Abstract
Gamma/delta T cell lymphomas (γ/δ TCL) represent rare, often aggressive types of T cell malignancy that are clinically and pathologically diverse. Most γ/δ TCL occur as a hepatosplenic or subcutaneous type. To date, analysis of the T cell receptor δ (TCRδ) gene repertoire of hepatosplenic γ/δ TCL (γ/δ HSTCL) and subcutaneous panniculitis-like γ/δ TCL (γ/δ SPTCL) has been reported only in a limited number of cases. In this study we analyzed 11 γ/δ HSTCL and 4 γ/δ SPTCL by polymerase chain reaction and immunostaining to determine their usage of the Vδ subtypes (Vδ1–6). It is noteworthy that 10 of 11 γ/δ HSTCL expressed the Vδ1 gene. The remaining case also expressed T cell receptor δ (TCRδ) as determined by flow cytometry and TCRδ rearrangement in Southern blot. However, the Vδ gene expressed by this lymphoma could not be determined, which suggests usage of an as yet unidentified Vδ gene. In striking contrast to the γ/δ HSTCL, all 4 γ/δ SPTCL expressed the Vδ2 gene. Our data demonstrate that γ/δ HSTCL are preferentially derived from the Vδ1 subset of γ/δ T lymphocytes, whereas γ/δ SPTCL are preferentially derived from the Vδ2 subset. The pattern of Vδ gene expression in HSTCL and SPTCL corresponds to the respective, predominant γ/δ T cell subsets normally found in the spleen and skin. This finding suggests that γ/δ TCL are derived from normal γ/δ T lymphocytes which reside in the affected tissues. Furthermore, the selective, lymphoma type-specific Vδ gene segment usage may provide a molecular tool to distinguish better among various types of γ/δ TCL lymphoma particularly in the clinically advanced, widely disseminated cases.
Similar to normal T lymphocytes, T cell lymphomas (TCL) may express two different types of T cell receptor (TCR), α/β or γ/δ. Four TCR genes (α, β, γ, and δ) are composed in their germline configuration of noncontiguous segments of variable (V), diversity (D), joining (J), and constant (C) regions. During T cell differentiation, somatic VDJ rearrangements occur and thereby generate variability of the TCR. 1 Only complete in-frame TCR gene rearrangements, consisting of V, D, and J regions, may form a functional TCR. Incomplete rearrangements between two D regions (D-D), between V and D region (V-D), and between D and J region (D-J) are nonfunctional.The majority of normal T lymphocytes express the α/β heterodimer; however, approximately 5% of the T cells express the γ/δ heterodimer. 2 In contrast to α/β T lymphocytes, development of γ/δ T cells is not dependent on expression of major histocompatibility complex (MHC) I or MHC II molecules. 3, 4 Unlike α/β T cells, which develop almost exclusively in thymus, γ/δ T cells can be generated in extrathymic sites such as intestinal epithelium, skin, spleen, and fetal liver. 5, 6, 7, 8 The exact function of γ/δ T lymphocytes has not been fully elucidated, but some studies suggest a role for these cells in early immune responses to infections, autoimmune disorders, and cancer immune surveillance. 9 γ/δ T cells share some features with CD8+ α/β T lymphocytes and with natural killer (NK) cells. They show MHC-dependent and MHC-independent cytotoxity, produce lymphokines, and exhibit NK-like lytic activity.
γ/δ TCL represent a rare type of T cell malignancy. They comprise less than 10% of peripheral T cell lymphomas 10 and occur mostly at extranodal sites in hepatosplenic, subcutaneous, or intestinal form. Hepatosplenic γ/δ TCL (γ/δ HSTCL) is recognized as a provisional subset of peripheral T cell lymphoma in the Revised European-American Classification of Lymphoid Neoplasms (REAL), 11 although a few identified cases of α/β HSTCL appear to have similar clinicopathological characteristics. Histologically, γ/δ HSTCL is characterized by a mixture of small to medium-sized atypical lymphocytes. To date only about 40 cases of γ/δ HSTCL have been reported. 10, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 These lymphomas frequently show two nonrandom chromosomal abnormalities, isochromosome 7q [i(7)(q10)] and trisomy 8 (8+). 19, 20 Affected individuals are usually young males. Patients commonly present with B symptoms and hepatosplenomegaly, but not lymphadenopathy. The disease usually follows an aggressive course with poor response to chemotherapy and short time of survival.
Subcutaneous panniculitis-like TCL (SPTCL) is an uncommon form of cutaneous lymphoma, involving mainly subcutis and often mimicking lobular panniculitis. 24 SPTCL also has been proposed as a provisional subset of peripheral T cell lymphoma in the REAL classification. 11 It is sometimes associated with aggressive clinical behavior and poor prognosis, particularly when accompanied by a hemophagocytic syndrome. 24, 25, 26 Based on TCR expression, SPTCL can be divided into α/β and γ/δ SPTCL subsets, which are not recognized as distinct entities in the REAL classification. To date only a few γ/δ SPTCL cases have been reported. 26, 27, 28, 29, 30, 31
The TCRδ gene consists of at least six Vδ gene segments. 32, 33 A detailed analysis revealed that over 95% of the γ/δ T cells express either Vδ1 or Vδ2 gene. 2, 9 Interestingly, normal γ/δ T lymphocytes present in spleen, thymus, and intestinal epithelium predominantly express the Vδ1 gene, whereas the majority of γ/δ T cells in peripheral blood, tonsils, and skin express the Vδ2 gene. 2 The reason for this dichotomy in the Vδ gene usage repertoire remains unclear. Vδ gene usage by γ/δ TCL has not been studied so far in the great detail, and the small number of the reported cases does not allow any definitive conclusions in regard to Vδ usage by the specific subtype of γ/δ TCL. In this study we analyzed the Vδ usage in 11 hepatosplenic and 4 subcutaneous γ/δ TCL using polymerase chain reaction (PCR) and flow cytometry. Preferential usage of Vδ1 gene was found in γ/δ HSTCL (10/11 cases) and of Vδ2 gene in γ/δ SPTCL (4/4 cases). Biological and diagnostic implications of this finding are discussed.
Materials and Methods
Patient Samples
We investigated 15 patients with γ/δ TCL of hepatosplenic (11 cases) and subcutaneous (4 cases) type in this study. The cases were derived from files of the participating institutions: the University of Pennsylvania, Vanderbilt University, Johns Hopkins University, the University of Pittsburgh, and Harvard University. Data on cases 1–3, 12, and 13 including Vδ subtype expression were previously reported in part. 20, 26
Cytogenetic Analysis and Fluorescence in Situ Hybridization (FISH)
Metaphase cytogenetic analysis was performed by standard trypsin Giemsa banding using unstimulated cell cultures of spleen or bone marrow cells. Slides for FISH were prepared from spleen or peripheral blood mononuclear cells according to a standard method. In brief, liquid nitrogen-stored, dimethyl sulfoxide-preserved frozen cells from three patients were cultured overnight, cytospun onto slides at a concentration of 104 cells/slide, air-dried, fixed in Carnoy’s for 20 minutes, and air-dried overnight. The cytospins were analyzed with VYSIS (Downers Grove, IL) CEP 8 probe to enumerate chromosome 8 centromeres or combination of CEP 7 and LSI D7S486 probes to detect simultaneously chromosome 7 centromere and band 7q31 on the chromosome’s long arm. The staining was performed as recommended by the probe manufacturer.
