Abstract
A new method is described for effecting ion/ion proton transfer reactions that involve storage of analyte ions while oppositely charged ions are transmitted through the stored ion population. In this approach, the products are captured and stored in the linear ion trap for subsequent mass analysis. Charge reduction of multiply charged protein ions is used as an example to illustrate the analytical usefulness of this method. In another variation of the transmission mode ion/ion reaction approach, two charge inversion experiments, implemented by passing analyte ions through a population of multiply charged reagent ions in a LIT, are also demonstrated. A pulsed dual ion source approach coupled with a hybrid triple quadrupole/linear ion trap instrument was used to demonstrate these two methods. The results for ion/ion reactions implemented using these so-called “transmission mode” experiments were comparable to those acquired using the more conventional mutual storage mode, both in terms of efficiency and information content of the spectra. An advantage of transmission mode experiments compared to mutual storage mode experiments is that they do not require any specialized measures to be taken to enable the simultaneous storage of oppositely charged ions.
Keywords: Proton Transfer, Transmission Mode, Charge Inversion, Ion/Ion Reaction, Dual Nano-electrospray Ionization, Linear Ion Trap
INTRODUCTION
The utility of gas-phase ion/ion reactions in the tandem mass spectrometry of biomolecules is becoming increasingly apparent [1, 2]. Among the various types of ion/ion reactions, proton transfer reactions have been the most extensively studied and a number of applications have been developed [3–7]. Most applications reported so far involve charge state manipulation of biomolecules. Ion/ion proton transfer reactions, for example, have been used to simplify the complexity associated with electrospray of mixtures of biomolecules by reducing spectral overlap due to ions of different nominal masses and charges but similar mass-to-charge ratios [3, 7]. Ion/ion proton transfer reactions have also been shown to be useful in simplifying product ion spectra from unimolecular dissociation of multiply charged ions [4], in concentration and purification of parent ions via either ion parking [5] or parallel ion parking techniques [6]. These applications were initially developed on 3-D quadrupole ion traps and have subsequently been implemented on linear ion traps (LITs). The LITs hold significant advantages over 3-D quadrupole ion traps in terms of dynamic range and the efficiency with which they can be coupled with ion sources, detectors, and other mass analyzers [8, 9]. Therefore, it is desirable to explore ion/ion proton transfer reaction techniques with LITs.
Four approaches to effect ion/ion reactions within a LIT have been proposed [10, 11], and these are listed in Table 1. These approaches can be used for both electron transfer dissociation (ETD) reactions and proton transfer reactions. These methods include: (I) continuously passing of both polarities of ions through the LIT, (II) storage of analyte ions while continuously passing reagent ions through the LIT, (III) storage of reagent ions while continuously passing analyte ions through the LIT, and (IV) mutual storage of both polarities of ions within a LIT. The rate constants for ion/ion reactions are expected to be lowest for Method I because the relative velocities of the ions are expected to be the highest in this mode, whereas Method IV is expected to result in the largest rate constants due to the lowest relative velocities of the ions. Rate constants of intermediate value are expected for Methods II and III. Among all the methods described, only ion/ion reactions performed in the mutual storage mode (Method IV) [12, 13] require the implementation of radio frequency (RF) voltages to the containment lenses of the LIT, or the application of unbalanced RF to the quadrupole array [13].
Table 1.
Four methods for effecting ion/ion reaction experiments in a LIT: (I) passage of both polarity ions, (II) analyte ion storage/reagent ion transmission, (III) analyte ion transmission/reagent ion storage, and (IV) mutual storage of both polarity ions. Note that RF requirement means the requirement of RF to the containment lenses of a LIT.
| Method | Analyte Ions | Reagent Ions | RF Requirement |
|---|---|---|---|
| I | passing | passing | No |
| II | trapping | passing | No |
| III | passing | trapping | No |
| IV | trapping | trapping | Yes |
To date, most ion/ion proton transfer reactions performed in a LIT have employed the mutual storage mode (Method IV). Method III for charge reduction of biomolecules via ion/ion proton transfer reaction has also been demonstrated by using distinct electrospray ionization (ESI) and atmospheric sampling glow discharge ionization (ASGDI) sources [10]. However, some important types of ion/ion proton transfer reactions, such as charge inversion [14–16] and protein/peptide complex formation [17, 18], could not be implemented using the ESI/ASGDI combination. This is because multiply charged reagent ions are normally required for charge inversion and complex formation while the ASGDI ion produces only singly charged ions. This limitation has been overcome by the development of a dual nano-ESI source [19]. The dual ion source produces and injects sequentially both analyte ions and proton transfer reagent ions into the LIT along the axial dimension, which makes the implementation of Methods II, III and IV straightforward. While ion/ion proton transfer reactions in LITs employing Method III and IV have been demonstrated and utilized in the analysis of proteins and peptides, ion/ion proton transfer reactions in a LIT performed using Method II have not been previously reported.
