Abstract
Background
Pituitary and adrenal responsiveness is suppressed in abstinent alcohol-dependent individuals. To clarify the specific organizational disruption in hypothalamic-pituitary-adrenal functioning during early abstinence, the authors separately assessed each level of the stress-response axis. In this second of a two-part study, ovine corticotropin-releasing factor (oCRH) was used to stimulate the pituitary corticotrophs, and naloxone was used to activate the axis at the hypothalamic level. In addition, pulsatile characteristics of corticotropin and cortisol were assessed over a 12-hr period (0800 to 2000 hr).
Methods
Eleven abstinent alcohol-dependent men and 10 healthy comparison participants were assessed. All participants were between the ages of 30 and 50 years, and alcohol-dependent patients were abstinent from 4 to 6 weeks. Basal concentrations of corticotropin and cortisol were obtained every 10 min from 0800 to 2000 hr and subjected to pulsatile analysis. Plasma corticotropin and cortisol concentrations were then obtained every 5 to 10 min after low-dose, intravenously administered doses of oCRH (0.4 μg/kg) or naloxone (0.125 mg/kg). Medications were administered at 2000 hr and the two challenge studies were separated by 48 hr.
Results
Pulsatile analysis revealed that the mean corticotropin amplitude was increased in alcohol-dependent patients relative to controls (p < 0.05). Other pulsatile characteristics of corticotropin and all cortisol pulsatile measures were not significantly different between the two groups. The integrated cortisol response to oCRH was significantly lower in alcohol-dependent patients compared with controls (p < 0.01), but the integrated corticotropin response was not significantly different. In contrast, neither the corticotropin nor the cortisol response to naloxone was significantly different between groups.
Conclusions
Adrenocorticoid hyposensitivity persists after oCRH infusion for at least 1 month after cessation of drinking, whereas hyporesponsiveness of the pituitary corticotrophs to CRH seems to resolve with continued abstinence. The authors suggest that adrenocortical hyporesponsiveness during prolonged abstinence may impact relapse risk.
Keywords: Adrenal Cortex, Alcoholism, Corticotropin-Releasing Hormone, Naloxone, Pituitary-Adrenal System
Hypercortisolemia is experienced during both chronic alcohol ingestion (Adinoff et al., 2003; Mendelson and Stein, 1966; Stokes, 1973) and repeated bouts of alcohol withdrawal (Adinoff et al., 1991; Iranmanesh et al., 1989; Keedwell et al., 2001). The prolonged state of glucocorticoid excess in heavy, daily drinkers (Adinoff et al., 2003; Mendelson and Stein, 1966) seems to induce pituitary and adrenocortical hyporesponsiveness that persists after cessation of drinking. Pharmacologic interventions that restore equilibrium to the hypothalamic-pituitary-adrenal (HPA) axis may have potential in the treatment of alcohol dependence. Therefore, isolating the specific level of axis disruption, the degree of physiological hyposensitivity at each organ level, and the persistence of these alterations upon abstinence assumes increasing importance. Such an understanding would allow a targeted, dose-appropriate pharmacologic intervention upon the critical axis disruption during a temporally relevant period of biological vulnerability.
The HPA cascade begins with central activation of hypothalamic corticotropin-releasing hormone (CRH), primarily in response to fearful or anxiety-inducing stressors. CRH then induces the synthesis and secretion of corticotropin from the anterior pituitary gland, which in turn stimulates the production of glucocorticoids from the adrenal cortex. The glucocorticoids (primarily cortisol in humans) have a negative feedback effect on both hypothalamic CRH and pituitary corticotropin secretion.
As noted, the HPA axis exhibits diminished responsiveness during early abstinence. For example, cerebrospinal fluid measures of CRH are reduced in abstinent alcohol-dependent individuals (Geracioti et al., 1994), suggesting a decrease in CRH hypothalamic release. In response to naloxone, which blocks opioid tonic inhibition of CRH secretion, Inder et al. (1995) observed a blunted corticotropin response in alcohol-dependent men who were 10 days to 6 weeks abstinent. This report suggested a down-regulation of hypothalamic CRH and/or pituitary corticotroph receptors. A number of investigators (Adinoff et al., 1990; Bardeleben et al., 1989; Costa et al., 1996; Ehrenreich et al., 1997; Loosen et al., 1991) have reported a similar blunting of the corticotropin response to ovine corticotropin-releasing hormone (oCRH), which directly stimulates pituitary corticotrophs. Adinoff et al. (1990) demonstrated that the corticotropin suppression seems to gradually resolve after 3 weeks of abstinence, although others have shown that this response persists for at least 12 weeks of abstinence (Ehrenreich et al., 1997). The pituitary corticotropin response has also been shown to be suppressed after nicotine (Coiro and Vescovi, 1999), hyperthermia (Vescovi et al., 1997), and insulin-induced hypoglycemia (Costa et al., 1996). Downstream adrenocortical secretion is suppressed after an alcohol challenge (Merry and Marks, 1972), insulin-induced hypoglycemia (Costa et al., 1996), operative trauma (Margraf et al., 1967), nicotine (Coiro and Vescovi, 1999), CRH stimulation (Bailly et al., 1989), cosyntropin stimulation (Wand and Dobs, 1991), hyperthermia (Vescovi et al., 1997), cold pressor (Errico et al., 1993), mental arithmetic (Errico et al., 1993), and public speaking (Lovallo et al., 2000) (for review, see Adinoff et al., 1998).
These cumulative studies implicate a similarly muted response at each level of the HPA axis for several days, if not weeks, after cessation of drinking in alcohol-dependent individuals. The relative consistency of this literature, however, does not define the relative contribution of each axis component. Previous studies, for example, have typically used only a single provocative measure of HPA axis reactivity, limiting concurrent assessments of the hierarchical levels of HPA axis functioning. Hypothalamic, pituitary, and adrenal sensitivity to both exogenous and endogenous stimulation also remains uncertain because high doses of biological stimuli have typically been used to assess the system. Such severe activation may produce a ceiling response, particularly of the adrenocortical response, thus masking more subtle disruptions. In addition, the resolution or persistence of axis disruption with more extended periods of abstinence remains uncertain. Most studies have assessed HPA axis functioning in a patient cohort exhibiting varied abstinence intervals, leaving the time frame of HPA persistence or recovery unanswered. Finally, many studies of HPA axis responsiveness include patients with psychiatric and/or other substance use disorders. Because disruptions in HPA axis alterations have been documented in other substance use disorders (for review, see Kreek and Koob, 1998; Sinha, 2001) and psychiatric disorders (Gold and Chrousos, 2002; Yehuda, 2001), it is critical to separate out the pathophysiology induced by chronic alcohol ingestion as opposed to concomitant disease processes.