Immunophenotype Analysis
Flow cytometry and frozen section or paraffin immunohistochemistry were used for immunophenotyping of the lymphomas. All cases were studied by flow cytometry and/or frozen section immunohistochemistry with a set of standard anti-T cell and anti-NK cell antibodies as previously described. 20, 26 To confirm the γδ phenotype, we used antibodies to the α/β and γ/δ TCR (Endogen, Woburn, MA). 20, 26 Vδ subtype expression was also determined by flow cytometry or frozen section immunohistochemistry using commercially available monoclonal antibodies specific for different V regions of the TCRδ chain (Vδ1 and Vδ2, Endogen; Vδ3, Immunotech, Westbrook, ME).
Southern Blot Analysis
Southern blot analysis for TCRδ gene rearrangements was performed on genomic DNA extracted from frozen tumor in five cases of γ/δ HSTCL. The DNA was treated with restriction enzymes EcoRI, HindIII and BamHI, transferred to a nylon membrane and hybridized to a TCRδ gene probe TCRDJ1 (Dako Corp., Carpinteria, CA), which corresponds to the Jδ1 exon and its 3′ flanking region. A nonoverlapping 3.0-kb probe, which corresponds to the Jδ2 exon and its 5′ flanking region (pjk 3.0s, kindly provided by Dr. Carlo Croce, Philadelphia, PA) 34 was also used in one case that did not show a TCRδ rearrangement using the TCRDJ1 probe.
PCR
PCR of 50 μl total volume was performed in a Trio-Thermoblock (Biometra, Goettingen, Germany) with 0.1 μg of genomic DNA, 10 pmol of each primer, 5 nmol each dATP, dCTP, dGTP, dTTP (Perkin Elmer-Cetus, Norwalk, CT), 1.5 U Taq polymerase, and PCR Buffer (Perkin Elmer-Cetus) including 10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2, and 0,001% (w/v) gelatin. After 3′ denaturation at 94°C, 35 PCR cycles were performed, each cycle consisting of denaturation at 94°C, annealing at 60°C, and extension at 72° (for 1 minute each), followed by a final 7-minute extension at 72°C. Oligonucleotide primers used for PCR were previously described 26, 35, 36, 37 and are listed in Table 1 . Their specificity was confirmed by sequencing of their products 36, 37 (and data not shown) and nested PCR which yielded products of expected molecular weight (data not shown). To exclude DNA contamination, negative controls were included, and to avoid false negative results an internal positive control was run in each reaction. Amplification of recombinase activating gene (RAG1) served as such a positive control. PCR products were visualized by 2% agarose gel electrophoresis containing ethidium bromide. Under these experimental conditions, lymphoma-derived, clonal TCRδ gene rearrangements, which are present in the large proportion of cells in the samples, are seen as distinct, well-defined bands in the gel. Because the incidence of a specific Vδ rearrangement is low in normal reactive polyclonal T cells, TCRδ genes from reactive T cells present in the samples are amplified, but not visible as distinct bands.
Table 1.
List of Primers
| Primers | Source | |
|---|---|---|
| 5′ primers | ||
| Vδ1 | 5′-ACT CAA GCC CAG TCA TCA GT | Yokota et al 35 |
| Vδ1b | 5′-GCA AAG TAC TTT TGT GCT CTT G | Yokota et al 35 |
| Vδ2 | 5′-GAG TCA TGT CAG CCA TTG AG | Yokota et al 35 |
| Vδ2b | 5′-GCA CCA TCA GAG AGA GAT GA | Yokota et al 35 |
| Vδ3 | 5′-ACA GCA GAT CAG AAG GTG CA | Przybylski et al 36 |
| Vδ4 | 5′-CCA GTG ATC CAA GTT ATG GTC | Przybylski et al 36 |
| Vδ5 | 5′-CTG AAG GTC CTA CAT TCC TG | Przybylski et al 36 |
| Vδ6 | 5′-TAT CAT GGA TTC CCA GCC TG | Przybylski et al 36 |
| Dδ2 | 5′-AGA GGG TTT TTA TAC TGA TGT | Schmidt et al 37 |
| 3′ primers | ||
| Jδ1 | 5′-GAG TTA CTT ACT TGG TTC CAC | Yokota et al 35 |
| Dδ3 | 5′-AGG GAA ATG GCA CTT TTG CC | Yokota et al 35 |
| Reference primers | ||
| RAG1(5′) | 5′-GCC ATG AAG AGC AGT GAA TTA | Salhany et al 26 |
| RAG1(3′) | 5′-AGG AAT TAA CTC ACA AAC TGC | Salhany et al 26 |
| RAG2(5′) | 5′-TTG GCA TAT ACC AGG AGA CAA T | Salhany et al 26 |
| RAG2(3′) | 5′-ACT ATT TGC TTC TGC ACT GA | Salhany et al 26 |
Results
Multiparameter Analysis of the γ/δ TCL
All cases were evaluated independently by at least two hematopathologists. According to the REAL classification, 11 cases were classified as γ/δ HSTCL and 4 cases as γ/δ SPTCL. Typical histological findings from two representative cases are shown in Figure 1 . Detailed clinical features of all cases are summarized in Table 2 . Thirteen of the patients were male and only two were female; patient age ranged from 18 to 68 years (median, 42 years). Most patients presented with B symptoms, anemia, thrombocytopenia, and/or leukopenia. Three γ/δ SPTCL patients developed severe hemophagocytic syndrome. All patients followed an aggressive clinical course with short survival of 1 to 36 months (median, 10 months). Immunophenotyping data of the cases are presented in Table 3 . All lymphomas expressed T-cell-associated markers, mainly CD3, CD2, and/or CD7. Most were negative for CD4 and CD8. The γ/δ T cell phenotype was confirmed in all cases by positive staining with anti-TCRγ/δ antibody and/or negative staining with anti-TCRα/β antibody. Although case 4 showed staining with anti-TCRβ in a small subset of cells, these probably represented reactive T cells, because most cells were TCRγ/δ+. Lymphoma cells usually expressed at least one of NK-associated marker, chiefly CD56. Interestingly, whereas the majority of γ/δ HSTCL expressed also CD16 and, to a lesser degree, CD11c, γ/δ SPTCL expressed only CD56.
Figure 1.
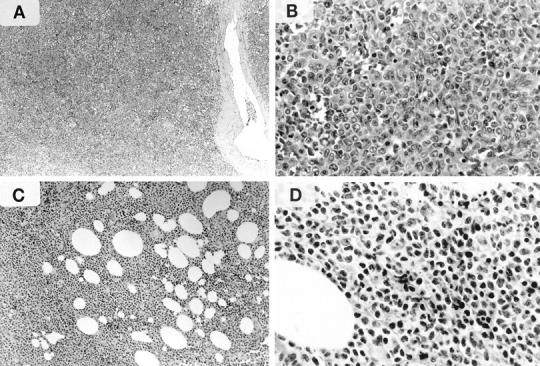
Histology of representative cases of γ/δ HSTCL (A and B, Patient 5 in Table 2 ) and γ/δ SPTCL (C and D, Patient 12). A: Diffuse involvement of splenic parenchyma. B: Relatively monomorphic population of medium-size lymphocytes with round to oval nuclei, small nucleoli, and a moderate amount of pale cytoplasm. C: Lobular panniculitis-like pattern. D: Predominance of pleomorphic, small lymphocytes with irregular and hyperchromatic nuclei and scant cytoplasm.
