Abstract
We report two cases of lipoblastoma with chromosome 8-related aberrations, ie, a 92,XXYY,t(7;8)(p22;q11.2)x2 [8]/46,XY[16] in Case 1 and a 46,XY,−8,−13,add(16)(q22),+mar, +r [cp13]/46,XY[7] in Case 2. Using spectral karyotyping and fluorescence in situ hybridization techniques, the karyotype of Case 2 was redesignated as 46,XY, r(8), del(13)(q12), der(16)ins(16;8)(q22;q24q11.2)[cp13]/46,XY[7]. This report delineates a new chromosome rearrangement, ie, der(16)ins(16;8)(q22;q24q11.2) in lipoblastoma, and also confirms the t(7;8)(p22;q11.2), reported only once previously, as a recurrent translocation involved in such a tumor. These findings provide valuable information for clinical molecular cytogenetic diagnosis of lipoblastoma. Furthermore, this report highlights the value of cytogenetic and molecular cytogenetic analysis in differential diagnosis of childhood adipose tissue tumors and adds to the number of lipoblastomas reported with chromosomal abnormalities at 8q11.2.
Lipoblastoma is a benign tumor of early childhood, usually occurring before age 3, and results from proliferation of primitive adipocytes. 1, 2 Although clinical and pathological features in some cases may suffice to distinguish lipoblastoma from myxoid or well-differentiated liposarcoma, this crucial differential diagnosis may be more difficult in cases of older children or those with atypical histology. Importantly, lipoblastoma is a benign lesion that may recur after local excision. 1, 2 In addition, the histopathological diagnosis of lipoblastoma can at times be difficult because some cases may mimic atypical lipoma. Recently, distinct cytogenetic abnormalities have been demonstrated for these childhood adipose tissue tumors, including lipoblastoma, atypical lipoma, and liposarcoma. Nonrandom rearrangements involving 8q11-q24 have been reported in 14 cases of lipoblastoma, with only 10 cases being clearly delineated with the breakpoints and rearrangements involved. 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 We present here two additional cases with chromosome 8-related anomalies and illustrate the utility of cytogenetics and molecular cytogenetic analyses in the differential diagnosis of these tumors.
Case Reports
Case 1
A Caucasian boy underwent marginal resection of a lobulated 6.5 × 3.5 × 3.5 cm fatty left shoulder mass at age 15 months. The cut surface showed a well-demarcated lesion with gray-pink, translucent, and mucoid appearing areas and adjacent yellow, lobulated tissue. Microscopically, the tumor was composed of variably sized lobules of fatty tissue separated by dense fibrous connective tissue septa. Varying stages of maturation were seen in the lobules, with many composed chiefly of mature adipocytes. Other lobules demonstrated increased cellularity and myxoid changes, with multivacuolated and univacuolar signet ring-like cells consistent with lipoblasts. Some of the myxoid areas displayed floret-like giant cells with multiple peripheral nuclei and abundant eosinophilic cytoplasm. An initial diagnosis of well-differentiated low-grade liposarcoma was rendered, but amended to intramuscular lipoblastoma with focal nuclear atypia after consultation. At age 5 1/3 years, the patient had a recurrent mass that did not limit his physical activity and was not associated with any constitutional symptoms. Magnetic resonance imaging revealed a mass approximately 3 cm in diameter completely within the deltoid muscle adjacent to the axillary nerve. The resection specimen consisted of a 9.8 g, 5.0 × 2.5 × 0.2 cm lobulated red-brown-tan soft tissue mass. The cut surface revealed a partial rim of skeletal muscle and a predominance of tan, fleshy soft tissue. Microscopic examination revealed a lipoblastoma similar to the initial specimen (Figure 1) . Significant cellular atypia and atypical mitoses were not prominent. The patient is currently doing well following the second procedure with a 2-year follow-up.
Figure 1.