In a series of studies, we assessed hypothalamic, pituitary, and adrenal responsiveness in 1-month alcohol-dependent men. Because of restrictions on the volume of blood withdrawal and the importance of obtaining all studies during a similar period of abstinence, two distinct groups of alcohol-dependent participants were compared with healthy control participants. In part 1 of the study (Adinoff et al., 2005), corticotropin and cortisol pulsatile characteristics were obtained from 2000 to 0800 hr, adrenocortical sensitivity was assessed in both the presence and the relative absence of endogenous pituitary corticotropin, and the pituitary corticotropin response to dexamethasone inhibition was obtained. In the current study (part 2), hypothalamic, pituitary, and adrenal activation was assessed with low doses of both oCRH and naloxone. In addition, corticotropin and cortisol pulsatility were determined from 0800 to 2000 hr. We hypothesized (1) that the net integrated corticotropin and cortisol responses to oCRH stimulation would be suppressed in the alcohol-dependent group compared with the healthy controls, indicating decreased pituitary responsiveness and (2) that the net integrated corticotropin and cortisol responses to naloxone stimulation would also be suppressed in the alcohol-dependent population compared with healthy controls, reflecting altered endogenous opioid activity at the hypothalamic level.
MATERIALS AND METHODS
Alcohol-Dependent Patients
Eleven male alcohol-dependent participants (aged 43.5 ± 4.8 years; range, 36–49 years) were recruited from patients requesting treatment for alcohol dependence at the Dallas VA Medical Center. Eight whites and three African-Americans participated. Because HPA axis functioning is altered with increasing age (Anton, 1987; Asnis et al., 1981; Davis et al., 1984), all participants were aged between 30 and 50 years. Patients reported an alcohol intake of at least 80 g of absolute alcohol (approximately one six-pack of beer or one half pint of 100% proof distilled spirits) on a daily basis for at least 2 weeks before the cessation of drinking and had at least a 10-year history of problematic drinking. To avoid the potential interaction of other disorders that may also disrupt HPA axis functioning, patients with a lifetime history of other DSM-IV (American Psychiatric Association, 1994) axis I psychiatric disorders (such as anxiety, post–traumatic stress disorder, or mood disorders) not associated with alcohol use, other substance use disorders within the previous 12 months (excluding caffeine or nicotine use disorders), medical disorders (i.e., hypertension, diabetes, chronic pain, or cardiac or pulmonary disorders), or a lifetime history of major head trauma were excluded from the study. Patients reporting at least weekly drug use were also excluded, even in the absence of a substance abuse or dependence diagnosis. In addition, patients taking medications that may interfere with HPA axis functioning (i.e., psychotropics, antihypertensives, hypoglycemic agents) were excluded. Patients with Beck Depression Inventory (BDI; Beck et al., 1979) scores above 15 the week before testing were also excluded, as were patients with alanine aminotransferase (ALT) or aspartate aminotransferase (AST) 3.0 times greater than the clinical laboratory’s upper limit of normal.
Healthy Controls
Ten healthy men (aged 38.2 ± 5.4 years; range, 30–47 years), including eight whites, one Hispanic, and one Asian, were studied. Controls reported no lifetime history of any DSM-IV axis I disorder (except caffeine or nicotine use disorders), reported no medical disorders, and were taking no medications. To avoid possible effects of family history of substance abuse or other psychiatric disorders on HPA axis functioning, controls with a single first-degree relative or two second-degree relatives with an axis I disorder were excluded.
Clinical Assessment
All participants underwent a history and physical examination, clinical laboratory testing (including complete blood count, liver function tests, routine blood chemistries, thyroid-stimulating hormone test, and HIV test), electrocardiography, and urine drug screening. Psychiatric and substance use disorders were assessed using the SCID-Lifetime (First et al., 1996). Alcohol-dependent patients were detoxified from alcohol on a locked psychiatric unit using diazepam and were then transferred to a residential treatment unit. Patients remained in the residential setting until the studies were initiated between approximately 4 to 5 weeks of abstinence. To assure abstinence, urine drug screening results were obtained three times weekly and Breathalyzer test results were obtained whenever the patient left the unit unaccompanied by staff. The study was approved by the Institutional Review Boards at both the University of Texas Southwestern Medical Center and the Dallas VA Medical Center. Informed consent was obtained after the study was fully explained, and participants were financially compensated for their participation. The Drinker Inventory of Consequences-Lifetime Consequences (DrInC-2L) (Miller et al., 1995) was used to assess lifetime severity of alcohol-related problems. A timeline follow back (Sobell and Sobell, 1978) was used to assess 1-month, 1-year, and lifetime drinking history.
Neuroendocrine Challenge Tests
Both neuroendocrine studies were performed at the General Clinical Research Center at the University of Texas Southwestern Medical Center. Both healthy control and alcohol-dependent participants arrived at the GCRC at least several hours before the studies. Alcohol-dependent patients were accompanied to the GCRC from their treatment site.
Pulsatile Characteristics of Corticotropin and Cortisol
Participants were admitted to the GCRC at 1800 hr on day 1. An intravenous catheter was inserted in each arm at 0700 hr on day 2. Blood sampling was initiated for pulsatile measures of corticotropin and cortisol 1 hr later (at 0800 hr) and continued through 2000 hr.
Ovine Corticotropin-Releasing Factor Stimulation Test
After the collection of the final basal measure of corticotropin and cortisol at 2000 hr, 0.4 μg/kg oCRH was administered intravenously over 1 min. Blood draws for corticotropin and cortisol were obtained every 5 min from 2000 to 2100 hr and every 10 min from 2100 to 2200 hr. Intravenous catheters were removed after the 2200 hr blood draws. Cortisol binding globulin (CBG) concentrations were also obtained at 2000 hr, just before oCRH administration. Alcohol-dependent patients were returned to their treatment site the next morning, and comparison participants left the GCRC that evening or the next morning. oCRH was administered at 2000 hr to coincide with the circadian trough to minimize the effect of basal hormonal concentrations.
Naloxone Stimulation Test
Participants returned to the GCRC by 1700 hr 2 days after the completion of the oCRH stimulation test. An intravenous catheter was placed in both arms at approximately 1800 hr on the day of the study. Basal measures of corticotropin and cortisol were obtained from 1900 to 2000 hr. Naloxone, 0.125 μg/kg, was administered intravenously over 1 min immediately after the 2000 hr blood draw. As described for the oCRH stimulation test, blood draws for corticotropin and cortisol were then obtained every 5 min from 2000 to 2100 hr and every 10 min from 2100 to 2200 hr. Intravenous catheters were removed after the 2200 hr blood draws. One patient did not return to the GCRC for the naloxone stimulation test after completion of the oCRH stimulation test.