Table 2.
Clinical Features of Hepatosplenic and Subcutaneous γ/δ T Cell Lymphomas
| Case | Age/sex | Primary site | Clinical symptoms | Cytopenias | Therapy and response | Survival |
|---|---|---|---|---|---|---|
| Hepatosplenic γ/δ T-Cell Lymphoma | ||||||
| 1 | 68 M | Spleen, liver, BM | Bacterial infections, fever, weight loss, hepatosplenomegaly | Neutropenia: WBC 800; ANC 128, anemia: Hgb: 7 g/l, thrombocytopenia: plt 35,000, polyclonal gammophaty | Splenectomy, CHOP: CR 22 mo | 22 mo; died of sepsis due to aplastic anemia NED at autopsy |
| 2 | 32 M | Spleen, liver, BM, PB | Fever, night sweats, weight loss, hepatosplenomegaly | Leukopenia: WBC 700 anemia: Hgb 8.3 thrombocytopenia: plt 43,000 circulating blasts in blood | Prednisone+ acyclovir: increase in platelet number, progressive hepatosplenomegaly and leukemia CHOP:NR | 5 mo; DOD |
| 3 | 59 M | Spleen, liver | Weight loss, splenomegaly, abdominal pain | Neutropenia: ANC 1440 anemia: Hgb 6 thrombocytopenia: plt 44,000 | CHOP: NR, progressive disease Fludarabine: minimal response | 10 mo; DOD |
| 4 | 42 F | Spleen, liver, BM, PB | Fever | Anemia: Hgb 7.6 | Reduction in immunosuppression: NR; modified CHOP: PR 4 mo. | 6 mo; DOD |
| 5 | 46 M | Spleen, liver, BM | Fever, chills, sweats | Anemia: Hgb 9.0, thrombocytopenia: plt 45,000 | Reduction in immunosuppression: NR CHOP: PR, rapid relapse allo-BMT with response | 5 mo; died 21 days after BMT from complications of BMT |
| 6 | 18 M | Spleen, liver | Autoimmune hepatitis | BMT: rapid relapse | ||
| 7 | 33 M | Spleen, liver | Fever, jaundice hepatosplenomegaly | WBC: 6600, thrombocytopenia: plt 70,000 | Splenectomy | 1 mo; died during surgery |
| 8 | 30 M | Spleen, liver, lung, axillary LN | Fever, night sweats, weight loss, hepatosplenomegaly | WBC: 7200, anemia: Hgb 9.1, thrombocytopenia: plt 80,000 | CHOP: NR BMT: PR XRT | 30 mo; died of pulmonary hemorrhage during radiotherapy |
| 9 | 41 M | Spleen, liver, BM, PB | Weight loss, abdominal pain | Leukopenia: WBC 1,200, anemia: Hct: 30%, thrombocytopenia: plt 72,000 | Splenectomy, multiagent chemotherapy: CR 19 mo. Relapse, untreated | 22 mo; DOD |
| 10 | 32 M | Spleen, liver, BM, PB | Fever, night sweats, hepatosplenomegaly | Leukopenia: WBC 2,000, anemia: Hct: 32%, thrombocytopenia: plt 8,000 | Splenectomy, Mega IV chemotherapy | 2 mo; died, NED on autopsy |
| 11 | 65 M | Spleen | Prolonged gingival bleeding post dental procedure, splenomegaly | Leukopenia: WBC 1,600, anemia: Hct: 34%, thrombocytopenia: plt 66,000 | Splenectomy, CHOP | 10 mo; NED |
| Subcutaneous γ/δ T-Cell Lymphoma | ||||||
| 12 | 47 M | skin/subcutis arms, back, abdomen, face | Fever, night sweats HPS | Neutropenia; ANC 1054, anemia; Hct 37%, thrombocytopenia: plt 52,000 | CHOP: PR | 3 mo; DOD |
| 13 | 53 F | skin/subcutis thighs | HPS | Neutropenia; ANC 1660, anemia: Hct 34% | CHOP: CR–2 mo, local recurrence XRT: CR 3 mo 2nd recurrence Polychemotherapy: CR | 18 mo; DOD |
| 14 | 36 M | skin/subcutis extremities, trunk | HPS | Leukopenia: WBC 1800, Hct, Hgb, plt count: normal | CHOP: PR, 2-CDA: PR, XRT: PR, Multiagent chemotherapy: NA | 36 mo; DOD |
| 15 | 45 M | skin/subcutis extremities, torso | Fever, night sweats, weight loss, arthralgias | Normal (ANC) to low normal (Hb, plt) counts | CHOP, ESHAP, FLAG-CR, 9 mo relapse ABMT, 13 mo relapse | 15 mo; DWD |
BM, bone marrow; PB, peripheral blood; HPS, hemophagocytic syndrome; WBC, white blood cells; ANC, absolute neutrophil count; plt, platelet count; Hct, hematocrit; Hgb, hemoglobin; NR, no response; CR, complete remission; PR, partial remission; XRT, radiation therapy, BMT, bone marrow transplantation; NED, no evidence of disease; DOD, dead of disease; DWD, dead with disease; NA, information not available.
Table 3.
Immunophenotype of Hepatosplenic and Subcutaneous γ/δ T Cell Lymphomas
| Case | T-cell-associated | TCR-specific | NK-associated | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD2 | CD3 | CD4 | CD5 | CD7 | CD8 | CD43 | CD45R0 | TCRα/β | TCR γ/δ | CD11c | CD16 | CD56 | CD57 | |
| Hepatosplenic γ/δ T cell lymphoma | ||||||||||||||
| 1 | ++++ | ++++ | + | + | +++ | − | − | ++++ | ++++ | + | − | − | ||
| 2 | ++++ | ++++ | − | − | + | − | − | ++++ | + | ++++ | +++ | − | ||
| 3 | ++++ | ++++ | − | − | ++++ | − | − | ++++ | − | ++++ | ++++ | − | ||
| 4 | ++++ | ++++ | + | + | ++++ | ++ | + | +++ | +++ | +++ | − | |||
| 5 | ++++ | ++++ | − | − | ++++ | − | − | ++++ | + | +++ | +++ | − | ||
| 6 | ++++ | ++++ | − | + | +++ | + | − | +++ | ||||||
| 7 | ++++ | − | − | ++ | + | − | − | − | ||||||
| 8 | ++++ | − | − | ++++ | +++ | − | − | − | ||||||
| 9 | +++ | − | +++ | +++ | − | ++ | − | |||||||
| 10 | ++++ | ++++ | − | +++ | ++++ | ++++ | +++ | − | − | ++++ | +++ | − | ||
| 11 | ++++ | ++++ | − | − | ++++ | − | − | ++++ | ++++ | ++++ | ||||
| Subcutaneous γ/δ T cell lymphoma | ||||||||||||||
| 12 | +++ | ++++ | − | − | ++ | − | +++ | +++ | − | ++++ | − | − | ++++ | − |
| 13 | ++++ | ++++ | −/+ | +++ | ++++ | + | ++++ | +++ | − | +++ | − | − | +++ | − |
| 14 | ++++ | ++++ | + | − | +++ | − | +++ | +++ | ||||||
| 15 | ++++ | ++++ | − | − | − | − | − | ++++ | − | ++++ | − | |||
TCR, T-cell receptor; NK, natural killer; −, <10% positive; +, 10–25% positive; ++, 26–50% positive; +++, 50–75% positive; ++++, >75% positive.
