Abstract
The optimal definition of the size, shape and location of gross tumour volume is one of the most important steps in the planning of radiation therapy, and necessitates a proper understanding of the procedure from both the oncologic radiologist and the radiation oncologist. This overview reports on the different terms and concepts that have been recommended in the ICRU Reports for this purpose; the latest Report 71 focuses on both previously given recommendations, and especially on electron beam therapy. This paper also highlights some of the problems that are encountered in the use of the International Commission on Radiation Units and Measurements (ICRU) recommendations in clinical practice, and at the interface between the radiation oncologist and the diagnostic oncologist.
Keywords: Target volume definition, radiotherapy
Background
The use of a common language and definitions of terms and concepts in radiotherapy is essential for accurate, effective, and safe exchange of information. It is a prerequisite for progress in the development of radiation therapy.
Until recently this was not the situation. There was confusion about the meaning of tumour volume. Dische et al.[1] reported in 1993 that the information (e.g. on volumes and dose specification points) given in two leading radiotherapy journals was acceptable and unambiguous in only 42% and 30% of the papers, respectively.
For several decades, the International Commission on Radiation Units and Measurements (ICRU) has been involved in an effort to improve harmonisation in reporting radiation treatments. In a series of Reports (nos. 29, 38, 50, 58, 62, and 71) recommendations for definition of different volumes and dose specification points in radiotherapy were developed[2–7].
The volume concepts
Several volume concepts were developed in the ICRU Reports (Fig. 1):
gross tumour volume (GTV),
clinical target volume (CTV),
internal target volume (ITV),
planning target volume (PTV),
organ at risk (OR),
planning organ at risk volume (PRV),
treated volume, and
irradiated volume.
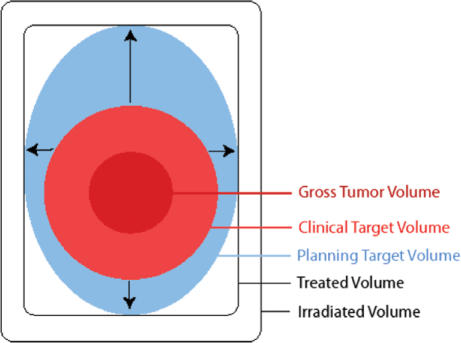
Figure 1.
Schematic illustration of the different volumes as defined in ICRU Report 50 (1993). Gross tumour volume (GTV) denotes the demonstrated tumour. Clinical target volume (CTV) denotes the demonstrated tumour (when present) and also volumes with suspected (subclinical) tumour considered to need treatment (e.g. a margin around the GTV and regional lymph nodes not invaded clinically). The CTV is thus a purely anatomical clinical concept. Planning target volume (PTV) consists of the CTV(s) and a margin to account for variations in size, shape, and position relative to the treatment beam(s). The PTV is thus a geometrical concept used to ensure that the CTV receives the prescribed dose, and it is defined in relation to a fixed coordinate system. Note that in the example shown, the magnitude of foreseen movements of the CTV is different in different directions. Treated volume is the volume that receives a dose that is considered important for local cure or palliation. The concept is useful for the evaluation of loco-regional relapses. Irradiated volume is the tissue volume which receives a dose that is considered significant in relation to normal tissue tolerance (other than those specifically defined as organs at risk) (reproduced by kind permission from the ICRU).
Only GTV, CTV, and OR represent tissues, whereas the others are geometric concepts and do not strictly represent tissue or organ volumes.
GTV (gross tumour volume)
The gross tumour volume (GTV) is the gross palpable or visible/demonstrable extent and location of the malignant growth.
The GTV may consist of primary tumour (GTV-T), metastatic lymphadenopathy (GTV-N), or other metastases (GTV-M). The GTV corresponds almost always to those parts of the malignant growth where the tumour cell density is the highest. Due to the high density of the cancer cells in the GTV, an adequate dose must be delivered to the whole GTV to obtain local tumour control in radical treatments. No GTV can be defined if the tumour has been completely removed, e.g. by previous surgery.
The shape, size, and location of a GTV may be determined by different methods such as clinical examination (e.g. inspection, palpation, endoscopy), and various imaging techniques (e.g. X-ray, computed tomography (CT), digital radiography, ultrasonography, magnetic resonance imaging (MRI), and radionuclide methods). The methods used to determine the GTV should meet the requirements for staging the tumour according to the clinical TNM[8,9] and American Joint Committee on Cancer (AJCC)[10,11] systems, and the definition of the GTV is then in full agreement with the criteria used for the TNM classification.
The GTV (primary tumour (GTV-T), metastatic lymphadenopathy (GTV-N), other metastases (GTV-M)), may be different in size and shape, sometimes significantly, depending on what examination technique is used for evaluation (e.g. palpation or mammography for breast tumours, and CT or MRI for some brain tumours). The radiation oncologists should therefore indicate in each case which methods have been used for evaluation and for the definition of the GTV. The problem has been highlighted recently[12]. The image format should make it possible to fuse information from different imaging equipments, using, e.g. DICOM format[13].
Since the description of a GTV on scans for radiation treatment planning usually is made subjectively, an interobserver variation can be expected.
The GTV should be described in standard topographical or anatomical terms, e.g. ‘tumour 18 mm in diameter in the left lobe of the prostate adjacent to but not breaching the capsule’. In many situations, a verbal description might be too cumbersome and, for the purpose of data recording and analysis, a classification system is needed. Several systems have been proposed for coding the anatomical description; some of them are mentioned in ICRU Report No. 50[4].