Assays
Plasma concentrations of corticotropin were measured by immunoradiometric assay (IRMA), using reagents from DiaSorin (Stillwater, MN). This assay has a low-end sensitivity of 1.5 pg/ml, with an intra-assay coefficient of variation of 2.5–5.4% in the concentration range of 33 to 773 pg/ml. The interassay coefficient of variation is 3.2–5.7% in the concentration range of 8.7 to 257 pg/ml. Serum concentrations of cortisol were measured by radioimmunoassay (RIA) using reagents from DiaSorin. This assay has a low-end sensitivity of 0.21 μg/dl, with an intra-assay coefficient of variation of 6.6–7.7% in the concentration range of 2.9 to 47.1 μg/dl. The interassay coefficient of variation is 8.8–9.8% in the concentration range of 3.7 to 36.9 μg/dl. Serum concentrations of CBG were assayed by RIA using reagents from BioSource Europe S.A. This assay has a low-end sensitivity of 0.25 μg/ml, with an intra-assay coefficient of variation of 3.3 to 7.7 in the concentration range of 33 to 109 μg/ml. The interassay coefficient of variation is 4.5–5.4% in the concentration range of 32 to 105 μg/ml.
Statistics
Demographics
Student’s t test (interval data) and χ2 contingency table analyses (nominal data) were used to compare demographic characteristics of the two groups. Items of interest included age, education, employment status, housing status, marital status, smoking status, and liver function. Descriptive statistics were used to quantify drinking characteristics of the alcohol dependent group, including years of problem drinking, drinking days (previous 30 days and lifetime), standard drinks (previous 30 days and lifetime), and days abstinent at the time of testing. Student’s t test was used to compare mean scores on the BDI and DrInC-2L.
Basal Corticotropin and Cortisol Pulsatile Characteristics
Because there is a circadian rhythm for the two hormones of interest, the assumption that all secretion was pulsatile leads to unreasonably long half-lives for corticotropin and cortisol, resulting in a poor fit of the calculated pulses to the actual data. Therefore, pulsatile characteristics were assessed by the Smoothing Baseline Pulse Pulses (SBPP) algorithm (Guo et al., 1999), which allows for a changing baseline. In general, missing values were not an issue in these data, but equally spaced observations are required for analysis in pulse-detection algorithms. Missing values were handled in the following way. If the concentration at the time point preceding the missing time point was lower than at the point after the missing time point, the missing concentration was replaced with the concentration value of the point preceding the missing time. This was done to avoid creating the possibility of a pseudopulse. Missing values that were part of a decreasing trend were linearly interpolated. For various reasons, some series had to be excluded from the secondary analysis, mainly because they did not show pulsatility. Pulsatile analysis was completed on seven alcohol-dependent patients and six controls for corticotropin analyses, and eight patients and seven controls for the cortisol analyses. Student’s t test was used to compare group means. A brief description of the summary measures from SBPP is given below.
Total probability
The sum of the probabilities of there being a pulse at each point.
Adjusted input
The sum of input at each time point times the probability of input at each point. It is a weighted total input.
Number of inputs
The number of input locations with a probability greater than 0.5. SBPP models the probability of input at each time point, and we used the 0.5 cutoff to distinguish large input (where input is most likely occurring) from small input (unlikely) locations. This measure allows the comparison of input frequencies between the two groups but is not a measure of pulse number.
Average baseline
The mean of the baseline values estimated at each point. SBPP allows for a changing baseline, so baseline itself is no longer a parameter.
Mean amplitude
The net mean height of pulses after subtracting the changing baseline.
Time × Group Analysis
Repeated-measures ANOVA on the mean hormone level over four 3-hr blocks (i.e., 0800–1050, 1100–1350, 1400–1650, and 1700–2000 hr) was performed to incorporate all participants into the circadian analysis. Because the distribution of data was skewed, each block mean of both corticotropin and cortisol was log (base-e) transformed before analysis.
Pharmacologic Challenge Studies
Corticotropin and cortisol secretion were compared between 2000 and 2200 hr by the area-under-the-curve method, or net integrated response, and by net peak corticotropin and cortisol for both the oCRF and the naloxone challenge. Because the net integrated response was similar to the net peak response, only the former is reported. The net integrated response was calculated by taking the average hormone concentration between consecutive measurement points, multiplying by the time interval between the points, summing across time, and netting out basal hormone levels multiplied by the total time interval over which measurement occurred (i.e., 120 min). For each of these analyses, Student’s t test was used to compare group means. Missing values (10 total) were estimated based on the mean of hormone measures taken directly before and after the missing values.
Correlation Analysis
The relation between drinking history (i.e., drinks in previous 30 days, lifetime drinks) and various neuroendocrine measures (basal cortisol and corticotropin concentrations before oCRH, integrated cortisol and corticotropin response after oCRH, and integrated cortisol and corticotropin response after naloxone) was assessed via Pearson correlational analysis.
RESULTS
Demographics
The 11 alcohol-dependent patients were more likely to be unemployed (p < 0.02), had fewer years of education (p < 0.001), and were more likely to be smokers (p < 0.001) relative to the healthy controls. Although the alcohol-dependent patients were a few years older than the controls (p < 0.05), mean ages were less than 5 years apart, and all participants were between the ages of 30 and 50 years. The patient group also had higher BDI (p < 0.001) scores at the time of the first study compared with controls. Alcohol-dependent patients reported an onset of drinking problems at 17.6 ± 3.0 years (range, 12–23 years) and had been drinking an average of 23.9 ± 5.4 years (range, 17–34 years). These patients reported drinking 26.9 ± 4.4 days (range, 19–30 days) in the 30 days before drinking cessation and drank 637 ± 331 standard drinks (range, 154–1234 standard drinks) of alcohol over that period (one standard drink = 1 oz spirits, 4 oz wine, or 12 oz beer). Alcohol-dependent patients were abstinent 29.3 ± 3.3 days (range, 24–35 days) at the time of the study. Total DrInC-2L (Miller et al., 1995) scores in the alcohol-dependent group (34.1 ± 10.7) were significantly higher (p < 0.001) than those of controls (3.1 ± 2.8). Despite their extensive drinking history, liver enzymes (ALT and AST) were not significantly different between alcohol-dependent and control groups, and levels of ALT and AST were not markedly higher than the clinical laboratory range for healthy individuals (ALT, 0–40 units/liter; AST, 0–37 units/liter).
Secretory Characteristics of Corticotropin and Cortisol
Secretory characteristics were determined by SBPP, an algorithm to assess pulsatility that allows for a changing baseline. Corticotropin mean amplitude (net mean height of pulses after subtracting the changing baseline) was significantly (p < 0.05) higher in alcohol-dependent (8.5 ± 5.7 pg/ml) compared with healthy control participants (2.9 ± 1.6 pg/ml). Therefore, corticotropin mean amplitude in the alcohol-dependent group was almost 3-fold greater than that of the controls. There was also a trend toward an increase in the average baseline (the mean of the baseline value estimated at each point) in the alcohol-dependent patients relative to the controls (p < 0.08). There were no other significant differences in corticotropin pulsatile characteristics between groups, and there were no differences in any of the cortisol measures. Time × group ANOVA using all participants (10 in each group) found that corticotropin concentrations tended to be higher in alcohol-dependent patients compared with controls over the 12-hr period (0800–2000 hr; p = 0.10), although the comparisons did not reach statistical significance. There was no significant group effect of cortisol concentrations (p = 0.072) or a group × time effect for either hormone. Both corticotropin and cortisol had a significant within-group time effect (p < 0.0001). (See Table 2 and Fig. 1.)