We were also able to perform cytogenetic analysis in three cases of γ/δ HSTCL (cases 2, 4, and 5). By FISH all three cases have shown cytogenetic abnormalities characteristic for HSTCL: isochromosome 7q and trisomy 8 19, 20, 38, 39 (Figure 2) . Standard metaphase cytogenetic analysis demonstrated an abnormal karyotype in case 2 including the presence of isochromosome 7q and trisomy 8 (46, X, −Y, i(7)(q10), +8, del(10)(q?22) and failed to show any abnormalities in the remaining two cases.
Figure 2.
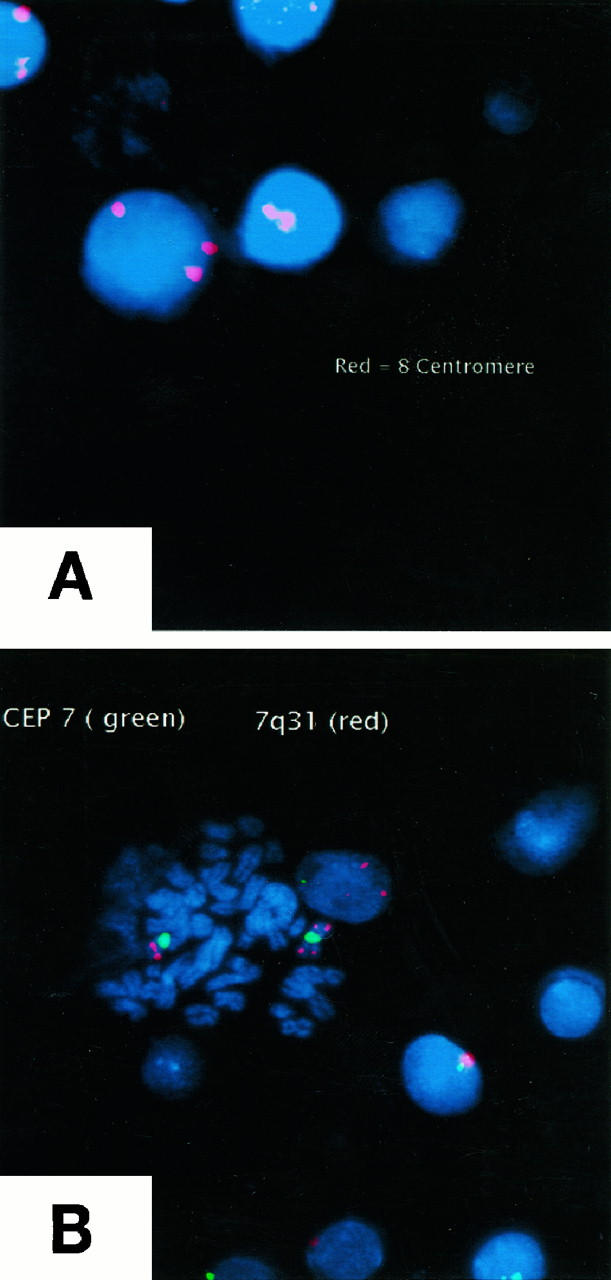
FISH analysis of γ/δ HSTCL (Patient 2). A: CEP 8 probe to enumerate chromosome 8 centromeres showed the presence of trisomy 8 in 20% of the analyzed cells. B: Combination of CEP 7 probe (green staining) and LSI D7S486 probe (red staining) to detect simultaneously chromosome 7 centromere and band 7q31 on the chromosome’s long arm showed the presence of isochrome 7 in 28% of the analyzed cells. A positive cell in metaphase is seen in the center of this photograph. Similar results showing the presence of both trisomy 8 and isochromosome 7 were obtained in patients 4 and 5 (not shown).
TCRδ Gene Rearrangements in γ/δ T Cell Lymphomas
Southern blot analysis using the TCRDJ1 probe and the pjk 3.0s probe revealed clonal TCRδ gene rearrangements in all 5 γ/δ HSTCL cases investigated (cases 1–5; see Table 4 ). In three cases two rearranged bands were visible, indicating rearrangement of both TCRδ alleles. In case 4 the presence of a rearranged and a germline band in Southern blot probably represented a monoallelic rearrangement or, less likely, contribution of the germline sequence by the non-lymphoma cells combined with deletion of one rearranged allele in the lymphoma cells. Finally, in case 2, a single rearranged band was observed but the germline band was absent, suggesting deletion of the second allele. Such deletion occurs on rearrangement of the surrounding TCRα gene; however, immunophenotyping indicated that the TCRα gene was not expressing functional protein in this case.
Table 4.
Immunophenotypic and Molecular Analysis of the TCR Vδ Gene Subset Expression in Hepatosplenic and Subcutaneous γ/δ T Cell Lymphoma
| Patient | Vδ gene rearrangement (Southern blot) | Vδ subtype protein (flow cytometry) | Vδ subtype DNA (PCR) | Vδ subset classification |
|---|---|---|---|---|
| Hepatosplenic γ/δ T Cell Lymphoma | ||||
| 1 | R /R | neg. Vδ(1–3) | neg. Vδ(1–6)Jδ (1,2) | undetermined |
| 2 | R /D | Vδ1+ | Vδ1Jδ1 | Vδ1 |
| 3 | R /R | Vδ1+ | Vδ1Jδ1/Dδ2Jδ1 | Vδ1 |
| 4 | R /G | Vδ1+ | Vδ1Jδ1 | Vδ1 |
| 5 | R /R | Vδ1+ | Vδ1Jδ1 | Vδ1 |
| 6 | Vδ1Jδ1 | Vδ1 | ||
| 7 | Vδ1Jδδ1 | Vδ1 | ||
| 8 | Vδ1Jδ1/Dδ2Jδ1 | Vδ1 | ||
| 9 | Vδ1Jδ1/Dδ2Jδ1 | Vδ1 | ||
| 10 | Vδ1Jδ1 | Vδ1 | ||
| 11 | Vδ1Jδ1/Dδ2Jδ1 | Vδ1 | ||
| Subcutaneous−/− T Cell Lymphoma | ||||
| 12 | Vδ2+ | Vδ2Jδ1 | Vδ2 | |
| 13 | Vδ2+ | Vδ2Jδ1/Vδ3Jδ1 | Vδ2 | |
| 14 | Vδ2Jδ1/Dδ2Jδ1 | Vδ2 | ||
| 15 | Vδ2Jδ1 | Vδ2 | ||
PCR, polymerase chain reaction; Sb, Southern blot; Vδ, variable region; Jδ, joining region; Dδ, diversity region; R, rearranged; D, deletion; G, germline; ND, not done.