GTV may be confined to only part of an organ (e.g. a T1 breast cancer), or involve a whole organ (e.g. in multiple metastases to the brain). The GTV may or may not extend outside the normal borders of the organ or tissue involved.
CTV (clinical target volume)
The clinical target volume (CTV) is a tissue volume that contains a demonstrable GTV and/or is considered to contain microscopic, subclinical extensions at a certain probability level. This volume thus has to be considered for therapy and, if included, should be irradiated adequately to achieve cure.
For the treatment of subclinical disease, two situations may be defined as below, and as illustrated in Fig. 2. In this situation the prescription is based on the assumption that in some anatomically definable tissues/organs, there may be cancer cells at a given probability level, even though the cannot be detected; they are subclinical. The level of probability is based on clinical experience from adequately documented treatments and follow-up. For the purpose of prescription of treatment, it can usually be described in terms of frequency of risk for later detectable mainfestations (failure rate), when not treated adequately in the “subclinical” situation.
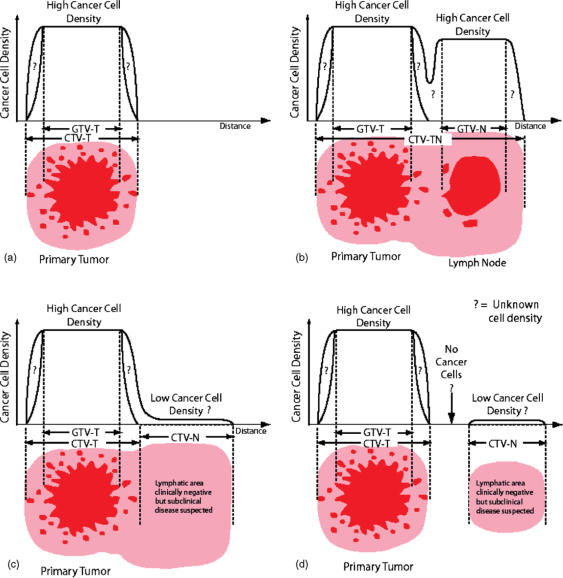
Figure 2.
Schematic illustration of the relations between GTV-T/N(s) and CTV-T/N(s) in different clinical situations (from ICRU, Report 71, 2004[7], with kind permission of Oxford University Press). (a) Simple case with one GTV and the corresponding CTV (e.g. a skin tumour). At the level of the GTV (dark red), the cellular density is the highest (as an average, about 106 cells/mm3), but may be heterogeneous (e.g. due to necrosis). The width of the safety margin (light red) is selected so that, in principle, no cancer cells are present outside the limits of the CTV. The cancer cell density decreases between the border of the GTV and the outer limit of the CTV, but the variation of the cell density with distance is not known (‘?’ in the figure) and depends on tumour type and location. In some situations a natural anatomical border may limit subclinical extensions, e.g. pariental pleura in mediastinal lymphomas. (b) Two GTVs are present: the primary tumour, GTV-T, and a metastatic fixed lymph node, GTV-N (e.g. a tumour of the tonsil with a homolateral cervical lymph node). A safety margin for microscopic invasion has to be taken around each GTV, which may lead to the definition of two CTVs: CTV-T corresponding to the primary tumour and its safety margin, and CTV-N for the lymph node and its safety margin. Actually, since in this example, the two CTVs are close to each other and because there are certainly malignant cells between the two GTVs, only a single CTV is selected (CTV-TN), which includes the two GTVs and a common safety margin. (c) The primary tumour and its safety margin define a first CTV (CTV-T). In addition, there is a high probability of invasion of the adjacent lymph node area, which leads to the definition of a second CTV (CTV-N). It is decided to keep the two CTVs adjacent to each other as there could be cancer cells all along between them (e.g. breast cancer and adjacent lymph node area(s), or tumour of the head and neck and adjacent lymph node area(s)). (d) A similar situation to that in (c), but there is free space between the CTV containing the primary tumour and the CTVs including the lymph node areas at risk. This is the case, e.g. for a tumour of the anus and the inguinal lymphatic areas, which can be treated with electron beams.
Prescription of treatment of subclinical extensions adjacent to a GTV
Clinical experience indicates that around a GTV (Fig. 2a,b) (primary tumour; GTV-T, or metastatic lymphadenopathy; GTV-N) there is generally subclinical involvement, i.e. individual malignant cells, small cell clusters, or microextensions, which cannot be detected by staging procedures. The GTV together with this surrounding volume of local subclinical involvement can be defined as a clinical target volume (CTV-T for primary tumour, and CTV-N for metastatic lymphadenopathy, etc.). If the same dose is prescribed for two such CTVs and if they are close to each other, they can be labelled CTV-TN. If different doses are prescribed, there will be one CTV-T and one CTV-N, respectively. If the GTV has been removed by seemingly radical surgery, but it is still felt that radiotherapy is needed for the tissues that remain close to the site of the removed GTV, this volume is also usually designated as CTV-T.
Prescription of treatment of subclinical extension at a distance from a GTV
Additional volumes (CTVs) with presumed subclinical spread (Fig. 2c,d) (e.g. regional lymph nodes, N0) may also be considered for therapy. They are also defined as clinical target volumes, and may topographically be designated CTV-N I, CTV-N II, etc. To stress that in such cases subclinical disease is treated ‘electively’, it may be useful to add ‘E’, e.g. CTV-EN. It may also be useful to differentiate between ‘CTV-E high-risk’ and ‘CTV-E low-risk’. A precise description of the terminology used should be available in the treatment protocol[14]. The anatomical localisation of different lymph node areas has been reported[15,16].