Table 2.
Corticotropin and Cortisol Pulsatile Characteristics as Determined by SBPP analysis and mean Corticotropin and Cortisol Concentrations Over 12 hr (0800–2000 hr) and in 3-hr Blocks
| Healthy Controls | Alcohol Dependent | t stat | df | p Value | |
|---|---|---|---|---|---|
| Corticotropin pulsatile characteristics | |||||
| Total probability | 17.1 ± 9.1 | 11.8 ± 9.3 | 1.04 | 11 | NS |
| Adjusted input | 50.8 ± 21.1 | 66.4 ± 50.0 | 0.7 | 11 | NS |
| Number of Inputs | 13.8 ± 10.6 | 8.3 ± 7.8 | 1.09 | 11 | NS |
| Average baseline | 18.5 ± 3.9 | 29.3 ± 12.9 | 1.97 | 11 | 0.075 |
| Mean amplitude | 2.9 ± 1.6 | 8.5 ± 5.7 | 2.29 | 11 | 0.043 |
| Half-life | 21.4 ± 7.1 | 42.1 ± 28.5 | 1.72 | 11 | NS |
| Mean corticotropin concentrations (pg/ml) | |||||
| 0800–2000 hr (total 12 hr) | 27.3 ± 8.6 | 35.4 ± 12.5 | 1.72 | 19 | NS |
| 0800–1050 hr | 29.8 ± 10.2 | 42.2 ± 16.7 | 2.03 | 19 | 0.057 |
| 1100–1350 hr | 28.1 ± 9.7 | 37.3 ± 14.4 | 1.69 | 19 | NS |
| 1400–1650 hr | 28.8 ± 8.3 | 37.7 ± 14.0 | 1.75 | 19 | 0.096 |
| 1700–2000 hr | 23.9 ± 7.2 | 28.3 ± 8.9 | 1.23 | 19 | NS |
| Cortisol pulsatile characteristics | |||||
| Total probabilitya | 19.2 ± 7.5 | 16.3 ± 7.7 | 0.71 | 13 | NS |
| Adjusted inputb | 35.3 ± 12.3 | 23.9 ± 10.5 | 1.9 | 13 | NS |
| Number of inputsc | 16.4 ± 6.1 | 13.1 ± 7.4 | 0.9 | 13 | NS |
| Average baselined | 4.4 ± 2.2 | 4.1 ± 0.7 | 0.3 | 13 | NS |
| Mean amplitudee | 2.0 ± 1.1 | 1.7 ± 0.9 | 0.7 | 13 | NS |
| Half-life | 36.3 ± 16.1 | 44.3 ± 18.3 | 0.9 | 13 | NS |
| Mean cortisol concentrations (μg/dl) | |||||
| 0800–2000 hr (total 12 hr) | 7.7 ± 1.6 | 7.3 ± 2.5 | 0.36 | 19 | NS |
| 0800–1050 hr | 10.7 ± 2.2 | 10.1 ± 3.4 | 0.48 | 19 | NS |
| 1100–1350 hr | 7.9 ± 1.7 | 7.6 ± 2.8 | 0.26 | 19 | NS |
| 1400–1650 hr | 7.5 ± 1.9 | 6.9 ± 2.4 | 0.65 | 19 | NS |
| 1700–2000 hr | 5.9 ± 2.0 | 5.7 ± 2.7 | 0.13 | 19 | NS |
See text for description of SBPP measures.
Fig. 1.
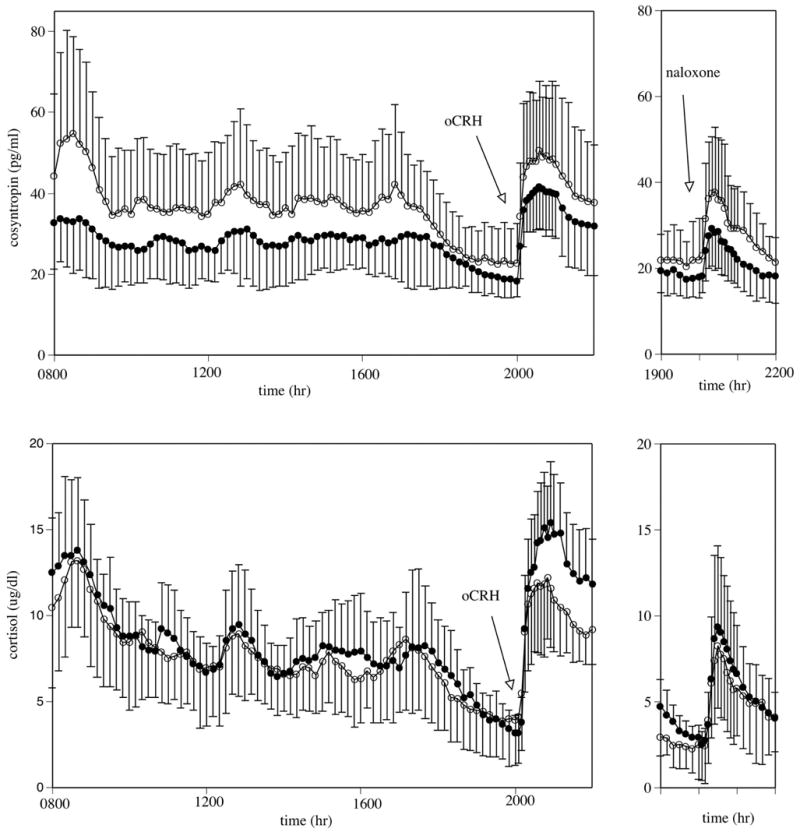
Mean ± SD of corticotropin (cosyntropin; top) and cortisol (bottom) concentrations. Left panels: Measures between 2000 and 0800 hr reflect basal concentrations; oCRH (0.4 μg/kg iv) was administered at 2000 hr. The integrated cortisol response to oCRH was significantly lower in the alcohol-dependent participants compared with the healthy controls (p < 0.02). Right panels: Naloxone (125 μg/kg iv) was administered at 2000, 48 hr after oCRH administration. Closed circles represent healthy control participants; open circles represent alcohol-dependent participants.