Usage of Vδ Segments in Hepatosplenic and Subcutaneous γ/δ T Cell Lymphomas
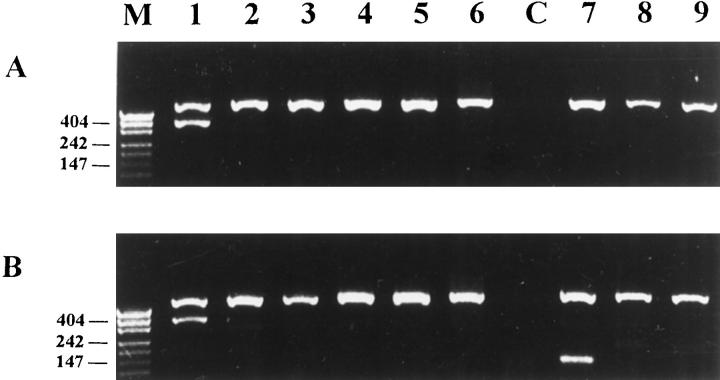
To determine the usage of TCRδ gene segments by γ/δ T cell lymphomas, PCR was performed with Vδ1-Vδ6 specific 5′ primers and a Jδ1 3′ primer. We also looked for incomplete TCRδ gene rearrangements: Dδ2Jδ1, frequently occurring in T-ALL, 37, 40 and Vδ2Dδ3 and Dδ2Dδ3 rearrangements, which were frequently found in B-precursor ALL. 41 . To each reaction control primers which amplify a 600-bp fragment of recombinase activating gene-1 (RAG1) were added to exclude false negative results. In this experimental setup, both TCRδ and RAG1 amplification products should be detected in the samples which contain a dominant T cell clone. In the reactive, polyclonal conditions detection of RAG1 but not TCRδ amplification product is expected. Because in cases 7–11 no fresh tissue was available, DNA was extracted from archival formalin-fixed, paraffin-embedded samples. Because tissue fixation frequently leads to partial DNA degradation, PCR was performed with internal Vδ1b and Vδ2b primers which amplify a shorter fragment (∼140 bp) and RAG2 control primers, which amplify a 220-bp DNA fragment. We were also able to perform analysis of the Vδ usage on the protein level by flow cytometry using anti-Vδ1, -Vδ2, and -Vδ3 antibodies in 7 cases. Representative results for γ/δ HSTCL PCR using fresh-frozen tissue are shown in Figure 3and results using formalin-fixed tissue in Figure 4 . Representative results for γ/δ SPTCL PCR are shown in Figure 5 . All PCR and flow cytometry data are summarized in Table 4 . Strikingly, 10 of 11 γ/δ HSTCL showed a Vδ1Jδ1 rearrangement and in 4 of them an additional, incomplete Dδ2Jδ1 rearrangement of the second allele was detected. In one γ/δ HSTCL (patient 1; see Table 4 ) no amplification product was obtained. This case expressed the TCRγ/δ (Table 3) ; however, it was negative for Vδ1–3 expression (Table 4) , as determined by immunophenotyping. Furthermore, on Southern blot, it showed a rearrangement pattern different from other γ/δ HSTCLs analyzed in this study. These findings suggest that a variable TCRδ gene segment other than Vδ1-Vδ6 might have been used in this case. In contrast to γ/δ HSTCL, all 4 γ/δ SPTCL showed rearrangement of the Vδ2Jδ1 gene segment (Figure 5 and Table 4 ). Case 14 showed also the Dδ2Jδ1 rearrangement and case 13 the Vδ3Jδ1 rearrangement of the second allele. Because flow cytometry analysis in case 13 showed a Vδ2 expression, the Vδ3Jδ1 rearrangement was recognized to be nonfunctional.
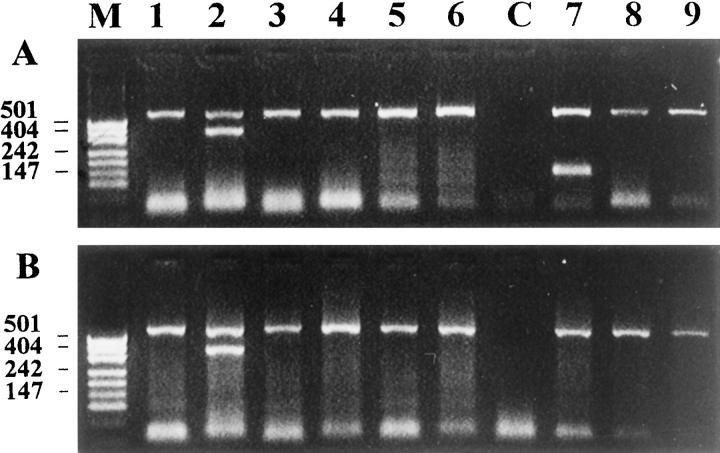
Figure 4.
PCR analysis of Vδ gene usage in archival paraffin-embedded samples of hepatosplenic γ/δ T cell lymphoma. Lane M, molecular weight marker; lanes 1–3, Patient 7; lanes 4–6, Patient 8; lane C, negative control. PCR was performed with the Jδ1 primer and the Vδ1b primer (lanes 1 and 4), the Vδ2b primer (lanes 2 and 5), and the Dδ2 primer- (lanes 3 and 6). The upper band of ∼220 bp represents RAG2 reference gene, bands of ∼120 bp in lanes 1 and 4 represent a Vδ1Jδ1 rearrangement, and the band of ∼140 bp in lane 6 represents an incomplete Dδ2Jδ1 rearrangement.
Figure 5.
PCR analysis of Vδ gene usage in subcutaneous panniculitis-like γ/δ T cell lymphoma. A: Patient 14. B: Patient 15. Lane M, molecular weight marker; lanes 1–6, PCR products obtained with Vδ1-Vδ6 specific primers, respectively, and the Jδ1 primer; lane C, negative control; lane 7, Dδ2 and Jδ1 primers, lane 8, Vδ2 and Dδ3 primers; lane 9, Dδ2 and Dδ3 primers. The upper band of ∼600 bp represents RAG1 reference gene, bands of ∼400 bp in lanes A2 and B2 represent a Vδ2Jδ1 rearrangement, and the band of ∼140 bp in lane A7 represents an incomplete Dδ2Jδ1 rearrangement.