If different doses are prescribed, different CTVs have to be defined for treatment planning. For any given situation there is often more than one CTV. One situation can be illustrated by considering a primary tumour and its regional lymphatics separately (e.g. in breast conserving procedures) where the primary tumour and its regional lymphatics are separated anatomically. In other situations the aim is to treat two or more CTVs to different dose levels. One common example of this is ‘boost’ therapy, where often the ‘high-dose’ volume (often containing the GTV or GTVs) is located inside the ‘low-dose’ volume.
The prescription of the GTV(s) and CTV(s) are based on general oncological principles, and are not specific to the field of radiation therapy. For instance, in surgery, a safety margin is taken around the gross tumour volume according to clinical judgment, and this implies the same use of the clinical target volume concept as in external beam radiation treatments.
In brachytherapy, volumes to be treated are also defined, and thus the concept of CTV is valid. The definition of GTV(s) and CTV(s) thus constitute the basic prescription of treatment for all radiation therapy techniques, and must precede the subsequent treatment planning.
ITV (internal target volume) and PTV (planning target volume)
Once the CTV(s) have been defined, in external beam radiation treatment, a suitable arrangement of radiation beam(s) must be selected to achieve an acceptable dose distribution. Today this calculation can only be done for a static representation, whereas in fact there are variations and uncertainties in the positions, sizes and shapes, and orientations of both the tissues, patient, and the beams in relation to the common coordinate system. This will be seen both during a single session and from one session to another. The variations and errors may be either random or systematic. A detailed analysis of this problem can be found in ‘Geometric uncertainties in Radiotherapy’[17]. Such variations and uncertainties may also occur when information for decision making is obtained (e.g. by CT scanning), and also between this part of the procedure and the first treatment. This creates a situation where the dose distribution is being calculated for a static situation which does not reflect the real dynamic situation. If no margins are added, some of the tissues will move out of the beam for part of the treatment resulting in underdosage. Other parts of the tissues may move around in a dose gradient, making it difficult to state the exact dose received by each tissue element. Too wide margins will result in unnecessary morbidity. There is no ideal solution, and acceptable compromises must be agreed. To assure that the CTV(s) in practice receive a dose that does not deviate significantly from the prescribed and planned dose, margins must be added to the CTV(s) for variations in tissue positions, sizes, and shapes, as well as for variations in patient position and beam geometries, both intrafractionally and interfractionally. This leads to the concept of planning target volume (PTV).
For the final treatment planning (definition of beam sizes, etc.), all the different variations and uncertainties must be considered, to define a static volume (planning target volume (PTV)) that will be used for treatment planning and for basic reporting of doses, which is considered representative of the corresponding CTV(s).
An internal margin (IM) must be added to the CTV to compensate for physiological variations in size, shape, and position of the CTV during therapy in relation to an internal reference point and its corresponding coordinate system. The IM, commonly asymmetric around the CTV, compensates for movements and variations in site, size and shape of the tissues which contain or are adjacent to the CTV, resulting from e.g.:
respiration,
variable filling of the bladder,
variable filling of the rectum,
swallowing,
heart beat,
movements of the bowel.
These internal variations are physiological ones, and they result in changes in site, size, and shape of the CTV. They cannot always be influenced easily. Techniques to take these into account (such as gating) are being tested. They do not depend on external uncertainties in patient day-to-day set-up or beam geometry.
The internal target volume (ITV) is the volume encompassing the CTV, which takes into account the fact that the CTV varies in position, shape and size.
The internal target volume (ITV) is defined by the internal margin (IM), as described above, and is referred to the patient coordinate system.
To account specifically for uncertainties and variability in the reproducibility of patient positioning and inaccuracies in the alignment of the therapeutic beams during treatment planning and throughout treatment, a set-up margin (SM) for each beam is needed. The uncertainties to be compensated for may vary on different anatomical directions, and thus the extent of such margins depends on the selection of beam geometries. The inaccuracies depend on factors such as:
variations in patient positioning,
lack of reproducibility of the equipment (worn bearings causing, e.g. sagging of gantry, collimators, and couch),
human factors (e.g. experience and precision of the radiographers/radiotherapists).
They may also vary from machine to machine. The use of patient immobilisation devices, the application of quality assurance programs for the physical aspects of the treatment equipment, and the skill and experience of the radiographers/radiotherapists are important factors which must be taken into account. The use of different record and verify systems (in real time or not) may also be important, and may significantly reduce the size of the set-up margins needed.
Each centre should evaluate its own set-up margins, at least for frequent treatment techniques, thus allowing for potential standardisations.
The net effect of combining an internal margin (referenced in the patient coordinate system) and a set-up margin (referenced to the external coordinate system) to the CTV leads to the concept of planning target volume (PTV).
The planning target volume (PTV) is a geometric concept, used for treatment planning, and it is defined to select appropriate beam sizes and beam arrangements, to ensure that the prescribed dose is actually delivered to the CTV.
Thus the border of the PTV must be clearly defined on charts or in files for treatment planning purposes. For dose specification for reporting, the margin that defines the PTV must be a closed line/surface, even if this may not be necessary for the proper selection of beam parameters.