In this note, we describe a new method of performing ion/ion proton transfer reactions in a LIT (i.e., Method II) where analyte ions are stored in the LIT while passing proton transfer reagent ions through the LIT. Charge reduction of multiply charged protein ions, with and without ion parking, is used as an example to demonstrate the analytical usefulness of this method. Also included in this note are two charge inversion experiments implemented via Method III, which were heretofore inaccessible by use of distinct ESI/ASGDI sources. A significant advantage of these transmission mode ion/ion proton transfer reaction approaches is that they do not require the superposition of RF to the containment lenses of the LIT.
EXPERIMENTAL SECTION
Materials
Methanol and glacial acetic acid were purchased from Mallinckrodt (Phillipsburg, NJ). Bovine ubiquitin and bradykinin were purchased from Sigma-Aldrich (St. Louis, MO) and used without further purification. Perfluoro-1-octanol (PFO), poly(propylenimine) [1,4-diaminobutane (DAB) core] dendrimers (generation 4.0), and carboxylate-terminated polyamidoamine (PAMAM) dendrimers (generation 1.5) were obtained from Sigma-Aldrich (Milwaukee, WI). Solutions of peptides or proteins were dissolved to 10 μM in 48/48/2 (vol/vol/vol) methanol/water/acetic acid for positive ESI or in 48/48/2 (vol/vol/vol) methanol/water/ammonium hydroxide for negative ESI. PFO, with a final concentration of 200 μM, was subjected to negative ESI from 1% ammonium hydroxide methanol solution. A positive ESI spray solution of DAB dendrimers with a concentration of 0.5 mg/mL was prepared in aqueous 5% acetic acid. A solution of PAMAM dendrimers was prepared in 48/48/2 (vol/vol/vol) methanol/water/ammonium hydroxide with a concentration of 1.0 mg/mL for negative ESI.
Mass Spectrometry
All experiments were performed using a prototype version of a Q TRAP mass spectrometer [9] (Applied Biosystems/MDS SCIEX, Concord, Ontario, Canada) equipped with a home-made dual nano-ESI source, as shown schematically in Scheme 1. All the electronics were controlled by Daetalyst 3.10 software, a research version of software provided by MDS SCIEX. The LINAC [20] function of Manitoba Q2 collision cell was turned off for this transmission mode proton transfer reaction study. The Q2 and Q3 quadrupole arrays are configured as LITs and operated at a drive RF of 1 MHz while Q0 and Q1 quadrupole arrays are operated at a drive RF of 650 kHz.
Scheme 1.
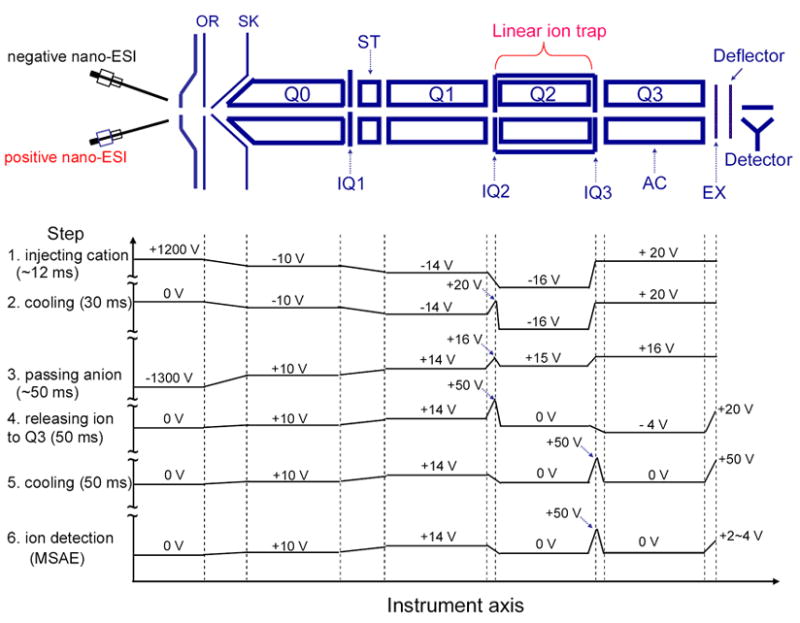
Schematic of the Q TRAP mass spectrometer equipped with a home-made dual nano-ESI source. The plots show the typical potentials along the instrument axis at different steps (first step = top, last step = bottom) for Method II ion/ion proton transfer reaction experiments. For each step, the solid lines reflect qualitatively the changes in voltages. These levels are not drawn to scale but the voltage values themselves are indicated.