Discussion
T cell lymphomas expressing γ/δ TCR represent recently recognized, rare subsets of non-Hodgkin’s lymphoma. 11 The literature on γ/δ T cell lymphomas remains sparse. 10, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Because only a few cases were analyzed in each of the published studies, any preferences in Vδ gene usage could not have been adequately addressed. Our present report, which describes 11 cases of γ/δ HSTCL and 4 cases of γ/δ SPTCL, is the largest study to date on γ/δ TCL. It is the first study focused on immunophenotypical and molecular subtyping of these rare but clinically distinct subtypes of peripheral TCL. There was an excellent concordance among results obtained by molecular and immunophenotypic approaches (Table 4) . Our data demonstrate that the two types of γ/δ TCL express different subset of γ/δ TCR. We found that 10 out of 11 γ/δ HSTCL expressed the Vδ1 gene, whereas all 4 γ/δ SPTCL used the Vδ2 gene (P = 0.001, Fisher’s exact test). This result indicates that selection of the specific Vδ subtype is lymphoma type-dependent. The results of two previous, rather limited studies on Vδ gene usage which evaluated a total of 6 γ/δ HSTCL 10, 15, 18 and 2 γ/δ SPTCL, 31 support our conclusion. In these studies, 4/6 γ/δ HSTCL expressed the Vδ1 gene, and in the remaining 2 γ/δ HSTCL cases, an unidentified V gene was used. In contrast, both γ/δ SPTCL expressed the Vδ2 gene. It is well established that normal γ/δ T lymphocytes which reside in spleen express predominantly the Vδ1 gene, whereas most γ/δ T lymphocytes present in subcutis express the Vδ2 gene. 42 The identified unique Vδ gene usage patterns in γ/δ HSTCL and γ/δ SPTCL reflects local predominance of either Vδ1+ or Vδ2+ subset within their normal T cell counterparts. This strongly suggests that both γ/δ HSTCL and γ/δ SPTCL are derived from the local lymphoid tissue. Vδ subtype analysis; rather limited studies on other γ/δ T cell lymphomas also suggested preferences in Vδ usage. Two out of three precursor T cell lymphomas involving lymph nodes expressed the Vδ1 (the third expressed an unidentified Vδ gene), 43 3/3 nasal γ/δ TCL expressed the Vδ2 gene, 31 2/3 gastro-intestinal γ/δ TCL expressed the Vδ3, and 1 expressed the Vδ2 gene. 31 In a large study on γ/δ T cell acute lymphoblastic leukemia, the vast majority (26/30) of cases, similarly to γ/δ HSTCL, expressed the Vδ1 gene, 2 cases used the Vδ2 gene, one case used the Vδ3 gene and 2 cases used the Vα gene rearranged to the Jδ1 segment. 44 The Vδ gene expression pattern in TCR γ/δ+ T-ALL resembled that of TCR γ/δ+ thymocytes and differed markedly from that of peripheral blood γ/δ+ T cells. These data support the conclusion that T-ALL is a malignant counterpart of thymocytes rather than peripheral blood γ/δ+ T cells.
A detailed immunophenotypic analysis, performed in γ/δ HSTCL and γ/δ SPTCL showed a similar pattern of T-cell associated antigens (CD3+, CD2+ CD7+, CD5−, CD4−, CD8−). All lymphomas expressed also NK-associated antigens, but some differences were observed. Five out of six γ/δ HSTCL tested expressed both CD16 and CD56, whereas all 3 γ/δ SPTCL tested expressed only CD56 and, finally, 1 γ/δ HSTCL, which used an unidentified Vδ gene, expressed neither CD16 nor CD56 but did express CD11c antigen. Furthermore, in contrast to γ/δ SPTCL, α/β SPTCL do not express CD56. 26 Taken together, the above data suggest a relationship between the type of TCR and a pattern of expression of NK cell-associated markers among various types of hepatosplenic and subcutaneous TCL. However, the number of the TCL cases analyzed by us and others is still too small to draw any definitive conclusions in this regard.
It is often difficult to diagnose TCL on histological grounds alone, especially the cases involving skin. Molecular analysis has proven to be very useful in this respect. Detection of a clonally rearranged TCRγ gene often allows to distinguish T cell lymphoma from benign, reactive T cell proliferation or B cell lymphoma highly enriched in reactive T lymphocytes. 45, 46 Our study indicates that analysis of Vδ gene usage may be helpful in diagnosis and proper classification of γ/δ TCL. The observed dichotomy in the Vδ gene usage between γ/δ HSTCL and γ/δ SPTCL indicates that analysis of expression of the Vδ gene subtype by either molecular or immunological method may permit better discrimination among different types of γ/δ TCL, particularly in the clinically advanced, generalized cases with multi-organ involvement. Furthermore, because Vδ gene rearrangements show an extensive diversity of the joining site, lymphoma-specific probes could be developed to monitor minimal residual disease in γ/δ TCL. 18
In summary, our results indicate that hepatosplenic and subcutaneous panniculitis-like γ/δ T cell lymphomas are derived from different Vδ subsets of γ/δ T lymphocytes. Whereas γ/δ HSTCL belong usually to the Vδ1 subset, γ/δ SPTCL represent the Vδ2 subset. The exact properties of either normal or malignant Vδ1+ and Vδ2+ γ/δ T cells leading to this different, tissue-specific expression of the Vδ subsets have not been determined. Whether the restricted, highly lymphoma type-specific Vδ gene expression in γ/δ HSTCL and γ/δ SPTCL plays a role in the pathogenesis of these lymphomas also remains to be determined.
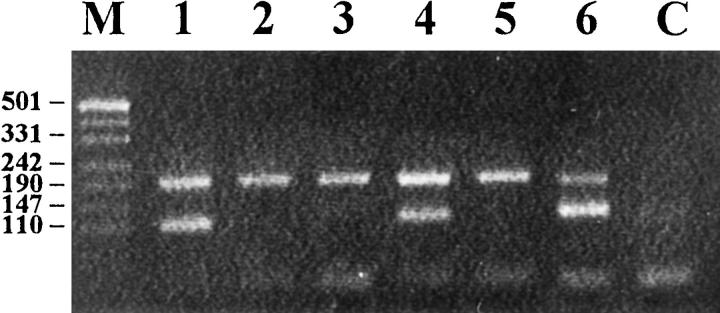
Figure 3.
PCR analysis of Vδ gene usage in hepatosplenic γ/δ T cell lymphoma. A: Patient 2. B: Patient 3. Lane M, molecular weight marker; lanes 1–6, PCR products obtained with Vδ1-Vδ6 specific primers, respectively, and Jδ1 primer; lane C, negative control; lane 7, Dδ2Jδ1, lane 8, Vδ2Dδ3; lane 9, Dδ2Dδ3. The upper band of ∼600 bp represents RAG1 reference gene, bands of ∼400 bp in lanes A1 and B1 represent a Vδ1Jδ1 rearrangement, band of ∼140 bp in lane B7 represents an incomplete Dδ2Jδ1 rearrangement.
Address reprint requests to Mariusz A. Wasik, M.D., 7.106 Founders Building, Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, 3400 Spruce Street, Philadelphia, PA 19104. E-mail: wasik@mail.med.upenn.edu.
Footnotes
Supported in part by grants from the Committee for Scientific Research (KBN PO5A 01516 to G. K. P.) and the National Cancer Institute (CA76627 to M. A. W.).
