]
- 26.Whiting PJ, Vincent A, Schluep M, Newsom-Davis J: Monoclonal antibodies that distinguish between normal and denervated human acetylcholine receptor. J Neuroimmunol 1986, 11:223-235 [DOI] [PubMed] [Google Scholar]
- 27.Heidenreich F, Vincent A, Roberts A, Newsom-Davis J: Epitopes on human acetylcholine receptor defined by monoclonal antibodies and myasthenia gravis sera. Autoimmunity 1988, 1:285-297 [DOI] [PubMed] [Google Scholar]
- 28.Missias AC, Chu GC, Klocke BJ, Sanes JR, Merlite JP: Maturation of acetylcholine receptor in skeletal muscle: regulation of the AChR-gamma-to-epsilon-switch. Dev Biol 1996, 179:223-238 [DOI] [PubMed] [Google Scholar]
- 29.Witzemann V, Barg B, Nishikawa Y, Sakman B, Numa S: Differential regulation of muscle acetylcholine receptor γ- and ɛ-subunit mRNAs. FEBS Lett 1987, 223:104-112 [DOI] [PubMed] [Google Scholar]
- 30.Hara H, Hayashi K, Ohta K, Itoh N, Ohta M: Nicotinic acetylcholine receptor mRNAs in myasthenic thymuses: association with intrathymic pathogenesis of myasthenia gravis. Biochem Biophys Res Commun 1993, 194:1269-1275 [DOI] [PubMed] [Google Scholar]
- 31.Marx A, Kirchner T, Hoppe F, O’Connor R, Schalke B, Tzartos S, Müller-Hermelink HK: Proteins with epitopes of the acetylcholine receptor in epithelial cell cultures of thymomas in myasthenia gravis. Am J Pathol 1989, 134:865-877 [PMC free article] [PubMed] [Google Scholar]
- 32.Kaminski HJ, Kusner LL, Block CH: Expression of acetylcholine receptor isoforms at extraocular muscle endplates. Invest Ophthalmol Vis Sci 1996, 37:345-351 [PubMed] [Google Scholar]
- 33.Hesselmans LFGM, Jennekens FGI, van den Oord CJM, Veldman H, Vincent A: Development of innervation of skeletal muscle fibers in man: relation to acetylcholine receptors. Anat Rec 1993, 236:553-562 [DOI] [PubMed] [Google Scholar]
- 34.Gattenloehner S, Vincent A, Leuschner I, Tzartos S, Müller-Hermelink HK, Kirchner T, Marx A: The fetal form of the acetylcholine receptor distinguishes rhabdomyosarcomas from other childhood tumors. Am J Pathol 1998, 152:437-444 [PMC free article] [PubMed] [Google Scholar]
- 35.Frascella E, Rosolen A: Detection of the MyoD1 transcript in rhabdomyosarcoma cell lines and tumor samples by reverse transcription polymerase chain reaction. Am J Pathol 1998, 152:577-583 [PMC free article] [PubMed] [Google Scholar]
- 36.Gattenloehner S, Müller-Hermelink HK, Marx A: PCR-based diagnosis of rhabdomyosarcomas: comparison of fetal type acetylcholine receptor subunits and myogenin. Diag Mol Pathol 1998, 7:129-134 [DOI] [PubMed] [Google Scholar]
- 37.Stratton MR, Reeves BR, Cooper CS: Misidentified cell. Nature 1989, 337:311-312 [DOI] [PubMed] [Google Scholar]
- 38.Chomczynski P, Sacchi N: Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 39.Beeson D, Brydson M, Newsom-Davis J: Nucleotide sequence of human muscle actelycholine receptor beta-subunit. Nucleic Acids Res 1989, 17:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beeson D, Brydson M, Betty M, Jeremiah S, Povey S, Vincent A, Newsom-Davis J: Primary structure of the human muscle acetylcholine receptor-cDNA. Cloning of the gamma-and epsilon-subunit. Eur J Biochem 1993, 215:229-238 [DOI] [PubMed] [Google Scholar]
- 41.Luther MA, Schopfer R, Whiting P, Casey B, Blatt Y, Montal MS, Montal M, Lindstrom J: A muscle acetylcholine receptor is expressed in human cerebellar medulloblastoma cell line TE 671. J Neurosci 1989, 9:1082-1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoepfer R, Luther M, Lindstrom J: The human medulloblastoma cell line TE 671 expresses a muscle-like acetylcholine-receptor: cloning of the alpha-subunit. FEBS Lett 1988, 226:235-240 [DOI] [PubMed] [Google Scholar]
- 43.Braun T, Boner E, Buschhausen-Denker G, Kohtz S, Grzeschik KH, Arnold HH, Kotz S: Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO 1989, 8:3617-3625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arcari P, Martinell R, Salvatore F: The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res 1984, 12:1979-1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch EF, Maniatis T: Molecular cloning 2. 1989. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY,
- 46.Bishop PW, McNally K, Harris M: Audit of bone marrow trephines. J Clin Pathol 1992, 45:1105-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]