A typical scan function for a Method II transmission mode ion/ion proton transfer experiment consists of the following steps: positive ion injection into Q2 (12-ms), anion transmission through Q2 LIT (50-ms), and transfer of ion/ion reaction product ions from Q2 to mass analyzer Q3 (50-ms) for mass analysis, as shown in Scheme 1. This scheme summarizes the potentials applied to the relevant ion optical elements of the system for the key steps in the process. The ordinate represents distance (not drawn to scale) with the dashed lines lining up with the corresponding ion optical elements shown above the plot. The abscissa is a series of voltage axes where the first step of the experimental sequence is represented at the top and the final step is at the bottom. For a transmission mode ion/ion proton transfer reaction experiment of this type, the first step involves the pulsed application of a positive high voltage to the nano-ESI wire to generate analyte ions. These positive analyte ions can be isolated by Q1 in RF/DC mode and injected axially into the Q2 LIT with nitrogen as the buffer gas at a pressure of 1–8 mTorr. These ions were cooled in Q2 for 30-ms, during which time the high voltage on this emitter was turned off. After the cooling step, the power supply connected to the other nano-ESI wire, which was operated in the negative polarity, was triggered on to generate the proton transfer reagent anions. These reagent anions were isolated by Q1 in RF/DC mode before they entered Q2 LIT with relatively low kinetic energies (Q2 DC offset was roughly 5 V attractive relative to the Q0 DC offset). At the same time, the DC potentials applied to the containment lenses (i.e., IQ2 and IQ3) of Q2 LIT were adjusted to a value that was about 1 V repulsive relative to the Q2 LIT DC offset. The 1 V difference in potential is high enough to trap the cooled analyte ions in the axial dimension. The reaction time for ion/ion proton transfer reaction in this approach is determined by the injection time of the anion into the Q2 LIT. After a defined anion transmission time, charge reduced product ions arising from ion/ion proton transfer reactions, were transferred from Q2 to Q3, and cooled for 50 ms before they were subjected to mass selective axial ejection (MSAE) [21] using a supplementary RF signal at a frequency of 380 kHz. Ion parking experiments were effected by applying a single frequency resonance excitation voltage generated by an external waveform generator (Agilent, Palo Alto, CA) to one pair of opposing rods of the Q2 quadrupole array during the anion transmission period.
A typical scan function for ion/ion charge inversion reactions performed by Method III, whereby charge inversion reagent anions are stored in Q2 while singly charged peptide cations are transmitted through Q2, is shown in Scheme 2. Since charge inverted product ions have the same ion polarity as the charge inversion reagent ions (i.e., negative), they were trapped as they formed in Q2, along with the reagent ions, by the DC potential applied to the containment lenses. These charge inversion experiments do not require an external device to collect the product ions. This stands in contrast to ion/ion charge reduction reactions performed via Method III, in which case the charge reduced products of interest are accumulated in the adjacent Q3 quadrupole array. The spectra shown here were typically the average of 20–50 individual scans. For ion/ion charge inversion of negatively charged peptides to positively charged species, the reaction sequence is similar, but the order in which anions and cations are injected in this experiment is reversed from that shown in Scheme 2.
Scheme 2.
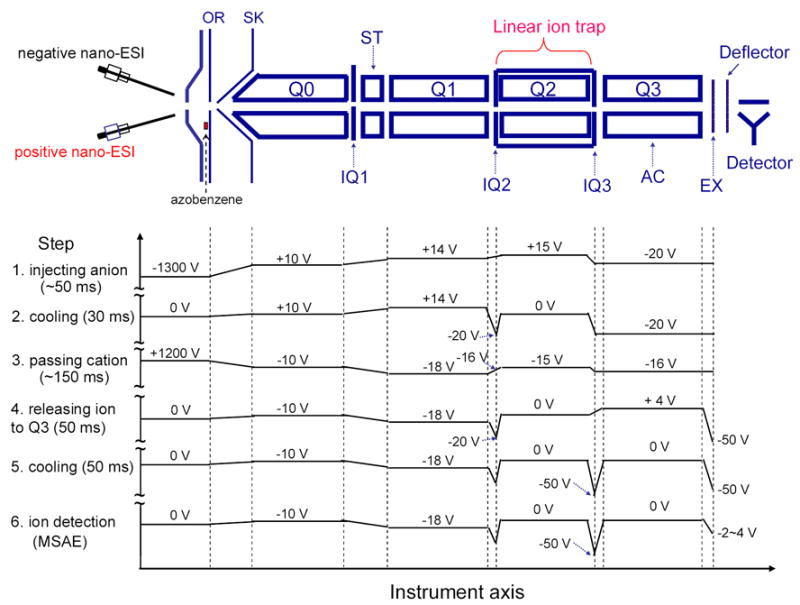
Schematic of the Q TRAP mass spectrometer equipped with a home-made dual nano-ESI source. The plots below show the typical potential along the instrument axis at different steps for ion/ion charge inversion reaction experiments of positively charged peptide ions to negatively charged species performed in Method III.
RESULTS AND DISCUSSION
From our previous study of ion/ion reactions implemented in the transmission mode (i.e., Method II and Method III for ETD [11], Method III for proton transfer reactions [10]) in a LIT, we have learned that optimized conditions for efficient ion/ion reactions are obtained with background pressures milliTorr range, relatively low ion injection energy, and relatively high ion injection q-value [22]. Such experimental conditions are expected to be applicable to this study of transmission mode ion/ion proton transfer reactions because experimental conditions for electron transfer and proton transfer ion/ion reactions are essentially the same [23].