W. Macon’s current address: Mayo Clinic, Rochester, MN.
R. Felgar’s current address: University of Rochester, Rochester, NY.
J. DiGiuseppe’s current address: Hartford Hospital, Hartford, CT.
K. Salhany is deceased.
References
- 1.Gellert M: Recent advances in understanding V(D)J recombination. Adv Immunol 1997, 64:39-64 [DOI] [PubMed] [Google Scholar]
- 2.Falini B, Flenghi L, Pileri S, Pelicci P, Fagioli M, Martelli MF, Moretta L, Ciccone E: Distribution of T-cells bearing different forms of the T cell receptor g/d in normal and pathological human tissues. J Immunol 1989, 143:2480-2488 [PubMed] [Google Scholar]
- 3.Correa I, Bix M, Liao NS, Zijlstra M, Jaenisch R, Raulet D: Most γδ T cells develop normally in β2-microglobulin-deficient mice. Proc Natl Acad Sci USA 1992, 89:653-657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bigby M, Markowitz JS, Bleicher PA, Grusby MJ, Simha S, Siebrecht M, Wagner M, Nagler Anderson C, Glimcher LH: Most γδ T cells develop normally in the absence of MHC class II molecules. J Immunol 1993, 151:4465-4475 [PubMed] [Google Scholar]
- 5.Palacios R, Samaridis J: Rearrangement patterns of T-cell receptor genes in the spleen of athymic (nu/nu) young mice. Immunogenetics 1991, 33:90-95 [DOI] [PubMed] [Google Scholar]
- 6.Ota Y, Kobata T, Seki M, Yagita H, Shimada S, Huang YY, Takagaki Y, Okumura K: Extrathymic origin of Vg1/Vd6 T cells in the skin. Eur J Immunol 1992, 22:595-598 [DOI] [PubMed] [Google Scholar]
- 7.McVay LD, Carding SR: Extrathymic origin of human gd T cells during fetal development. J Immunol 1996, 157:2873-2882 [PubMed] [Google Scholar]
- 8.Bandeira A, Itohara S, Bonneville M, Burlen Defranoux O, Mota Santos T, Coutinho A, Tonegawa S: Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gd. Proc Natl Acad Sci USA 1991, 88:43-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas W, Pereira P, Tonegawa S: Gamma/delta cells. Annu Rev Immunol 1993, 11:637-685 [DOI] [PubMed] [Google Scholar]
- 10.Gaulard P, Bourquelot P, Kanavaros P, Haioun C, Le Couediac JP, Divine M, Goossens M, Zafrani ES, Farcet JP, Reyes F: Expression of the a/b and g/d T-cell receptors in 57 cases of peripheral T-cell lymphomas: identification of a subset of gd T-cell lymphomas. Am J Pathol 1990, 137:617-628 [PMC free article] [PubMed] [Google Scholar]
- 11.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American Classification of Lymphoid Neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 12.Kadin ME, Kamoun M, Lamberg J: Erythrophagocytic T γ lymphoma: a clinicopathologic entity resembling malignant histiocytosis. N Engl J Med 1981, 304:648-653 [DOI] [PubMed] [Google Scholar]
- 13.Gaulard P, Kanavaros P, Farcet JP, Rocha FD, Haioun C, Divine M, Reyes F, Zafrani ES: Bone marrow histologic and immunohistochemical findings in peripheral. T-cell lymphoma: a study of 38 cases. Hum Pathol 1991, 22:331-338 [DOI] [PubMed] [Google Scholar]
- 14.Wong KF, Chan JK, Matutes E, McCarthy K, Ng CS, Chan CH, Ma SK: Hepatosplenic γδ T-cell lymphoma: a distinctive aggressive lymphoma type. Am J Surg Pathol 1995, 19:718-726 [DOI] [PubMed] [Google Scholar]
- 15.Ohno T, Komada F, Yamaguchi M, Oka K, Nishii K, Tsuda M, Katsuta K, Yamaguchi T, Kita K, Shirakawa S: Gamma/delta T-cell lymphoma with hepatosplenomegaly: report of a case. Int J Hematol 1993, 57:269-276 [PubMed] [Google Scholar]
- 16.Alonsozana EL, Stamberg J, Kumar D, Jaffe ES, Medeiros LJ, Frantz C, Schiffer CA, O’Connell BA, Kerman S, Stass SA, Abruzzo LV: Isochromosome 7q: the primary cytogenetic abnormality in hepatosplenic gamma delta T cell lymphoma (letter). Leukemia 1997, 11:1367-1372 [DOI] [PubMed] [Google Scholar]
- 17.Wang CC, Tien HF, Lin MT, Su IJ, Wang CH, Chuang SM, Shen MC, Liu CH: Consistent presence of isochromosome 7q in hepatosplenic T γ/δ lymphoma: a new cytogenetic-clinicopathologic entity. Genes Chromosomes Cancer 1995, 12:161-164 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Sanchez F, Menarguez J, Cristobal E, Cantalejo A, Gil J, Algara P, Vicario JL: Hepatosplenic γ-δ T-cell malignant lymphoma: report of the first case in childhood, including molecular minimal residual disease follow-up. Br J Haematol 1995, 90:943-946 [DOI] [PubMed] [Google Scholar]
- 19.Cooke CB, Krenacs L, Stetler Stevenson M, Greiner TC, Raffeld M, Kingma DW, Abruzzo L, Frantz C, Kaviani M, Jaffe ES: Hepatosplenic T-cell lymphoma: a distinct clinicopathologic entity of cytotoxic γδ T-cell origin. Blood 1996, 88:4265-4274 [PubMed] [Google Scholar]
- 20.Salhany KE, Feldman M, Kahn MJ, Peritt D, Schretzenmair RD, Wilson DM, DiPaola RS, Glick AD, Kant JA, Nowell PC, Kamoun M: Hepatosplenic gammadelta T-cell lymphoma: ultrastructural, immunophenotypic, and functional evidence for cytotoxic T lymphocyte differentiation. Hum Pathol 1997, 28:674-685 [DOI] [PubMed] [Google Scholar]
- 21.Farcet JP, Gaulard P, Marolleau JP, Le Couedic JP, Henni T, Gourdin MF, Divine M, Haioun C, Zafrani S, Goossens M: Hepatosplenic T-cell lymphoma: sinusal/sinusoidal localization of malignant cells expressing the T-cell receptor γδ. Blood 1990, 75:2213-2219 [PubMed] [Google Scholar]
- 22.Sallah S, Smith SV, Lony LC, Woodard P, Schmitz JL, Folds JD: Gamma/delta T-cell hepatosplenic lymphoma: review of the literature, diagnosis by flow cytometry and concomitant autoimmune hemolytic anemia. Ann Hematol 1997, 74:139-142 [DOI] [PubMed] [Google Scholar]
- 23.Nosari A, Oreste PL, Biondi A, Costantini MC, Santoleri L, Intropido L, Muti G, Pungolino E, Gargantini L, Morra E: Hepato-splenic gd T-cell lymphoma: a rare entity mimicking the hemophagocytic syndrome. Am J Hematol 1999, 60:61-65 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez CL, Medeiros LJ, Braziel RM, Jaffe ES: T-cell lymphoma involving subcutaneous tissue: a clinicopathologic entity commonly associated with hemophagocytic syndrome. Am J Surg Pathol 1991, 15:17-27 [DOI] [PubMed] [Google Scholar]