Reporting ITV is not considered to be compulsory in the last ICRU Report[7].
In some cases, the internal margin approaches a very low value, (e.g. with brain tumours), and in other cases the set-up margin may be very small (e.g. with on-line correction for the different set-up errors and variations).
Ideally, the size of the margins should be determined in an iterative way during the selection of an optimal beam arrangement, e.g. in beam's eye view (as when planning both co-planar and non-coplanar conformal therapy). In practice this may not always be feasible, and as a compromise one can specify the margins for uncertainty in such a way that they can be used for different types of beam arrangement (e.g. one beam, two opposed beams, box technique, orthogonal beams, moving beams). In daily clinical use, this is probably most appropriate for defining the PTV for treatment planning and for basic dose specification for reporting, and it is the approach recommended in ICRU Report No. 50, 1993[4]. Note that intensity modulated radiotherapy (IMRT) presents special difficulties for checking beam geometry.
When delineating the PTV, consideration should also be given to the presence of any radiosensitive normal tissue (see next section). This may lead to choice of alternative beam arrangements and/or shapes as part of an optimisation procedure (Fig. 3). In some cases it may be necessary to change the prescription (for volumes and/or doses), and accept a smaller benefit. If, for radical treatments, the probability of benefit approaches a low value, the aim of therapy may shift from radical to palliative.
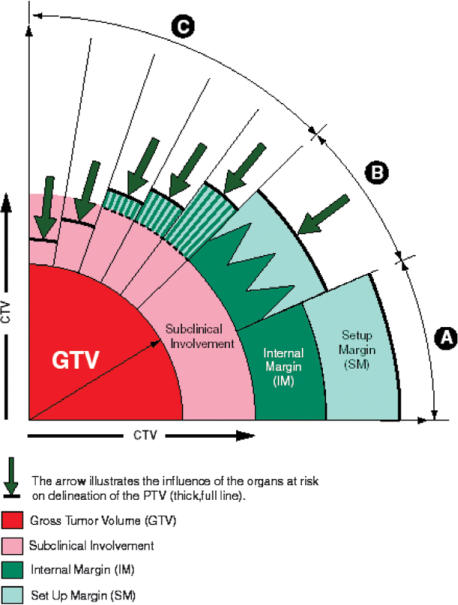
Figure 3.
Schematic illustration of the relationship between GTV-T/N(s) and CTV-T/N(s) in different clinical situations[6]. (Reproduced by kind permission from the ICRU). Scenario A. A margin is added around the gross tumour volume (GTV) to take into account potential ‘subclinical’ invasion. The GTV and this margin define the clinical target volume (CTV). In external beam therapy, to ensure that all parts of the CTV receive the prescribed dose, additional margins for geometric variations and uncertainties must be considered. An internal margin (IM) is added for the variations in position and/or shape and size of the CTV. This defines the internal target volume. A set-up margin is added to take into account all the variations/uncertainties in patient-beam positioning. CTV+IM+SM define the planning target volume (PTV) on which the selection of beam size and arrangement is based. Scenario B. The simple (linear) addition of all factors of geometric uncertainty, as indicated in scenario A, often leads to an excessively large PTV, which would be incompatible with the tolerance of the surrounding normal tissues. In such instances, instead of adding linearly the internal margin and the set up margin, compromise combinations are used, e.g., 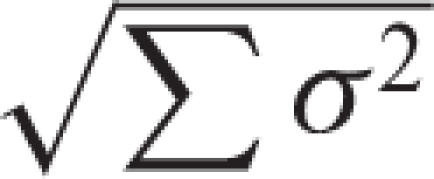 formalism). This quantitative evaluation is only possible if all uncertainties and their σ are available, i.e., in a few sophisticated protocols. Scenarios C and D. In the majority of clinical situations, a ‘global’ safety margin is adopted. In some cases, the presence of an organ at risk dramatically reduces the width of the acceptable safety margin (e.g., presence of the spinal cord, optical nerve, etc.). In other situations (scenario C), larger safety margins may be accepted. Since the incidence of subclinical invasion may decrease with distance from the GTV (see Fig. 2.4), a reduction of the margin for subclinical invasion may still be compatible with chance for cure, albeit at a lower probability rate. It is important to stress that the thickness of the different safety margins may vary with the angle at which one looks at the PTV (e.g., bony structures or fibrotic tissue may prevent, at least temporarily, malignant cell dissemination). (Note that if an adequate dose cannot be given to the whole GTV, the whole aim of therapy shifts from radical to palliative).
formalism). This quantitative evaluation is only possible if all uncertainties and their σ are available, i.e., in a few sophisticated protocols. Scenarios C and D. In the majority of clinical situations, a ‘global’ safety margin is adopted. In some cases, the presence of an organ at risk dramatically reduces the width of the acceptable safety margin (e.g., presence of the spinal cord, optical nerve, etc.). In other situations (scenario C), larger safety margins may be accepted. Since the incidence of subclinical invasion may decrease with distance from the GTV (see Fig. 2.4), a reduction of the margin for subclinical invasion may still be compatible with chance for cure, albeit at a lower probability rate. It is important to stress that the thickness of the different safety margins may vary with the angle at which one looks at the PTV (e.g., bony structures or fibrotic tissue may prevent, at least temporarily, malignant cell dissemination). (Note that if an adequate dose cannot be given to the whole GTV, the whole aim of therapy shifts from radical to palliative).