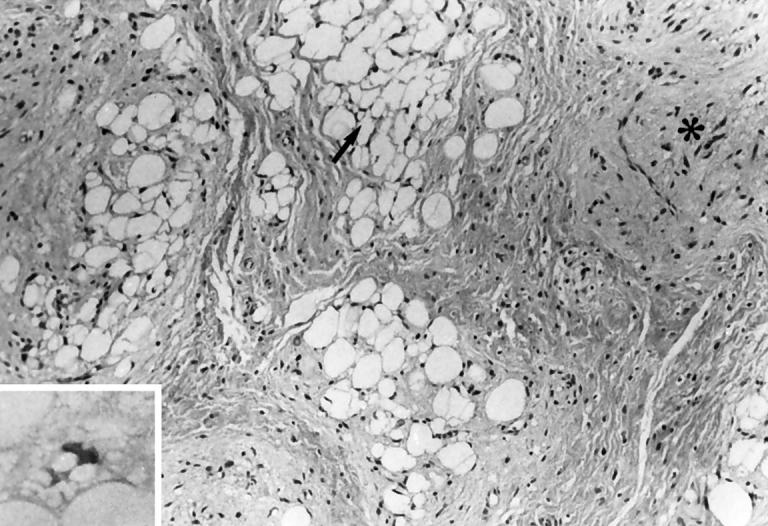
Multivacuolated lipoblasts (arrow, inset) persisted in the recurrent tumor from Case 1.
Case 2
A 10-month-old Caucasian boy presented with a 2 × 3 cm soft tissue mass of the left medial thigh. The mass had increased in size over a 3-month period. Magnetic resonance imaging demonstrated a mass apparently arising from the fascia and primarily involving the subcutaneous space. There was no obvious tumor within the muscle, and the lesion was located directly over the neurovascular bundle at the junction of the mid and proximal thirds of the thigh. The resection specimen was a 12.2 g, 3.7 × 3.2 × 2.1 cm soft tissue mass partially covered by skeletal muscle on one side and fibroadipose tissue on the other. The cut surface was composed of a finely nodular tan-yellow mass. Microscopic examination revealed a lipoblastoma. The partially encapsulated, lobular proliferation of mature and maturing adipose tissue infiltrated the skeletal muscle and fibrous tissue. The mass was traversed by prominent fibrous septa, and although mature adipose tissue predominated, several myxoid areas containing lipoblasts were seen (Figure 2) . Atypical cells were not prominent. The patient is doing well 10 months after the surgery.
Figure 2.
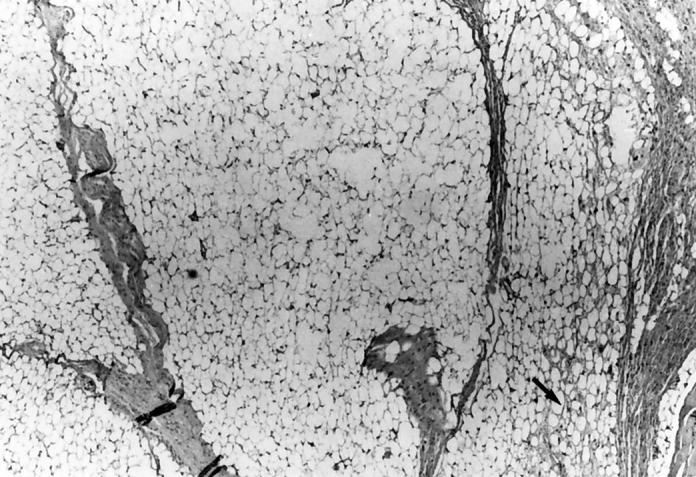
Mature adipocytes predominate in this portion of a lipoblastoma, with focal myxoid area (arrow). The lobular architecture divided by fibrous septa seen in both figures is more characteristic of lipoblastoma than liposarcoma.