Charge Reduction of Multiply Protonated Protein Ions
Figure 1 gives an example of transmission mode ion/ion charge reduction reactions between cations formed from bovine ubiquitin and anions formed from perfluoro-1-octanol (PFO), which were both generated by the pulsed dual nano-ESI source. The first step of the experiment involved the accumulation of bovine ubiquitin [M+12H]12+ ions formed via positive nano-ESI in Q2 LIT. The resulting mass spectrum is shown in Figure 1a. The second step is the injection of the deprotonated PFO [PFO-H]− anions, which was generated by negative nano-ESI and isolated by Q1 using the RF/DC mode, into the same linear ion trap. Figure 1b shows the mass spectrum of [PFO-H]− ions in the negative mode (no cations present). The injection q-value of the [PFO-H]− ions was kept at 0.65 since we found that a high q-value is necessary for optimum spatial overlap of analyte cations and reagent anions, corresponding to a higher ion/ion reaction rate [11]. Figure 1c shows the post-ion/ion mass spectrum in the positive mode after passing deprotonated PFO anions through the whole population of bovine ubiquitin cations for 100-ms. The [M + H]+ ions of ubiquitin were the most abundant species in the product ion distribution, indicating ion/ion reaction rates for the transmission mode experiment similar to those typically observed for mutual storage mode.
Figure 1.
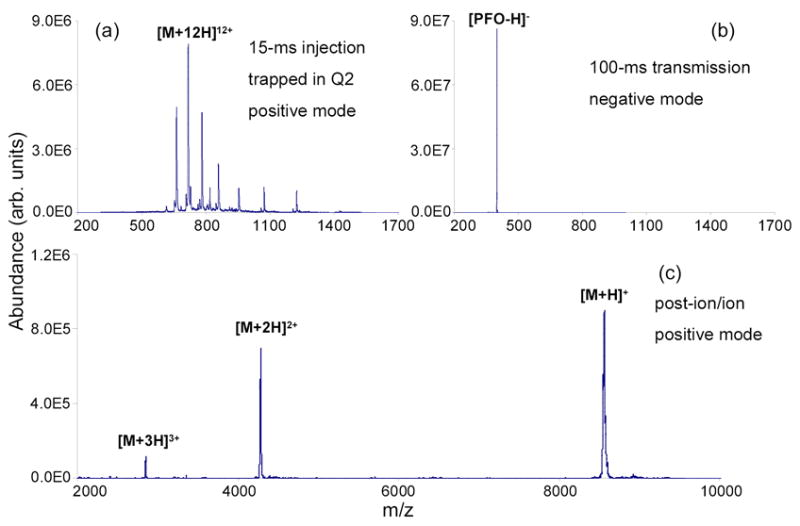
Mass spectra derived from transmission mode ion/ion charge reduction reaction: (a) 15-ms positive nano-ESI of 10 μM bovine ubiquitin (trapped), (b) 100-ms negative nano-ESI of 200 μM PFO (passing), and (c) post-ion/ion spectrum acquired at 50 kHz ejection frequency after 100-ms reaction of ions shown in (a) and (b).
When ion/ion reactions are conducted using electrodynamic ion traps as reaction vessels, the reaction rates of specified ions can be inhibited in a selective fashion due to the mass-to-charge dependent frequencies of ion motion. The selective inhibition of the ion/ion reaction rate of one charge state of an analyte species has been referred to as ion parking [5]. This technique has been used to concentrate ions dispersed over multiple charge states into a single charge state. In a particular case, ion parking coupled with ion/ion reactions can also be used to create relatively abundant ions with a charge state which can not be directly generated by ESI. In the case of a linear ion trap, ion parking is implemented by applying to the ion trap electrodes a supplementary RF voltage that is on resonance with the ion of interest. Under the current instrument setup, a dipolar auxiliary RF signal is coupled directly to one set of opposing rods of the Q3 quadrupole array. Due to the capacitive coupling of Q3 and Q2, the RF signal is also applied across opposing rods of Q2 but with lower amplitude.
Figure 2 illustrates the ion parking experiment with the ion/ion proton transfer reaction conducted in the Method II transmission mode. This experiment differs from the experiment illustrated in Figure 1 in that a dipolar RF signal was applied during the anion transmission period. The isolated amount of bovine ubiquitin [M+12H]12+ is shown in mass spectrum Figure 2a. Figure 2b shows the post-ion/ion mass spectrum in the positive mode after passing deprotonated PFO anions (data not shown) through bovine ubiquitin [M+12H]12+ cations for 50-ms. Figure 2c shows results of the ion parking experiment applied to the [M+10H]10+ charge-state ions using the same ion/ion reaction conditions as in Figure 2b except that a resonance excitation signal at 75.8 kHz, 3.7 V0-p was applied for 50-ms to an opposing set of rods in the Q2 LIT.