- 25.Mehregan DA, Su WP, Kurtin PJ: Subcutaneous T-cell lymphoma: a clinical, histopathologic, and immunohistochemical study of six cases. J Cutan Pathol 1994, 21:110-117 [DOI] [PubMed] [Google Scholar]
- 26.Salhany KE, Macon WR, Choi JK, Elenitsas R, Lessin SR, Felgar RE, Wilson DM, Przybylski GK, Lister J, Wasik MA, Swerdlow SH: Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of a/b and g/d subtypes. Am J Surg Pathol 1998, 22:881-893 [DOI] [PubMed] [Google Scholar]
- 27.Burg G, Dummer R, Wilhelm M, Nestle F, Ott MM, Feller A, Hefner H, Lanz U, Schwinn A, Wiede J: A subcutaneous delta-positive T-cell lymphoma that produces interferon γ. N Engl J Med 1991, 325:1078-1081 [DOI] [PubMed] [Google Scholar]
- 28.Fujita M, Miyachi Y, Furukawa F, Toichi E, Furukawa I, Nakajima N, Imamura S: A case of cutaneous T-cell lymphoma expressing γδ T-cell receptors. J Am Acad Dermatol 1993, 28:355-360 [DOI] [PubMed] [Google Scholar]
- 29.Avinoach I, Halevy S, Argov S, Sacks M: Gamma/delta T-cell lymphoma involving the subcutaneous tissue, and associated with a hemophagocytic syndrome. Am J Dermatopathol 1994, 16:426-433 [DOI] [PubMed] [Google Scholar]
- 30.Munn SE, McGregor JM, Jones A, Amlot P, Rustin MH, Russell Jones R, Whittaker S: Clinical and pathological heterogeneity in cutaneous γ-δ T-cell lymphoma: a report of three cases and a review of the literature. Br J Dermatol 1996, 135:976-981 [DOI] [PubMed] [Google Scholar]
- 31.Arnulf B, Copie Bergman C, Delfau Larue MH, Lavergne Slove A, Bosq J, Wechsler J, Wassef M, Matuchansky C, Epardeau B, Stern M, Bagot M, Reyes F, Gaulard P: Nonhepatosplenic gd T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood 1998, 91:1723-1731 [PubMed] [Google Scholar]
- 32.Takihara Y, Tkachuk D, Michalopoulos E, Champagne E, Reimann J, Minden M, Mak TW: Sequence and organization of the diversity, joining, and constant region genes of the human T-cell delta-chain locus. Proc Natl Acad Sci USA 1988, 85:6097-6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takihara Y, Reimann J, Michalopoulos E, Ciccone E, Moretta L, Mak TW: Diversity and structure of human T cell receptor delta chain genes in peripheral blood γ/δ-bearing T lymphocytes. J Exp Med 1989, 169:393-405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards RH, Wasik MA, Finan J, Rodriguez R, Moore J, Kamoun M, Rennert H, Bird J, Nowell PC, Salhany KE: Evidence for hematopoietic progenitor cell involvement in acute promyelocytic leukemia. Am J Clin Pathol 1999, 112:819-827 [DOI] [PubMed] [Google Scholar]
- 35.Yokota S, Hansen-Hagge TE, Ludwig WD, Reiter A, Raghavachar A, Kleihauer E, Bartram CR: Use of polymerase chain reaction to monitor minimal residual disease in acute lymphoblastic leukemia patients. Blood 1991, 77:331-339 [PubMed] [Google Scholar]
- 36.Przybylski G, Oettle H, Ludwig WD, Siegert W, Schmidt CA: Molecular characterization of illegitimate tcrδ gene rearrangements in acute myeloid leukemia. Br J Haematol 1994, 87:301-307 [DOI] [PubMed] [Google Scholar]
- 37.Schmidt CA, Przybylski G, Tietze A, Oettle H, Siegert W, Ludwig WD: Acute myeloid and T-cell acute lymphoblastic leukaemia with aberrant antigen expression exhibit similar TCR? gene rearrangements. Br J Haematol 1996, 92:929-936 [DOI] [PubMed] [Google Scholar]
- 38.Jonveaux P, Daniel MT, Martel V, Maarek O, Berger R: Isochromosome 7q, and trisomy 8 are consistent primary, non-random chromosomal abnormalities associated with hepatosplenic T γ/delta lymphoma. Leukemia 1996, 10:1453-1455 [PubMed] [Google Scholar]
- 39.Coventry S, Punnett HH, Tomczak EZ, Casher D, Koehler M, Borowitz MJ, Griffin CA, de Chadar vian JP: Consistency of isochromosome 7q and trisomy 8 in hepatosplenic gammadelta T-cell lymphoma: detection by fluorescence In situ hybridization of a splenic touch-preparation from a pediatric patient. Pediatr Dev Pathol 1999, 2:478-483 [DOI] [PubMed] [Google Scholar]
- 40.Breit TM, Wolvers Tettero IL, Beishuizen A, Verhoeven MA, van Wering ER, van Dongen JJ: Southern blot patterns, frequencies, and junctional diversity of T-cell receptor-δ gene rearrangements in acute lymphoblastic leukemia. Blood 1993, 82:3063-3074 [PubMed] [Google Scholar]
- 41.Biondi A, Francia di Celle P, Ross Vi,, Casorati G, Matullo G, Giudici G, Foa R, Migone N: High prevalence of T-cell receptor Vδ2-(D)-Dδ3 or Dδ1/2-Dδ3 rearrangements in B-precursor acute lymphoblastic leukemias. Blood 1990, 75:1834-1840 [PubMed] [Google Scholar]
- 42.Alaibac M, Daga A, Harms G, Morris J, Yu RC, Zwingerberger K, Chu AC: Molecular analysis of the γ delta T-cell receptor repertoire in normal human skin and in Oriental cutaneous leishmaniasis. Exp Dermatol 1993, 2:106-112 [DOI] [PubMed] [Google Scholar]
- 43.de Villartay JP, Pullman AB, Andrade R, Tschachler E, Colamenici O, Neckers L, Cohen DI, Cossman J: γ/δ lineage relationship within a consecutive series of human precursor T-cell neoplasms. Blood 1989, 74:2508-2518 [PubMed] [Google Scholar]
- 44.Langerak AW, Wolvers-Tettero IL, van den Beemd MW, van Wering ER, Ludwig WD, Hahlen K, Necker A, van Dongen JJ: Immunophenotypic and immunogenotypic characteristics of TCRγ/δ+ T cell acute lymphoblastic leukemia. Leukemia 1999, 13:206-214 [DOI] [PubMed] [Google Scholar]
- 45.Curco N, Servitje O, Llucia M, Bertran J, Limon A, Carmona M, Romagosa V, Peyri J: Genotypic analysis of cutaneous T-cell lymphoma: a comparative study of Southern blot analysis with polymerase chain reaction amplification of the T-cell receptor-γ gene. Br J Dermatol 1997, 137:673-679 [PubMed] [Google Scholar]
- 46.Bakels V, van Oostveen JW, van der Putte SC, Meijer CJ, Willemze R: Immunophenotyping and gene rearrangement analysis provide additional criteria to differentiate between cutaneous T-cell lymphomas and pseudo-T-cell lymphomas. Am J Pathol 1997, 150:1941-1949 [PMC free article] [PubMed] [Google Scholar]