OR (organs at risk) and PRV (planning organ at risk volume)
Organs at risk are normal tissues whose radiation sensitivity may significantly influence treatment planning and/or prescribed dose (e.g. spinal cord).
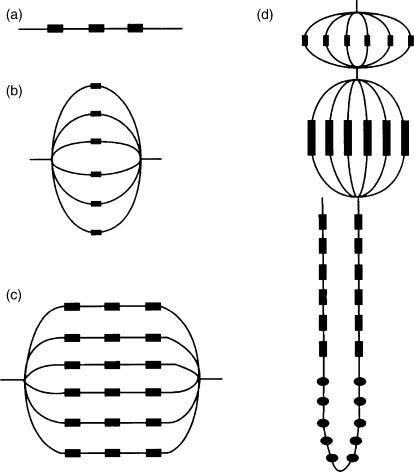
The dose–volume response of normal tissues is a complex process, which changes progressively. It has been suggested that the tissues of an organ at risk can be considered to be organised in functional sub units (FSUs), and the concepts of ‘serial’, ‘parallel’, and ‘serial-parallel’ organisation of the normal structures (Fig. 4) has been suggested. For example, the spinal cord has a high ‘relative seriality’, implying that a dose above the tolerance limit to even a small volume of the organ at risk may be deleterious, whereas the lung usually has a low ‘relative seriality’, meaning that it may be the relative size of the volume that is irradiated above tolerance level that is the most important parameter.
Figure 4.
Schematic examples of tissue organisation structures in the parallel–serial model. (a) A serial string of subunits (e.g., the spinal cord), (b) a parallel string of subunits (e.g., the lungs), (c) a serial–parallel string of subunits (e.g., the heart), (d) a combination of parallel and serial structures (e.g., a nephron). Modified from Withers et al.[35] and Källman et al.[36], citied in ICRU[6] (reproduced by kind permission from the ICRU).
For example, the late effects from (partial) irradiation of the lungs (a parallel tissue) in H.D. were much less serious than those from the heart (a combined serial [coronary arteries] and parallel [myocardium] tissue)[18,19].
As is the case with the planning target volume, any movements of the organ(s) at risk during treatment, as well as uncertainties in the set-up during the whole treatment course must be considered.
An integrated margin has to be added to the OR to compensate for these variations and uncertainties, using the same principles of internal and set-up margins as for the PTV. This leads, in analogy with the PTV, to the concept of planning organ at risk volume (PRV). Note that a PTV and a PRV may overlap.
Treated volume and irradiated volume
Due to the limitations of irradiation techniques and in some specific clinical situations, the volume receiving the prescribed dose may not accurately match the PTV; it may be larger (sometimes much larger) and in general of a simpler shape. This leads to the concept of treated volume. It is defined when the treatment planning procedure is completed and the beam arrangement as well as all the other irradiation parameters have been selected.
The treated volume is the tissue volume which is planned to receive at least a dose selected and specified by the radiation oncologist as being appropriate to achieve the purpose of the treatment, e.g. tumour eradication, or palliation.
The treated volume is thus a volume enclosed by the isodose surface corresponding to that dose level. For example, if the prescribed dose is 60 Gy, and the minimum dose (considered to be adequate) was 5% below the central dose (which was normalised to 100%), the treated volume is then enclosed by the 57 Gy isodose surface.
Normally, in the patient, the tissue volume which actually receives that dose level (i.e. ‘actual’ treated volume) should match the ‘planned’ treated volume (‘conformal therapy’).
It is important to identify the treated volume and its shape, size, and position in relation to the PTV for various reasons. One is to distinguish causes of local recurrences (‘in-field’ [=too low dose] versus ‘marginal’ [=too small volume] ones). Another issue is to evaluate complications in normal tissues encountered outside the PTV but within the treated volume. These problems require imaging during the follow-up, so the relevant correlations can be made.
The irradiated volume is the tissue volume which receives a dose that is considered significant in relation to normal tissue tolerance.
If the irradiated volume is reported, the significant dose must be expressed either in absolute values (in Gy) or relative to the specified dose to the PTV. The irradiated volume depends on the treatment technique used.
Note that when the treated volume is made smaller by use of many beams (e.g. in IMRT) the irradiated volume may be larger, and the integral dose higher.
Tumour volume definitions
The prescription of radiation treatment includes the designation of the pertinent volumes and the prescription of dose and fractionation. The correct designation and contouring of the volumes for radiotherapy planning is becoming increasingly important with the increasing conformality of modern radiotherapy. Today, dose distributions can be obtained, and are routinely employed in most radiotherapy clinics, which fit quite accurately in all three dimensions to the volume contoured on the planning CT scan. In this way, less radiation is given to normal tissues. However, if all tumour tissue is not included in the planning volumes there is real danger that the chance of tumour control will decrease compared to earlier and less conformal techniques.
The correct designation and contouring of the planning volumes are ultimately the responsibility of the radiation oncologist. However, assistance is often needed from specialists in the different imaging modalities, and close collaboration is essential to obtain the benefit of modern techniques in imaging and treatment planning[20].
Contouring the GTV
CT scans are employed for treatment planning. Because the CT numbers are correlated with the electron density of the corresponding tissues at each voxel relative to the electron density of water, the information from the CT scan can be employed by the dose calculation algorithm of the treatment planning system for correct calculation of the absorption and scattering of the radiation in the tissues. Contouring the GTV on the planning CT scan is therefore the radiation oncologist's prescription of the volume in the patient that should receive the prescribed radiation dose.