Figure 2.
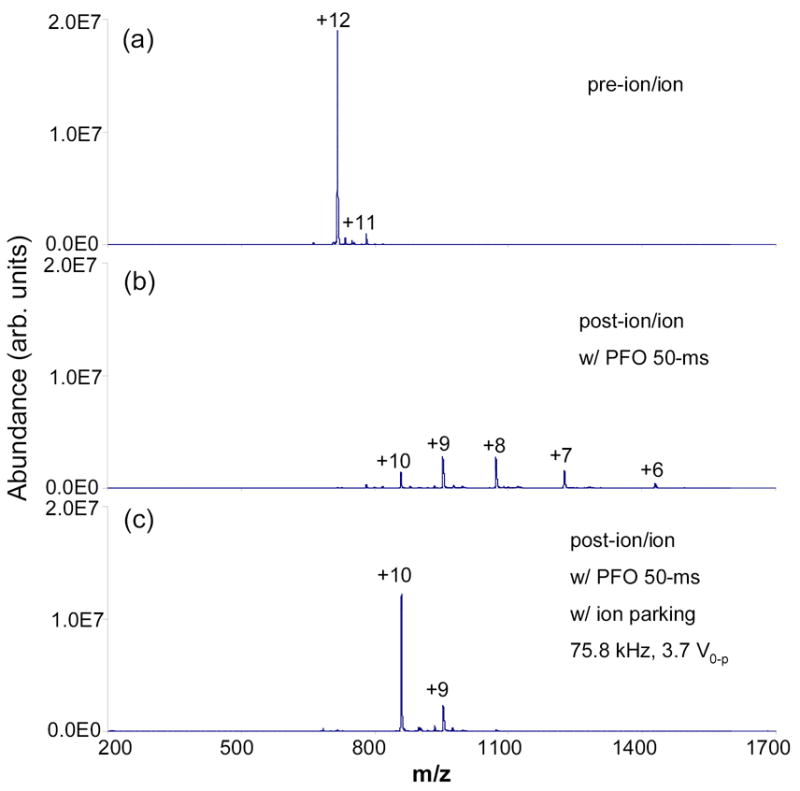
Mass spectra derived from transmission mode ion/ion proton transfer reaction: (a) 12-ms positive nano-ESI of 10 μM bovine ubiquitin (trapped), (b) post-ion/ion spectrum after 50-ms negative nano-ESI of 200 uM PFO passing through a population of ions shown in (a), and (c) ion parking (75.8 kHz, 3.7.0 V0-p) mass spectrum.
We note also that a Method III ion parking experiment, whereby the reagent anions are stored in Q2 while ubiquitin cations are transmitted through Q2 in the presence of dipolar excitation applied across opposing rods, has also been implemented (data not shown). This mode also allows for ion parking and showed comparable performance in terms of the resolution with which charge states can be parked. In these preliminary experiments, however, there appeared to be greater losses of the analyte ions with Method III than with Method II. That is, the product ion/precursor ion fraction after ion parking was roughly a factor of five lower with Method III than with Method II. This may well reflect the poorer transmission of ions through the Q2 exit aperture for ions that are undergoing resonance excitation in Q2. This problem is circumvented by use of Method II because the parked ions are transferred from Q2 to Q3 after the resonance ejection voltage is turned off and the analyte ions can be cooled to the center of Q2 prior to ejection. The Method III ion parking experiment, on the other hand, requires that the resonance excitation signal be on during transmission of the analyte species from Q2 to Q3.
Charge Inversion of Peptides from Positive/Negative Polarity to Negative/Positive Polarity
Charge reduction of protein or peptide ions via ion/ion proton transfer reactions often employs singly charged reagent ions with polarity opposite to that of the analyte ions. However, when multiply charged reagent ions are employed, it is possible to transfer multiple protons to the analyte ions in a single reaction step, which could result in the inversion of analyte ion polarity. Benefits from charge inversion experiments include the ion formation in a polarity which could not favorably be generated by certain ion sources [24] and net ion charge increase [14, 15] by using two sequential charge inversion reactions in the gas phase. To date, all charge inversion studies conducted in electrodynamic ion traps employ mutual storage mode, in which ions with both polarities including the analyte ions, reagent ions, and charge inverted product ions are simultaneously trapped. Here, Method III transmission mode ion/ion reactions are demonstrated to implement charge inversion experiments in a LIT while no auxiliary RF signals are applied to the containment lenses.