Ovine Corticotropin-Releasing Factor Stimulation Test
Mean basal corticotropin and cortisol concentrations (1930 to 2000 hr) were not significantly different between abstinent alcohol-dependent patients and healthy controls. CBG concentrations in the alcohol-dependent and healthy control men were also similar between the two groups at 2000 hr, as was the calculated free cortisol index (le Roux et al., 2002; serum cortisol in μM/CBG in μM). Alcohol-dependent patients had a significantly lower integrated cortisol response to oCRH stimulation compared with healthy controls (p < 0.01), whereas the integrated corticotropin response to oCRH stimulation was similar in the two groups. The net integrated corticotropin response to net integrated cortisol response (i.e., the ratio of net integrated corticotropin response/net integrated cortisol response) was 87% greater in the alcohol-dependent patients (3.91 ± 2.70) compared with the controls (2.09 ± 0.96). This finding suggests that for each unit of corticotropin secreted after oCRH stimulation, controls responded with almost twice as much cortisol as did the patient group. Although this difference did not reach statistical significance [t(19) = 2.01, p < 0.06], the percent increase in net peak corticotropin to percent increase in net peak cortisol in alcohol-dependent patients (67.4 ± 22.6%) was significantly increased relative to the controls (51.3 ± 8.4%) [t(19) = 2.20, p < 0.05]. (See Table 3 and Fig. 1.)
Table 3.
Plasma Basal Concentrations and Stimulated Corticotropin and Cortisol Response to oCRH (0.4 μg/kg) and Naloxone (125 μg/kg) in 1-Month-Abstinent Male Alcohol-Dependent Participants and Age-Matched Healthy Controls
| oCRH Stimulation Test
|
Naloxone Stimulation Test
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Alcohol Dependent | Controls | t (19) | p | Alcohol Dependent | Controls | t (18) | p | |
| Basal corticotropin (pg/ml) (1930–2000 hr) | 23.0 ± 8.9 | 18.9 ± 4.5 | 1.31 | NS | 21.4 ± 7.8 | 17.8 ± 4.6 | 1.26 | NS |
| Basal cortisol (μg/dl) (1930–2000 hr) | 3.9 ± 2.4 | 3.6 ± 1.2 | 0.42 | NS | 2.4 ± 1.5 | 3.1 ± 0.7 | 1.27 | NS |
| Cortisol binding globulin (μg/ml) | 33.0 ± 7.2 | 36.3 ± 6.3 | 1.10 | NS | 32.6 ± 7.5 | 36.3 ± 6.3 | 1.16 | NS |
| Free cortisol index | 0.17 ± 0.09 | 0.14 ± 0.03 | 1.03 | NS | 0.11 ± 0.06 | 0.12 ± 0.03 | 0.80 | NS |
| Net integrated corticotropin response (pg/ml.2 hr) | 2518 ± 1242 | 2051 ± 801 | 1.01 | NS | 878 ± 528 | 538 ± 392 | 1.64 | NS |
| Net integrated cortisol response (μg/dl.2 hr) | 715 ± 261 | 1027 ± 235 | 2.86 | 0.01 | 346 ± 305 | 326 ± 271 | 0.15 | NS |
Naloxone Stimulation Test
As observed before oCRH infusion, mean basal concentrations of corticotropin and cortisol (1930 to 2000 hr) were not significantly different between the alcohol-dependent and healthy controls before naloxone administration. Mean basal calculated free cortisol index, using CBG measures obtained before the oCRH infusion, were also not significantly different between the two groups. There was not a significant difference in the net integrated corticotropin or cortisol response to naloxone stimulation between the two study groups. There were no significant group differences in corticotropin response to cortisol response after naloxone. (See Table 3 and Fig. 1.)
Correlation Between Neuroendocrine and Clinical Variables
We assessed the relation between relevant neuroendocrine measures (e.g., integrated cortisol response to oCRH and the corticotropin response to cortisol response after both oCRH and naloxone) and measures of previous drinking history (e.g., number of drinks in 30 days before abstinence, 1 year before abstinence, and lifetime drinking). There were no significant correlations between any of the plasma hormone responses and drinking variables.
DISCUSSION
Our findings reveal that 1-month-abstinent alcohol-dependents exhibit (1) an increase in the corticotropin amplitude and average baseline by SBPP but similar cortisol pulsatile characteristics and (2) a significantly blunted glucocorticoid response after oCRH stimulation in the presence of an corticotropin response not significantly different from that of the control group. These findings support a decrease in glucocorticoid responsiveness relative to corticotropin release in abstinent alcohol-dependent patients. In contrast, the corticotropin and cortisol responses to naloxone did not significantly differ between the two groups.
Alcohol-dependent patients revealed a relative increase in 0800 to 2000 hr corticotropin amplitude and baseline concentrations (by SPBB) and mean plasma concentrations in the presence of cortisol pulsatile characteristic and plasma concentrations within the control range. In part 1 of this study (Adinoff et al., 2005), we reported that cortisol amplitude and mean concentrations were decreased in alcohol-dependent patients compared with controls from 2000 to 0800 hr, whereas corticotropin measures were similar. Both sets of findings suggest a decrease in cortisol responsiveness relative to corticotropin stimulation. Although two different alcohol-dependent patient samples were used for parts 1 and 2, these two groups were similar in age [t(20) = 0.34, p = 0.74], years of alcohol problems [t(20) = 1.30, p = 0.21], drinks over the previous 30 days [t(19) = 1.03, p = 0.31], and DrInC scores [t(19) = 0.91, p = 0.37]. Six controls were used for both studies (after at least a 6-week interval between parts 1 and 2). In addition, the leading and terminal points (0800 and 2000 hr) of the two 12-hr measures were in relatively close proximity.
Unlike the current study, Adinoff et al. (1991), Iranmanesh et al. (1989), and Loosen et al. (1991) did not observe differences in the pulsatile characteristics of corticotropin or cortisol during abstinence in alcohol-dependent patients compared with controls. The SBPP modeling technique may have uncovered group differences in corticotropin amplitude not observed by others. The careful selection of our patient population, including the exclusion of patients with a lifetime history of other psychiatric disorders and/or a recent history of drug dependence and the specific 1-month time frame of abstinence, may also explain our findings. However, our patient data showed a fair amount of variability, and comparisons met only relatively low levels of statistical significance or trend levels.