Materials and Methods
Cytogenetic Analysis
Tumor specimens from each case were processed using a routine procedure. 14 Briefly, the tumor specimens were disaggregated in collagenase, then the suspension seeded in flasks and on coverslips. The cells were cultured in RPMI-1640 (Fisher Scientific, Houston, TX) supplemented with 16% fetal bovine serum and Amnio Max supplemented media (Life Technologies, Grand Island, NY). Primary cultures were harvested after 3 to 7 days of growth. Air-dried chromosome preparations were GTG-banded and karyotypes described according to the ISCN 1995.
Spectral Karyotyping
Metaphase preparations were used for spectral karyotyping (SKY) studies for Case 2. Five metaphases of the abnormal clone were analyzed. SKY probes were obtained from Applied Spectral Imaging (Carlsbad, CA) and hybridized according to the manufacturer’s instructions. By using multiple differentially-labeled probes in one single hybridization, SKY can distinguish each chromosome in a single display based on distinct color visualization.
Fluorescence in Situ Hybridization (FISH)
Metaphase preparations were also used for FISH analysis for Case 2. Whole chromosome painting probes for chromosomes 8, 13, and 16 (Vysis, Downers Grove, IL) were used to confirm the aberrations detected by SKY. Hybridization and detection of hybridization signals were performed according to the manufacturer’s protocols.
Results
Case 1
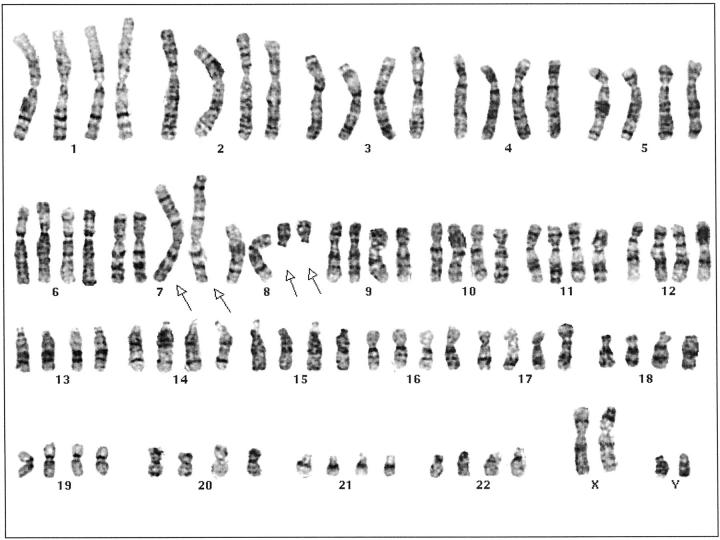
Evaluation of 4- to 5-day cultures of tumor from this patient revealed two cell populations. One population (8 cells) showed a tetraploid complement with two copies of an apparently balanced translocation between chromosomes 7 and 8. The remaining 16 cells showed a normal male chromosome complement. The abnormal karyotype was described as 92,XXYY, t(7;8)(p22;q11.2)x2[8]/46,XY[16] (Figure 3) .
Figure 3.
Karyotype of the lipoblastoma of Case 1 showing 92, XXYY,t(7;8)(p22;q11.2)x2.