The data in Figure 3 demonstrate charge inversion of bradykinin peptide ions from negative ion to positive ion polarity via Method III. The first step involved the accumulation of poly(propylenimine) 1,4-diaminobutane (DAB) dendrimer generation 4.0 [DAB+7H]7+ ions in the Q2 LIT. These charge inversion reagent ions were formed via positive nano-ESI with ions isolated by Q1 using the RF/DC mode. The resulting mass spectrum is shown in Figure 3b. After reagent cations were cooled in Q2 for 30-ms, bradykinin [M H] ions (Figure 3a), formed via negative nano-ESI and isolated by Q1, were transmitted through the Q2 LIT for 80-ms. Ion/ion reaction products resulting from the passing of analyte anions through the reagent cations is shown in Figure 3c. Charge inversion of bradykinin shows a more abundant [M+2H]2+ product ion than the [M+H]+ ion, as shown in Figure 3c. The ratio of abundance of the [M+2H]2+ ion to that of the [M+H]+ ion of bradykinin obtained in the transmission mode is very similar to that obtained in the mutual storage mode [19].
Figure 3.
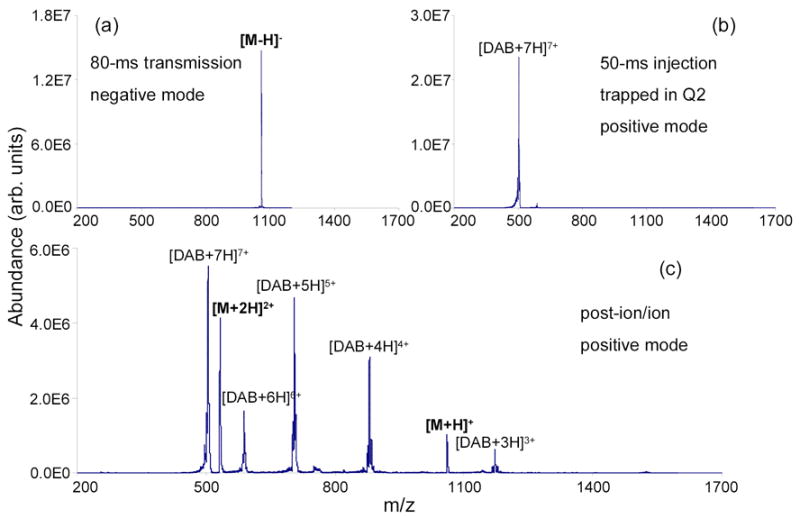
Mass spectra derived from Method III transmission mode ion/ion proton transfer reaction: (a) isolated [M–H]− ions of bradykinin from negative nano-ESI (passing), (b) isolated [DAB+7H]7+ ions of DAB dendrimer generation 4.0 from positive nano-ESI (trapped), and (c) Post-ion/ion spectrum acquired after 80-ms transmission of ions shown in (a) through a population of ions shown in (b).
Figure 4 illustrates positive ion to negative ion charge inversion of peptide ions. In this case, carboxylate-terminated polyamidoamine (PAMAM) dendrimer generation 1.5 was used to generate reagent anions for charge inversion experiments. In negative nano-ESI mode (Figure 4b), charge states of PAMAM dendrimers ranging from −3 to −5 were observed, denoted as Pn− (n = 3–5), as well as peaks arising from faulty synthesis products (not labeled in the spectrum). Within each charge state of PAMAM, mixtures of sodium ions and protons as counter-ions were also present, which can be expressed as [P–(n+m)Na+mH]n− [m = 0–(16–n)]. Since PAMAM dendrimer anion signal was spread over a wide variety of anionic species, no ion isolation via Q1 was employed so that the ion/ion reaction rate could be maximized. After the PAMAM denderimer Pn− reagent anions were cooled in Q2 for 30-ms, [M+H]+ ions of bradykinin, formed via positive nano-ESI and isolated by Q1 using the RF/DC mode, were transmitted through the entire PAMAM anion distribution for 150-ms. In the post-ion/ion spectrum of Figure 4c (negative mode), [M–H]− ions of bradykinin can be clearly identified while the [M–2H]−2 ions of bradykinin are not obvious. The formation of a highly abundant bradykinin dianion is not expected because bradykinin has only one strongly acidic site, i.e., the C-terminus.
Figure 4.
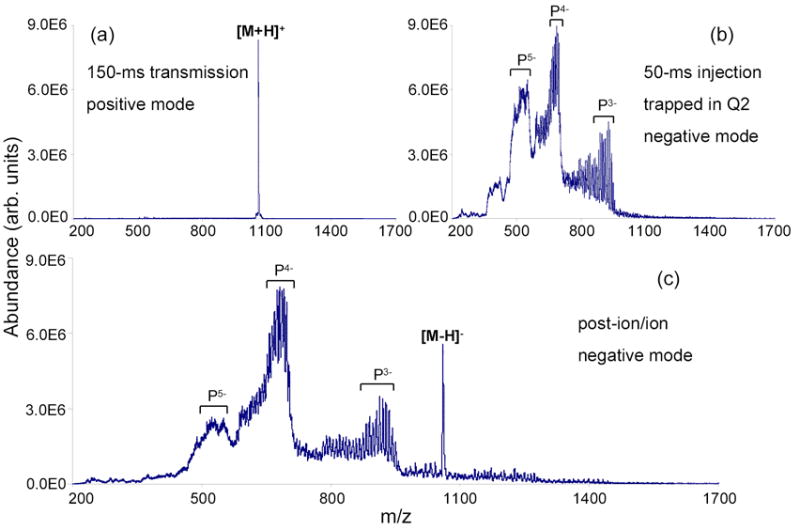
Mass spectra derived from Method III transmission mode ion/ion proton transfer reaction: (a) isolated [M + H]+ ions of bradykinin from positive nano-ESI (passing), (b) ions of PAMAM dendrimer generation 1.5 from negative nano-ESI (trapped), and (c) Post-ion/ion spectrum acquired after 150-ms transmission of ions shown in (a) through a population of ions shown in (b).