Previous studies of the pituitary responsiveness in alcohol-dependent participants have consistently demonstrated a diminished response to various physical and psychological stressors. The specific time after abstinence, however, seems critical in understanding this literature. For example, Adinoff et al. (1990) assessed corticotropin and glucocorticoid responsiveness to 1 μg/kg oCRH in alcohol-dependent men at 1 week, 3 weeks, 3 weeks to 6 months, and more than 6 months of abstinence. A marked suppression of the corticotropin response was observed at 1 week of abstinence compared with controls. Alcohol-dependent participants at 3 weeks of abstinence showed a significant increase in their corticotropin response compared with 1 week, nevertheless still significantly less than controls. The group that was abstinent for more than 3 weeks to 6 months revealed a corticotropin response similar to that of controls. This was similar to the work of Costa et al. (1996), who showed a blunted corticotropin and cortisol response to oCRH in 1-week to 2-weeks-abstinent subjects. Other investigators who showed a blunted corticotropin response to oCRH or human CRH studied subjects with varied periods of abstinence (Inder et al., 1995: 10 days to 6 weeks; Loosen et al., 1993: 15 to 39 days; Bardeleben et al., 1989: 2 to 6 weeks), thus possibly mixing early abstinent (e.g., 3 weeks or fewer) corticotropin-suppressed subjects with later abstinent (e.g., more than 3 weeks) corticotropin-normalized subjects. In confirmation, Bailly et al. (1989) did not describe a blunted corticotropin response in alcohol-dependent subjects assessed at 4 weeks of abstinence, although a blunted cortisol response was observed. However, the mean abstinent periods in the studies of Inder et al. (1995) (25 ± 3 days) and Loosen et al. (1993) (42.9 ± 25.7 days) were similar to ours (29.6 ± 1.0 days), and Ehrenreich et al. (1997) showed a blunted corticotropin response to oCRH in 12-weeks-abstinent subjects. Other differences between our study and previous studies of the HPA axis in alcohol-dependent subjects include our rigorous exclusion criteria, including any other substance use disorders in the previous year (other than nicotine) and a lifetime history of axis I disorders. To our knowledge, the other studies referenced did not incorporate such stringent diagnostic criteria.
In our study, the absence of a decreased pituitary corticotropin response to oCRH in alcohol-dependent participants may also be related to the relatively low dose of CRH administered. The lower dose of CRH was used to avoid a ceiling glucocorticoid response to oCRH, thus potentially masking diminished adrenocortical reactivity in the alcohol-dependent group. Therefore, the current study administered an oCRH bolus of 0.4 μg/kg, compared with 1.0 μg/kg in Adinoff et al. (1990), Costa et al. (1996), Inder et al. (1995), and Loosen et al. (1993); 100 μg in Bardeleben et al. (1989), and 150 μg in Ehrenreich et al. (1997). The higher oCRH doses initially provoke a larger corticotropin and cortisol response, presumably inducing a more robust glucocorticoid inhibitory effect on the pituitary corticotrophs and suppressing further corticotropin release. Such a mechanism of diminished corticotropin responsivity has been posited in depression (Gold and Chrousos, 2002), where a heightened adrenal response is thought to quickly suppress pituitary corticotropin production and release. We have recently observed that 1-month-abstinent alcohol-dependent patients exhibit increased corticotropin suppression after high-dose dexamethasone (Adinoff et al., 2005), suggesting increased sensitivity of the pituitary glucocorticoid receptors. A less robust adrenocorticoid response to the lower dose of oCRH (0.4 μg/kg) in our patient population may therefore not have reached levels necessary to suppress pituitary secretion. In fact, as observed in Fig. 1, the corticotropin response in alcohol-dependent patients tended to be greater (not smaller) than in controls. However, this finding did not reach significance, perhaps because of the small sample size and substantial variance.
The administration of naloxone also did not induce an attenuated pituitary response in alcohol-dependent patients, as previously reported by Inder et al. (1995). Again, a more variable length of abstinence, a higher dose of naloxone (20 mg), and/or less restrictive selection criteria (including the ongoing use of disulfiram in the patient population) of Inder et al. (1995) may account for these differences. Although the diminished plasma cortisol response to oCRH in alcohol-dependent patients would also be expected after the naloxone challenge 2 days later, we did not observe a blunted adrenocorticoid response to naloxone. It seems that the absence of this finding may have been due to the somewhat higher corticotropin response to naloxone in the alcohol-dependent patients (Fig. 1). However, differences in the corticotropin response did not reach significance between groups, possibly because of the small sample size and variance in the data. The heightened corticotropin response to naloxone may reflect premorbid differences, as Wand et al. (2001) has reported that the corticotropin response to naloxone is increased in the children of alcohol-dependent individuals. Also unexpected was the absence of a significant correlation between the net integrated corticotropin response/net integrated cortisol response to oCRH relative to the net integrated corticotropin response/net integrated cortisol response to naloxone (r =−0.25, p = 0.289). Although the preceding oCRH stimulation test may have altered the subsequent naloxone stimulation test, the 48-hr intervening interval would be expected to minimize any carryover effect. Unfortunately, we did not counterbalance the oCRH and naloxone infusions.
The naloxone and oCRH challenge studies presented here were accompanied by a series of other basal and stimulated assessments of HPA axis reactivity in alcohol-dependent subjects (Adinoff et al., 2005). These studies revealed significantly attenuated basal mean concentrations and pulsatile amplitude of plasma cortisol from 2000 to 0800 hr, a blunted glucocorticoid response to low-dose cosyntropin after dexamethasone pretreatment, and a heightened pituitary response to dexamethasone negative feedback. In toto and in context with other studies referenced above, these studies suggest the following scenario: Central activation of the HPA axis by chronic drinking and repeated withdrawal states induce a prolonged state of hypercortisolemia. The HPA axis adapts to the persistently increased plasma cortisol concentrations by down-regulating the hypothalamic, pituitary, and adrenal responses. At 4 weeks of abstinence, increased sensitivity of the pituitary corticotrophs to glucocorticoid negative feedback and diminished adrenocortical reactivity persists, accompanied by normalization of the hypothalamic and pituitary response to higher-level stimulation.
Our design had several notable strengths. Patients and controls were similar in age and race. Although ages were significantly different between the two groups, the mean ages were within 5 years of each other and were all within an age range during which HPA axis functioning remains stable. Neither group had significant medical disorders and were not taking any psychotropic or other medications that are known to alter HPA axis functioning. Alcohol-dependent patients were assessed within a relatively constricted period of abstinence, had no lifetime history of other non–substance-related psychiatric disorders, had no other substance use disorders in the previous year or frequent drug use not meeting DSM-IV criteria (except nicotine dependence), and reported extensive drinking histories. Controls had no personal or family history of substance use disorders (except nicotine) and reported no lifetime history of other axis I disorders. These findings may not extend to alcohol-dependent females. In addition, alcohol-dependent patients were smokers, and controls were not. However, a previous study has reported that cigarette smoking in abstinent alcohol-dependent subjects did not alter the pituitary-adrenal response to a pharmacological challenge (Anthenelli et al., 2001).