However, CT scanning may not always be the best modality for the precise delineation of the extent of the tumour. The radiation oncologist needs to make sure that all relevant information on the extent of the tumour tissue is employed when contouring the GTV on the planning CT scans. One important source of information is the clinical examination of the patient and findings from endoscopies and operations. In many instances (e.g. tumour spreading in a thin layer within the mucous membrane in the aerodigestive tract) the tumour cannot be seen on the CT scan, but naturally the volume known to be involved must be contoured to ensure that it gets the prescribed tumour dose. Other imaging modalities, e.g. MR scans, positron emission tomography (PET) scans, single photon emission computed tomography (SPECT) scans and ultrasound, may also add crucial information on the extent of the tumour tissue[21]. Image fusion with the planning CT scan is essential for the full exploitation of these imaging modalities. Specialists in diagnostic imaging are important collaborators in the contouring process, but it must be stressed that ultimately the contouring of the volume which shall receive the prescribed tumour dose is the responsibility of the radiation oncologist.
At present, new imaging modalities are appearing which are able to provide images of the physiology of the tissues. In the future we will be able to image volumes with different tumour biology with respect to hypoxia, angiogenesis, proliferation, or apoptosis. We will then be able to contour not only a morphological target volume but also different biological target volumes that might need higher doses or different fractionation of the radiotherapy. Hence, we will end up performing multidimensional treatment planning, contouring target volumes with different biological characteristics within the gross tumour volume with the aim of delivering radiation dose distributions tailored to biological differences within the individual tumour.
Contouring in the setting of combined modality treatment
One issue that is not covered in the ICRU guidelines is the increasingly common situation where radiotherapy is combined with chemotherapy. Chemotherapy may be used in a neoadjuvant fashion (i.e. before radiotherapy), concomitantly (i.e. during radiotherapy) or adjuvantly (i.e. after radiotherapy). In many situations, e.g. in the treatment of certain types of lymphomas, head and neck cancers and lung tumours, chemotherapy is the initial treatment followed by radiotherapy to the ‘initial tumour volume’, sometimes followed by a boost to the residual tumour volume after chemotherapy. In this situation contouring the initial tumour volume on the planning CT scan means contouring a GTV that was present before chemotherapy but which is no longer present (or is much smaller) at the time of the planning. The goal is to contour the tissue volume that contained the GTV before chemotherapy, as this is the tissue volume with a high risk of containing residual tumour cells.
Accurate description of the initial tumour volume (GTV) and imaging before the start of chemotherapy are essential for the planning of post-chemotherapy radiotherapy. The radiation oncologist must be involved before any chemotherapy is instituted to ensure that adequate clinical examinations and imaging necessary for later treatment planning are carried out. Pre-chemotherapy images, which will be employed for post-chemotherapy contouring, should be acquired with the patient in the same position as will later be used for radiotherapy. This is particularly important if image fusion of pre-chemotherapy CT, PET, or MR scans with post-chemotherapy planning CT scans is used. Guidelines for radiotherapy of Hodgkin's lymphoma according to these principles have recently been published by the EORTC Lymphoma Group[22]. An example of the contouring of the pre-chemotherapy tumour volume and the post-chemotherapy residual tumour volume in a patient with Hodgkin's lymphoma is shown in Fig. 5.
Figure 5.
Target volume contouring in a patient with Hodgkin's lymphoma. Pre-chemotherapy tumour volume contoured in blue, post-chemotherapy residual tumour volume contoured in red (Varian, Eclipse®).
Contouring the CTV
The CTV is the GTV plus a volume that is considered to contain microscopic disease with some significant probability. Hence, the CTV is not a structure that can be seen per se on the planning CT scan. The definition and contouring of the CTV therefore depends on the radiation oncologist's knowledge and experience of the typical growth and spreading pattern of the particular tumour type. Important publications on CTV definitions in different tumour types have appeared in recent years to aid the radiation oncologist in this respect[16]. Contouring of the CTV also depends on an accurate knowledge of image-based cross-sectional anatomy. Here, again, close collaboration with specialists in diagnostic imaging is essential.
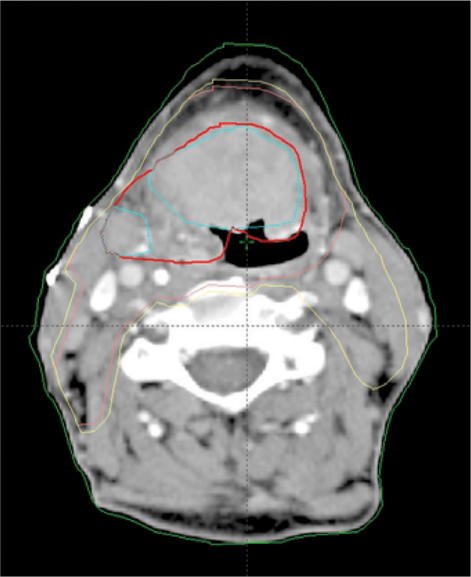
The dose, which needs to be prescribed for the CTV outside the GTV, has to be determined from past experience with radiotherapy of the particular tumour type. However, it is important to realise that with previous less conformal techniques, significant volumes close to the GTV received radiation doses that were significantly higher than the dose stated to be necessary for the CTV. This may actually have been an advantage, since the density of microscopic disease is likely to be highest near the GTV. In order not to lose efficacy of treatment it may therefore be desirable to define more than one CTV, e.g. a high-risk CTV close to the GTV, which should receive a relatively high dose, and a low-risk CTV that should receive only the previously accepted minimum dose for microscopic disease. Hence, additional terms not specified in the ICRU guidelines may have to be defined, e.g. CTV-high-risk and CTV-low-risk, with the aim of enabling the treatment planning system to optimise radiation doses to the CTV to different levels. This has been implemented in the treatment protocols for highly conformal (intensity modulated) radiotherapy for certain tumour types, e.g. head and neck cancers[14,23]. An example of the contouring of the GTV, CTV-high-risk, and CTV-low-risk in a patient with head and neck cancer is shown in Fig. 6.