Both charge inversion experiments of peptide ions either from negative to positive polarity or vice versa were implemented in a way that charge inversion reagent ions were trapped in the LIT while passing the analyte ions through it. This is necessary since the charge inverted product ions have the same ion polarity as the reagent ions, which are trapped in the LIT by the DC voltage. In other words, charge inverted product ions will be lost if analyte ions were trapped first while passing the reagent ions through the LIT. Due to this limitation, two step charge inversion ion/ion reactions performed in the transmission mode (Method II and III) can not be used to increase the absolute charge of a peptide ion. This type of two step charge inversion experiments has been performed in the mutual storage mode (Method IV) because there is no discrimination against either ion polarity from the RF signal. The main advantage of the transmission mode ion/ion reaction approach, relative to the mutual storage mode, is the lack of a requirement for superposition of RF signal on the containment lenses. Another possible advantage may be a lower sensitivity to space charge for the transmitted ion population. The latter effect is reflected by relatively lower sensitivity to the numbers of reagent ions in the ion parking experiment for the transmission mode experiment than for the mutual storage experiment. Much greater care must be taken in the mutual storage case (Method IV) to avoid the use of sufficiently large numbers of reagent ions to affect the frequencies of motion of the oppositely charged ions being parked.
With the instrumentation used here to illustrate the transmission mode ion/ion reactions, the time associated with executing a single experiment is on the other of 0.5–1 s. The time-frames of the experiments were not minimized for use with on-line chromatographic separations. The time associated with some of the steps could be reduced somewhat. For example, the cooling steps of 30 ms and 50 ms indicated in Schemes 1 and 2 could be reduced to 10 ms and 20–30 ms, respectively. However, the most time-consuming step in the process is the roughly 500 ms mass analysis step. With a faster mass analysis technique, a time of less that 0.5 s/scan could be realized without any reduction in the ion/ion reaction time-frames noted in these studies.
CONCLUSIONS
A new method for effecting ion/ion proton transfer reactions has been described that involves storage of analyte ions while oppositely charged ions are transmitted through the stored ion population. Charge reduction of multiply charged protein ions is used as an example to illustrate the analytical usefulness of this method (Method II). Method III transmission mode ion/ion proton transfer reactions have been previously reported for charge reduction of protein ions but this method was not shown for charge inversion experiments due to the limitation of available ion sources at that time. In this note, two charge inversion experiments are demonstrated to expand the usage of Method III transmission mode ion/ion proton transfer reaction. An advantage of the transmission mode ion/ion proton transfer methods is that they do not require instrument modification to allow for the mutual storage of both ion polarities. The transmission mode proton transfer reaction is facilitated by a dual nano-ESI source, which is able to alternately generate and inject the analyte and proton transfer reagent ions (either singly or multiply charged) into the LIT. The results for ion/ion reactions implemented in the transmission mode were comparable to those acquired using the more conventional mutual storage mode, both in terms of efficiency and information content of the spectra. While these methods were demonstrated here with a hybrid triple quadrupole/LIT instrument, they can, in principle, be used with any type of instrument that employs a quadrupole collision cell.
Acknowledgments
This work was sponsored by MDS SCIEX, an Industrial Associate of the Department of Chemistry, and the National Institutes of Health, Institute of General Medical Sciences under Grant GM 45372. We would like to acknowledge Dr. Frank A. Londry of MDS SCIEX, for helpful discussions and Dr. Min Yang of MDS SCIEX for providing custom instrument control software.