Case 2
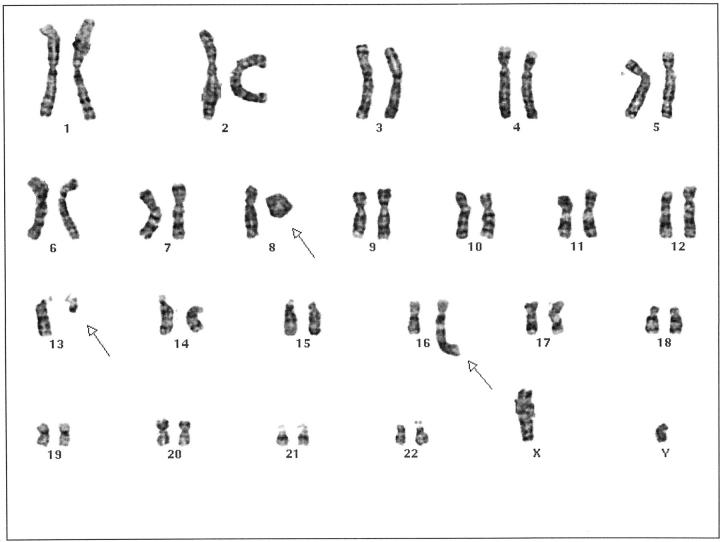
Analysis of 3- to 7-day cultures of tumor from this patient revealed two cell populations. One population (13 cells) showed a composite karyotype with several numerical and structural chromosome abnormalities. The remaining 7 cells were normal. The abnormal karyotype was initially characterized as 46,XY,−8,−13,add(16)(q22),+mar, +r[cp13]/46,XY[7].
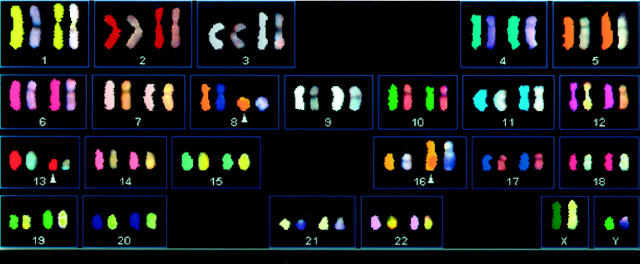
SKY revealed abnormalities that could not be identified by standard G-banded analysis. In five abnormal cells analyzed, SKY, combined with inverted DAPI G-bands consistently indicated the following abnormalities:
First, the add(16)(q22) is a rearrangement involving inverted insertion of chromosome 8 segment into chromosome 16, ie, a der(16)ins(16;8)(q22;q24q11.2).
Second, the ring chromosome is composed entirely of chromosome 8 material with unknown breakpoints. Based on the size of this ring chromosome, it appears reasonable to speculate that the r(8) is composed of duplication of the short arm and proximal long arm material remaining following the insertion between chromosomes 8 and 16.
Third, the marker chromosome is a del(13)(q12).
All of the rearrangements identified by SKY were further confirmed by use of FISH with painting probes. Therefore, the karyotype was revised as 46,XY, r(8), de(13)(q12), der(16)ins(16;8)(q22;q24q11.2) [cp13]/46,XY[7] (Figures 4 and 5 ).
Figure 4.
Karyotype of the lipoblastoma of Case 2 showing 46, XY, r(8), del(13)(q12), der(16)ins(16;8)(q22;q24q11.2).
Figure 5.
SKY shows a metaphase from the lipoblastoma of Case 2 with chromosomes assigned pseudocolors according to the measured spectra. Arrows indicate abnormal chromosomes with pseudocolors.
Discussion
Recently, distinct chromosome abnormalities have been well documented in some adipose tissue tumors, and include translocations between 12q13–15 and other chromosomes, del(13)(q12q22), and rearrangements of 6p22–23 in lipomas, a t(12;16)(q13;p11) or t(12;22)(q13;q12) in myxoid liposarcoma, and large marker chromosomes, rings, and double minutes in well-differentiated liposarcoma. 15
Though cytogenetic studies in lipoblastoma have been limited to 14 cases, nonrandom involvement of the long arm of chromosome 8 has been a consistent finding. 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13 The partner chromosomes associated with the 8q rearrangements include 7p22, 7q31, 2q23, 3q12, 1p13, 6p21, 9p22, 14q24, and 6q13q27. Each has been reported only once in the literature. 3, 4, 5, 6, 7, 8, 9, 10 The present study reveals a new partner chromosome, ie, 16q22, involved in the 8q-related rearrangements (Case 2), and further confirms the t(7;8)(p22;q11.2) as a recurrent translocation in lipoblastoma 3 (Case 1). No doubt, these findings provide solid bases for clinical molecular cytogenetic diagnosis of lipoblastoma. Breakpoints clustered within 8q11.2q13 have been suggested to characterize lipoblastoma. 3, 4, 5, 6, 7, 8, 9, 10 Because lipoblastoma is a benign tumor, a gene or genes located at 8q11.2-q13 may be more important for the regulation of cellular growth than for malignant transformation.