Figure 6.
Target volume contouring in a patient with head and neck cancer (base of tongue. fluorodeoxyglucose (FDG)-PET positive tumour volume contoured in blue, gross tumour volume based on both CT and PET contoured in red, high-risk CTV contoured in pink, and low-risk CTV contoured in yellow (Varian, Eclipse®).
The correct delineation of the target volume(s), as well as the organs at risk, is a prerequisite for a successful treatment outcome, i.e. a high tumour control probability with acceptable late side effects. It is probably the most important step in the radiotherapy procedure but probably also one of the weakest links in the radiotherapy chain at present, containing the largest uncertainties. Delineation errors stay constant during the whole course of the treatment and therefore have a large impact on the dose to the tumour especially when state-of-the art radiation delivery techniques, with highly conformal beams and sharp dose gradients, are used. A review on volume uncertainties in radiotherapy has been published by Hamilton and Ebert[24].
Delineation of the GTV and CTV on the treatment planning computer is based on clinical examinations and information from various diagnostic imaging techniques (CT, MR, PET, etc.). The interpretation of the data from these examinations is often performed by the radiation oncologist alone. Hence, the level of education and the oncologist's knowledge in interpretation of images from different image modalities is of utmost importance. Simple items like scanning protocols, grey-scale settings and window or level settings (especially in the case of lung tumours) may contribute to variations in target delineation. A close collaboration with the department of radiology is essential. The shortcomings in the GTV delineation often have to be compensated by an increase in CTV especially in tumour locations where the radiological extension of the tumour can be difficult to assess, e.g. atelectasis of the lung, mucosal infiltration of an oesophageal cancer, diffuse infiltration in the base of the tongue.
In some departments the radiation oncologist is routinely present when the patient undergoes clinical examinations, endoscopic exploratory surgery and when staging procedures of the patient are performed. This is especially common for the staging of head and neck cancer patients. Participation of the radiation oncologist at these examinations helps to correctly interpret the endoscopic and staging reports and should thus decrease the errors in target delineation.
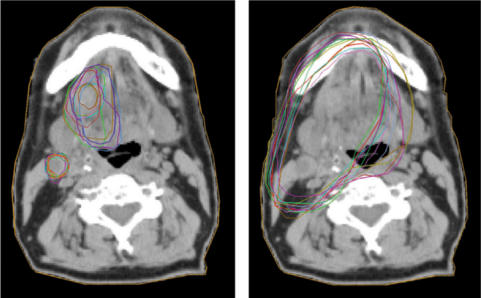
It is well known that the interpretation of the tumour extent from CT images varies from doctor to doctor. This is illustrated in Fig. 7, where the delineation of the GTV, performed by 11 radiation oncologists from different centres, and the resulting PTVs are shown for a patient with cancer of the tongue. The result is from a dummy run within the Swedish head and neck trial (ARTSCAN) which is a randomised controlled phase III trial studying the effect of overall treatment time in head and neck cancer (Zackrisson, personal communication, 2006).
Figure 7.
Example of interobserver variation (11 radiation oncologists) in the delineation of (a) GTV-T and GTV-N and (b) the resulting PTVs for a patient with cancer of the tongue with a homolateral lymph node deposit. From a ‘dummy-run’ in the ARTSCAN project (Zackrisson, personal communication, 2006). The images were produced with the CERR software package[37].
Several studies have been published focussing on the geometric uncertainties in the delineation of the GTV; predominantly interdoctor variations but also intradoctor variations have been reported. The magnitude of these variations have been studied for several tumour locations such as brain[25], head and neck[26], oesophagus[27], breast[28], lung[29], and prostate[26].
Multimodality imaging with co-registration of CT with other imaging modalities, e.g. MR[30] and PET[31] has been shown to increase the agreement between observers for some diagnoses.
An essential, but sometimes overlooked issue, is the availability of a detailed, concise and unambiguous study protocol with guidelines for delineation of the target volumes[32]. A dummy run is often performed in order to study, and hopefully improve, the compliance of participating institutions to the radiotherapy guidelines. Before the start of the trial a patient data set (CT images and patient charts) is sent out to the participating centres with the request to outline target volumes and organs at risk according to the protocol guidelines. A considerable improvement in protocol compliance is often achieved after such a dummy run procedure. The dummy run illustrates the difficulties in interpreting different imaging procedures but also differences between departments in clinical practice.
The main problem persists however; the extent and position of the target and organs at risk volumes are based on information from different imaging techniques and these often give different answers. A typical example is the prostate which often is visualised smaller on ultrasound than on MR which in turn usually depicts the prostate as smaller than on CT.