Footnotes
Prepared for as submission as JASMS
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLuckey SA, Stephenson JL., Jr Ion/ion chemistry of high-mass multiply charged ions. Mass Spectrom Rev. 1998;17:369–407. doi: 10.1002/(SICI)1098-2787(1998)17:6<369::AID-MAS1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.Pitteri SJ, McLuckey SA. Recent developments in the ion/ion chemistry of high-mass multiply charged ions. Mass Spectrom Rev. 2005;24:931–958. doi: 10.1002/mas.20048. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson JL, Jr, McLuckey SA. Ion/ion proton transfer reactions for protein mixture analysis. Anal Chem. 1996;68:4026–4032. doi: 10.1021/ac9605657. [DOI] [PubMed] [Google Scholar]
- 4.Stephenson JL, Jr, McLuckey SA. Simplification of product ion spectra derived from multiply charged parent ions via ion/ion chemistry. Anal Chem. 1998;70:3533–3544. doi: 10.1021/ac9802832. [DOI] [PubMed] [Google Scholar]
- 5.McLuckey SA, Reid GE, Wells JM. Ion parking during ion/ion reactions in electrodynamic ion traps. Anal Chem. 2002;74:336–346. doi: 10.1021/ac0109671. [DOI] [PubMed] [Google Scholar]
- 6.Chrisman PA, Pitteri SJ, McLuckey SA. Parallel ion parking of protein mixtures. Anal Chem. 2006;78:310–316. doi: 10.1021/ac0515778. [DOI] [PubMed] [Google Scholar]
- 7.Scalf M, Westphall MS, Smith LM. Charge reduction electrospray mass spectrometry. Anal Chem. 2000;72:52–60. doi: 10.1021/ac990878c. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz JC, Senko MW, Syka JE. A two-dimensional quadrupole ion trap mass spectrometer. J Am Soc Mass Spectrom. 2002;13:659–669. doi: 10.1016/S1044-0305(02)00384-7. [DOI] [PubMed] [Google Scholar]
- 9.Hager JW. A new linear ion trap mass spectrometer. Rapid Commun Mass Spectrom. 2002;16:512–526. doi: 10.1002/rcm.1020. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Hager JW, Xia Y, Londry FA, McLuckey SA. Positive ion transmission mode ion/ion reactions in a hybrid linear ion trap. Anal Chem. 2004;76:5006–5015. doi: 10.1021/ac049359m. [DOI] [PubMed] [Google Scholar]
- 11.Liang X, Hager JW, McLuckey SA. Transmission mode ion/ion electron transfer dissociation in a linear ion trap. Anal Chem. 2006 doi: 10.1021/ac062295q. [DOI] [PubMed] [Google Scholar]
- 12.Syka JE, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci USA. 2004;101:9528–9533. doi: 10.1073/pnas.0402700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia Y, Wu J, McLuckey SA, Londry FA, Hager JW. Mutual storage mode ion/ion reactions in a hybrid linear ion trap. J Am Soc Mass Spectrom. 2005;16:71–81. doi: 10.1016/j.jasms.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 14.He M, McLuckey SA. Two ion/ion charge inversion steps to form a doubly protonated peptide from a singly protonated peptide in the gas phase. J Am Chem Soc. 2003;125:7756–7757. doi: 10.1021/ja0354521. [DOI] [PubMed] [Google Scholar]
- 15.He M, McLuckey SA. Increasing the negative charge of a macroanion in the gas phase via sequential charge inversion reactions. Anal Chem. 2004;76:4189–4192. doi: 10.1021/ac496087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He M, Emory JF, McLuckey SA. Reagent anions for charge inversion of polypeptide/protein cations in the gas phase. Anal Chem. 2005;77:3173–3182. doi: 10.1021/ac0482312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells JM, Chrisman PA, McLuckey SA. Formation and characterization of protein-protein complexes in vacuo. J Am Chem Soc. 2003;125:7238–7249. doi: 10.1021/ja035051l. [DOI] [PubMed] [Google Scholar]
- 18.Gunawardena HP, McLuckey SA. Synthesis of multi-unit protein hetero-complexes in the gas phase via ion-ion chemistry. J Mass Spectrom. 2004;39:630–638. doi: 10.1002/jms.629. [DOI] [PubMed] [Google Scholar]
- 19.Xia Y, Liang X, McLuckey SA. Pulsed dual electrospray ionization for ion/ion reactions. J Am Soc Mass Spectrom. 2005;16:1750–1756. doi: 10.1016/j.jasms.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Thomson BA, Jolliffe CL. 5,847,386 US Patent.
- 21.Londry FA, Hager JW. Mass selective axial ion ejection from a linear quadrupole ion trap. J Am Soc Mass Spectrom. 2003;14:1130–1147. doi: 10.1016/S1044-0305(03)00446-X. [DOI] [PubMed] [Google Scholar]
- 22.Dawson PH. Quadrupole Mass Spectrometry and Its Applications. In: Dawson PH, editor. Quadrupole Mass Spectrometry and Its Applications. Woodbury, New York: American Institute of Physics; 1995. [Google Scholar]
- 23.Gunawardena HP, He M, Chrisman PA, Pitteri SJ, Hogan JM, Hodges BD, McLuckey SA. Electron transfer versus proton transfer in gas-phase ion/ion reactions of polyprotonated peptides. J Am Chem Soc. 2005;127:12627–12639. doi: 10.1021/ja0526057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunawardena HP, Emory JF, McLuckey SA. Phosphopeptide anion characterization via sequential charge inversion and electron-transfer dissociation. Anal Chem. 2006;78:3788–3793. doi: 10.1021/ac060164j. [DOI] [PMC free article] [PubMed] [Google Scholar]