Lipoblastomas may be either circumscribed or diffuse and infiltrative (diffuse lipoblastomatosis), 1, 2 and local recurrence is described in 14% of cases. 16, 17 Both of the lipoblastomas reported here were diagnosed based primarily on their clinicopathological features, including age early in the first decade of life, well-defined lobules delineated by fibrous septa, central concentration of mature adipocytes within lobules, focal rather than abundant mucinous pools, and pleomorphism. However, Case 1 highlights the difficulty sometimes encountered in differentiating lipoblastoma from liposarcoma, particularly in an older child or in a diffuse lipoblastoma, which may not display well-formed lobules and septa. Chromosome analysis can be very valuable in the differential diagnosis of these tumors. The molecular cytogenetics findings presented here strongly supported and confirmed the pathological diagnoses in these two patients, based on a t(7; 8)(p22;q11.2) in Case 1 and an 8q11.2-related anomaly, ie, der(16)ins(16;8)(q22;q24q11.2) in Case 2.
SKY has recently been developed to complement FISH and conventional cytogenetics in karyotype analysis. SKY permits the simultaneous visualization of all human chromosomes, with each chromosome being painted with a single fluorochrome or multifluorochrome combination and displaying a different color. This method combines the karyotype screening ability with the ability of FISH to characterize marker or derivative chromosomes and other chromosomal structures (such as double minutes and homogeneously staining regions) as well as numerical chromosome changes. In Case 2, SKY consistently revealed the abnormalities in all of the cells analyzed, strongly supporting our interpretation. Therefore, this case further highlights the value of SKY in conjunction with conventional cytogenetic analysis in the genetic diagnosis of childhood adipose tissue tumors. This report may represent the first study to analyze lipoblastomas by using SKY techniques in the literature.
The molecular genetics of lipoblastoma has not been elucidated. However, the characteristics of 8q11.2-q13-related rearrangements, being specifically involved in the pathogenesis of lipoblastoma, warrant further molecular studies to delineate the molecular aspects of lipoblastoma.
Address reprint requests to Dr. Zhong Chen, Cytogenetics Laboratory, Division of Medical Genetics, Department of Pediatrics, University of Utah School of Medicine, Room 1C204, University Medical Center, Salt Lake City, Utah 84132. E-mail: zhong.chen@hsc.utah.edu, or Dr. Amy Lowichik, Department of Pathology, University of Utah School of Medicine, University Medical Center, Salt Lake City, Utah 84132.