Raison and Miller (2003) have posited that “hypoadrenal states” may be due to decreased glucocorticoid bioavailability, attenuated glucocorticoid responsiveness, and/or accentuated inhibitory feedback on the pituitary corticotrophs. Such hypoadrenal states have been observed in atypical depression, chronic fatigue, post–traumatic stress disorder, and fibromyalgia (see reviews in Heim et al., 2000a; Raison and Miller, 2003) Although the clinical relevance of the hypoadrenal states remains speculative, decreased concentrations of glucocorticoids are associated with early trauma, depressive mood states, dysphoric arousal, and behavioral dyscontrol and inhibition (Heim et al., 2000b; King et al., 1990; Tennes and Kreye, 1985; Vanyukov et al., 1993; Virkkunen, 1985; Yehuda, 2001). With respect to substance use disorder, glucocorticoid administration increases drug intake in animal models (Goeders, 2002; Piazza et al., 1993), possibly through activation of mesolimbic dopaminergic neurotransmission (Barrot et al., 2000; Piazza et al., 1996). Conversely, Nash and Maickel (1988) have demonstrated that the unrestrained increase (e.g., absence of glucocorticoids) of hypothalamic CRH and pituitary corticotropin release after stress is causally related to increased drinking, and the increased drinking behavior was suppressed by the administration of exogenous glucocorticoids. Human studies demonstrate that inhibition of glucocorticoid synthesis in methadone-maintained patients is associated with the increased use of cocaine (Kosten et al., 2002), and Kiefer et al. (2002) have presented preliminary data suggesting a relation between low basal concentrations of glucocorticoids and early relapse. The positive relation between HPA axis disinhibition by naltrexone and craving in alcohol-dependent subjects (O’Malley et al., 2002) suggests that glucocorticoid compensation in abstinent subjects may have therapeutic relevance.
Our findings offer further evidence for the persistent attenuation of adrenocortical reactivity in alcohol-dependent men. The diminished response is also sensitive to low levels of stimulation and is present for at least 1 month after cessation of drinking. Future studies should explore whether glucocorticoid hyposensitivity response persists with even longer periods of abstinence and confirm whether this disruption in neuroendocrine functioning is associated with a heightened relapse risk.
Table 1.
Demographic and Clinical Characteristics of Alcohol-Dependent Participants and Healthy Controls
| Alcohol Dependent (n = 11) | Controls (n = 10) | t or χ2 | dF | p Value | |
|---|---|---|---|---|---|
| Age (years) | 43.45 ± 4.80 | 38.20 ± 5.4 | 2.358 | 19 | 0.029 |
| Race | 4.96 | 3 | NS | ||
| Asian | 0 | 1 | |||
| White | 8 | 8 | |||
| African-American | 3 | 0 | |||
| Hispanic | 0 | 1 | |||
| Marital status | 14.16 | 3 | 0.003 | ||
| Single | 1 | 0 | |||
| Married | 0 | 5 | |||
| Separated | 1 | 0 | |||
| Divorced | 9 | 1 | |||
| Employed | 6 | 10 | 5.97 | 1 | 0.015 |
| Education (years) | 12.3 ± 1.7 | 16.9 ± 2.8 | 4.52 | 18 | 0.000 |
| Homeless | 3 | 0 | 3.18 | 1 | 0.074 |
| Nicotine dependent | 10 | 0 | 17.35 | 1 | 0.000 |
| Beck Depression Score | 10.3 ± 7.8 | 0.7 ± 1.6 | 3.81 | 18 | 0.001 |
| ALT (units/liter) | 24.8 ± 18.2 | 27.7 ± 12.0 | 0.40 | 18 | NS |
| AST (units/liter) | 21.6 ± 8.5 | 24.1 ± 6.0 | 0.74 | 18 | NS |
| DrInC | 34.1 ± 10.7 | 3.1 ± 2.8 | 8.45 | 17 | 0.000 |
| Years problem drinking | 23.9 ± 5.4 | ||||
| Drinks in past 30 days | 637 ± 331 | ||||
| Lifetime drinks | 146,193 ± 82,485 |
Data are mean ± SD. Comparisons between groups were by t test or χ2.
Acknowledgments
The authors thank the Dallas VA Substance Abuse Team for assistance in the recruitment and clinical care of patients and the UT Southwestern GCRC staff for their excellent patient care and meticulous attention to research protocol. The authors also thank Ali Iranmanesh, MD, for his conceptual and instructional assistance and Dr. Joan Reisch and Alan C Elliott, MAS, for their statistical expertise.
Footnotes
Supported by Grant AA1570 from the National Institute on Alcohol Abuse and Alcoholism and Grant M01–RR00633 from USPHS. Presented at the 57th Annual Convention of the Society of Biological Psychiatry, May 18, 2002, Philadelphia, Pennsylvania; the 2003 Scientific Meeting of the Research Society on Alcoholism, June 22, 2003, Ft. Lauderdale, Florida; and the 12th World Congress on Biomedical Alcohol Research, October 1, 2004, Heidelberg/Mannheim, Germany.
References
- Adinoff B, Iranmanesh A, Veldhuis JD, Fisher L. Disturbances of the stress response: The role of the HPA axis during alcohol withdrawal and abstinence. Alcohol Health Res World. 1998;22:67–72. [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men: I. Adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005:29. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Martin PR, Bone GHA, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GHA, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- Adinoff B, Ruether K, Krebaum S, Iranmanesh A, Williams MJ. Increased salivary cortisol concentrations during chronic alcohol intoxication in a naturalistic clinical sample of men. Alcohol Clin Exp Res. 2003;27:1420–1427. doi: 10.1097/01.ALC.0000087581.13912.64. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Anthenelli RM, Maxwell RA, Geracioti TDJ, Hauger R. Stress hormone dysregulation at rest and after serotonergic stimulation among alcohol-dependent men with extended abstinence and controls. Alcohol Clin Exp Res. 2001;25:692–703. [PubMed] [Google Scholar]
- Anton RF. Urinary free cortisol in psychotic depression. Biol Psychiatry. 1987;22:24–33. doi: 10.1016/0006-3223(87)90126-0. [DOI] [PubMed] [Google Scholar]
- Asnis GM, Sachar EJ, Halbreich U, Nathan RS, Novacenko H, Ostrow LC. Cortisol secretion in relation to age in major depression. Psychosom Med. 1981;43:235–242. doi: 10.1097/00006842-198106000-00005. [DOI] [PubMed] [Google Scholar]
- Bailly D, Dewailly D, Beuscart R, Couplet G, Dumont P, Racadot A, Fossati P, Parquet PJ. Adrenocorticotropin and cortisol responses to ovine corticotropin-releasing factor in alcohol dependence disorder. Horm Res. 1989;31:72–75. doi: 10.1159/000181090. [DOI] [PubMed] [Google Scholar]
- Bardeleben UV, Heuser I, Holsboer F. Human CRH stimulation response during acute withdrawal and after medium-term abstention from alcohol abuse. Psychoneuroendocrinology. 1989;14:441–449. doi: 10.1016/0306-4530(89)90043-7. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–979. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1979;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Coiro V, Vescovi PP. Effect of cigarette smoking on ACTH/cortisol secretion in alcoholic after short- and medium-term abstinence. Alcohol Clin Exp Res. 1999;23:1515–1518. [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo-pituitary-adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–275. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Davis KL, Davis BM, Mathe AA, Mohs RC, Rothpearl AB, Levy MI, Gorman LK, Berger P. Age and the dexamethasone suppression test in depression. Am J Psychiatry. 1984;141:872–874. doi: 10.1176/ajp.141.7.872. [DOI] [PubMed] [Google Scholar]
- Ehrenreich H, Schuck J, Stender N, Pilz J, Gefeller O, Schilling L, Poser W, Kaw S. Endocrine and hemodynamic effects of stress versus systemic CRF in alcoholics during early and medium term abstinence. Alcohol Clin Exp Res. 1997;21:1285–1293. [PubMed] [Google Scholar]
- Errico AL, Parson OA, King AC, Lovallo WR. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–398. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- First MH, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. patient ed (SCID-I/P, version 2.0) New York State Psychiatric Institute; New York: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Geracioti TDJ, Loosen PT, Ebert MH, Ekhator NN, Burns D, Nicholson WE, Orth DN. Concentrations of corticotropin-releasing hormone, norepinephrine, MHPG, 5-hydroxyindoleacetic acid and tryptophan in the cerebrospinal fluid of alcoholic patients: Serial sampling studies. Neuroendocrinology. 1994;60:635–642. doi: 10.1159/000126807. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Guo W, Wang Y, Brown MB. A signal extraction approach to modeling hormone time series with pulses and a changing baseline. J Am Stat Assoc. 1999;94:746–756. [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000a;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000b;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Inder WJ, Joyce PR, Ellis MJ, Evans MJ, Livesey JH, Donald RA. The effects of alcoholism on the hypothalamic-pituitary-adrenal axis: Interaction with endogenous opioid peptides. Clin Endocrinol (Oxf) 1995;43:283–290. doi: 10.1111/j.1365-2265.1995.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Veldhuis JD, Johnson ML, Lizarralde G. 24-hour pulsatile and circadian patterns of cortisol secretion in alcoholic men. J Androl. 1989;10:54–63. doi: 10.1002/j.1939-4640.1989.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Keedwell PA, Poon L, Papadopoulos AS, Marshall EJ, Checkley SA. Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addict Biol. 2001;6:247–256. doi: 10.1080/13556210120056580. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Schick M, Wiedemann K. Alcohol self-administration, craving and HPA-axis activity: An intriguing relationship. Psychopharmacology (Berl) 2002;164:239–240. doi: 10.1007/s00213-002-1255-3. [DOI] [PubMed] [Google Scholar]
- King RJ, Jones J, Scheuer JW, Curtis D, Zarcone VP. Plasma cortisol correlates of impulsivity and substance abuse. Person Individ Diff. 1990;11:287–291. [Google Scholar]
- Kosten TR, Oliveto A, Sevarino KA, Gonsai K, Feingold A. Ketoconazole increases cocaine and opioid use in methadone maintained patients. Drug Alcohol Depend. 2002;66:173–180. doi: 10.1016/s0376-8716(01)00198-3. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: Stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Sivakumaran S, Alaghband-Zadeh J, Dhillo W, Kong WM, Wheeler MJ. Free cortisol index as a surrogate marker for serum free cortisol. Ann Clin Biochem. 2002;39:406–408. doi: 10.1258/000456302760042182. [DOI] [PubMed] [Google Scholar]
- Loosen PT, Chambliss B, Pavlou SS, Orth DN. Adrenal function in abstinent alcoholic men. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:771–780. doi: 10.1016/0278-5846(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Loosen PT, Ekhato N, Geraciotil TD, Bums D, Orthl DN. Blunted ACTH response in abstinent alcoholic men: Locus of disturbance. Biol Psychiatry. 1993;33:67A. [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Margraf HW, Moyer CA, Ashford LE, Lavalle LW. Adrenocortical function in alcoholics. J Surg Res. 1967;7:55–62. doi: 10.1016/0022-4804(67)90035-2. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Stein S. Serum cortisol levels in alcoholic and nonalcoholic subjects during experimentally induced ethanol intoxication. Psychosom Med. 1966;XXVIII:616–626. doi: 10.1097/00006842-196601000-00001. [DOI] [PubMed] [Google Scholar]
- Merry J, Marks V. The effect of alcohol, barbiturate, and diazepam on hypothalamic, pituitary/adrenal function in chronic alcoholics. Lancet. 1972;2:990–992. doi: 10.1016/s0140-6736(72)92403-8. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrlnC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse. National Institutes of Health; Rockville, MD: 1995. [Google Scholar]
- Nash JFJ, Maickel RP. The role of the hypothalamic-pituitary-adrenocortical axis in post-stress induced ethanol consumption by rats. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:653–671. doi: 10.1016/0278-5846(88)90010-3. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminiere JM, Maccari S, LeMoal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Marinelli M, Rouge-Pont F, Deroche V, Maccari S, Simon H, Moal M. Stress, glucocorticoids, and mesencephalic dopaminergic neurons: A pathophysiological chain determining vulnerability to psychostimulant abuse. NIDA Res Monogr. 1996;163:277–299. [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: The role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC. Behavioral Treatment of Alcohol Problems. Plenum Press; New York: 1978. [Google Scholar]
- Stokes PE. Adrenocortical activation in alcoholics during chronic drinking. Ann NY Acad Sci. 1973;215:77–83. doi: 10.1111/j.1749-6632.1973.tb28251.x. [DOI] [PubMed] [Google Scholar]
- Tennes K, Kreye M. Children’s adrenocortical responses to classroom activities and tests in elementary school. Psychosom Med. 1985;47:451–460. doi: 10.1097/00006842-198509000-00005. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: Associations with cortisol. Psychiatry Res. 1993;46:9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Vescovi PP, DiGennaro C, Coiro V. Hormonal (ACTH, cortisol, beta-endorphin, and met-enkephalin) and cardiovascular responses to hyperthermic stress in chronic alcoholics. Alcohol Clin Exp Res. 1997;21:1195–1198. [PubMed] [Google Scholar]
- Virkkunen M. Urinary free cortisol secretion in habitually violent offenders. Acta Psychiatr Scand. 1985;72:40–44. doi: 10.1111/j.1600-0447.1985.tb02568.x. [DOI] [PubMed] [Google Scholar]
- Wand G, McCaul ME, Gotjen D, Reynolds J, Lee S. Confirmation that offspring from families with alcohol-dependent individuals have greater hypothalamic-pituitary-adrenal axis activation induced by naloxone compared with offspring without a family history of alcohol dependence. Alcohol Clin Exp Res. 2001;25:1134–1139. [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry (Suppl 17) 2001;62:41–46. [PubMed] [Google Scholar]