References
- 1.Coffin CM: Lipoblastoma: an embryonal tumor of soft tissue related to organogenesis. Semin Diagn Pathol 1994, 11:98-103 [PubMed] [Google Scholar]
- 2.Colins MH, Chatten J: Lipoblastoma/lipoblastomatosis: a clinicopathologic study of 25 tumors. Am J Surg Pathol 1997, 21:1131-1137 [DOI] [PubMed] [Google Scholar]
- 3.Sandberg AA, Gibas Z, Saren E, Li FP, Limon J, Tebbi CK: Chromosome abnormalities in two benign adipose tumors. Cancer Genet Cytogenet 1986, 22:55-61 [DOI] [PubMed] [Google Scholar]
- 4.Ohjimi Y, Iwasaki H, Kaneko Y, Ishiguro M, Ohgami A, Kikuchi M: A case of lipoblastoma with t(3;8)(q12;q11.2). Cancer Genet Cytogenet 1992, 62:103-105 [DOI] [PubMed] [Google Scholar]
- 5.Fletcher JA, Kozakewich HP, Schoenberg ML, Morton CC: Cytogenetic findings in pediatric adipose tumors: consistent rearrangement of chromosome 8 in lipoblastoma. Genes Chromosomes Cancer 1993, 6:24-29 [DOI] [PubMed] [Google Scholar]
- 6.Sawyer JR, Parsons EA, Crowson SS, Smith S, Erickson S, Bell JM: Potential diagnostic implications of breakpoints in the long arm of chromosome 8 in lipoblastoma. Cancer Genet Cytogenet 1994, 76:39-42 [DOI] [PubMed] [Google Scholar]
- 7.Dal Cin P, Sciot R, De Wever I, Van Damme B, Van den Berghe H: New discriminative chromosomal marker in adipose tissue tumors: the chromosome 8q11–q13 region in lipoblastoma. Cancer Genet Cytogenet 1994, 78:232-235 [DOI] [PubMed] [Google Scholar]
- 8.Kanazawa C, Mitsui T, Shimizu Y, Saitoh E, Kawakami T, Shiihara T, Yokoyama S, Yamagiwa I, Hayasaka K: Chromosomal aberration in lipoblastoma: a case with 46,XX,ins(8;6)(q11.2;q13q27). Cancer Genet Cytogenet 1997, 95:163-165 [DOI] [PubMed] [Google Scholar]
- 9.Posey Y, Valdivia E, Persons DL, Ally S, Smith DL, Pantazis CG, Smith SD: Lipoblastoma presenting as a mesenteric mass in an infant. J Pediatr Hematol Oncol 1998, 20:580-582 [PubMed] [Google Scholar]
- 10.Panarello C, Rosanda C, Morerio C, Russo I, Dallorso S, Gambini C, Ricco AS, Storlazzi T, Archidiacono N, Rocchi M: Lipoblastoma: a case with t(7;8)(q31;q13). Cancer Genet Cytogenet 1998, 102:12-14 [DOI] [PubMed] [Google Scholar]
- 11.Francois A, Bodenant C, Rives N, Bachy B, Mitrofanoff P, Mace B, Hemet J: Mesenteric lipoblastoma with changes in chromosome 8: use of cytogenetics in the diagnosis of adipocytic tumors in children. Ann Pathol 1997, 17:406-411 [PubMed] [Google Scholar]
- 12.Fletcher CD, Akerman M, Dal Cin P, de Wever I, Mandahl N, Mertens F, Mitelman F, Rosai J, Rydholm A, Sciot R, Tallini G, van den Berghe H, van de Ven W, Vanni R, Willen H: Correlation between clinicopathological features and karyotype in lipomatous tumors: a report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol 1996, 148:623-630 [PMC free article] [PubMed] [Google Scholar]
- 13.Miller GG, Yanchar NL, Magee JF, Blair GK: Lipoblastoma and liposarcoma in children: an analysis of 9 cases and a review of the literature. Can J Surg 1998, 41:455-458 [PMC free article] [PubMed] [Google Scholar]
- 14.Fletcher JA: Cytogenetics. Cancer Treat Res 1993, 67:23-35 [DOI] [PubMed] [Google Scholar]
- 15.Sandberg AA, Bridge JA: The Cytogenetics of Bone and Soft Tissue Tumors. Edited by Sandberg AA, Bridge JA. Austin, TX, RG Landes, 1994, pp 147–216
- 16.Jimenez JF: Lipoblastoma in infancy and childhood. J Surg Oncol 1986, 32:238-244 [DOI] [PubMed] [Google Scholar]
- 17.Mahour GH, Bryan BJ, Isaacs H: Lipoblastoma and lipoblastomatosis: a report of six cases. Surgery 1988, 104:577-579 [PubMed] [Google Scholar]