Some practical issues pertaining to imaging for radiation treatment planning
CT scanning for radiation therapy planning differs from diagnostic CT scanning. The patient's medical status must be thoroughly evaluated prior to the therapy scan using all the relevant diagnostic modalities available. These images should be interpreted by experts in the field and the subsequent reports made available for consideration by the therapy team. Radiation therapy scanning is a tool intended solely for use in virtual simulation, and is not necessarily read and reported as a diagnostic CT would be. The therapy scan can be used in generating GTV, CTV, PTV, and organs at risk. A radiation oncologist, ideally the same person who evaluated the patient clinically, in cooperation with a radiologist, performs this task.
The therapy CT scan should be performed with the patient immobilised in the treatment position on a flat table top similar to the table top at the treatment machine. Body fixation in a position appropriate for the tumour type and site and the individual patient is an important feature in therapy scanning.
The image quality of the therapy CT scan should be equivalent to that of a diagnostic scan. Note that intravenous contrast and oral contrast are not considered to interfere significantly with dose calculation.
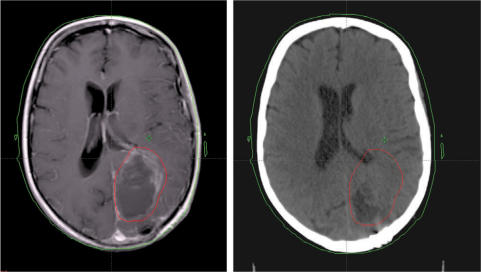
For treatment of brain tumours a therapy scan is obtained with the patient's head positioned in a moulded plastic net fixation device that is directly attached to the scanner flat-top cradle. If stereotactic radiation therapy is required, a surgically attached metal framework is placed surrounding the patient's cranium. CNS primary or metastatic tumours are known to be difficult to evaluate with CT alone, therefore fusion with a recent MR scan is optimal and can achieve accuracy of GTV definition to within a few millimetres (Fig. 8).
Figure 8.
Patient with residual tumour after resection for glioblastoma. The GTV is delineated on the MRI scan (left), and by means of image fusion transferred to the CT scan (right) that is used for dose calculation (Varian, Eclipse®).
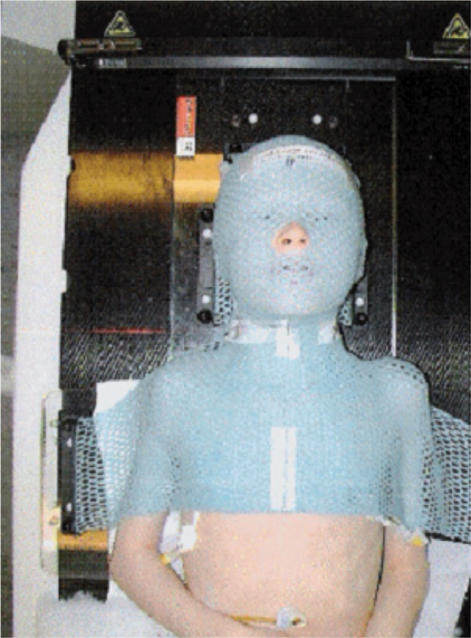
Patients with head and neck tumours are usually placed in a moulded plastic fixation net which includes the whole head, neck and shoulders (Fig. 9). The device is securely attached to the flat-top cradle. The therapy CT is performed with the patient's arms along the side of the body. This setup can be used in conventional radiation therapy planning as well as in IMRT., Any palpable nodes may be marked with wire. Combined PET/CT therapy data is often used as additional PET information may significantly improve tumour localisation which is especially important for conformal therapies like IMRT. Intravenous contrast is prescribed whenever possible and the scanning region includes not only the region of interest but the entire trunk in the evaluation for distant spread. When PET scanning is performed, a nuclear medicine physician is also involved in the treatment planning. This cooperation between specialities is crucial in head and neck treatment as the oral mucosa is not easily visualised with conventional CT or MR scanning. Preservation of normal mucosa is important for the post-therapy comfort for the patient.
Figure 9.
Patient immobilisation net used in the treatment for head and neck tumours.
Oesophageal and stomach cancer patients may benefit from a PET/CT scan. Intravenous contrast is used when possible, and water (350 ml as a peroral contrast agent) is given to the patient just before scanning to visualise the gastric mucosa. The patient's pre-op diagnostic CT scan and input from the surgeon is also extremely useful in treatment planning.
Breast cancer patients are scanned in the treatment position. This can vary greatly from patient to patient but the most common standard positioning is with the arm up over the head, the opposite arm along the side of the body, and the patient's head slightly rotated away from the treatment side.
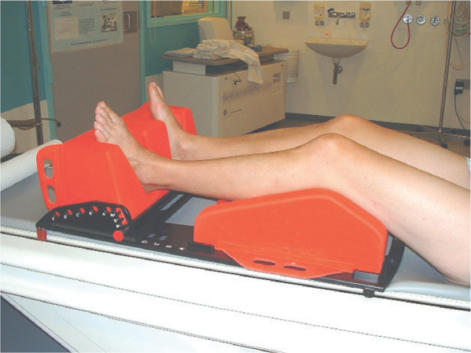
For gynaecologic cancer patients PET/CT scans are obtained with the arms up above the head. Intravenous and peroral contrast is prescribed when needed, and a special stabilising foam pillow for pelvic, leg, and foot fixation during scanning is used (Fig. 10).
Figure 10.
Patient immobilisation used in treatments for pelvic tumours.
Patients with rectal cancer are scanned in the prone position. Combined PET/CT scanning may be useful, and if possible fusion of these images with a supplemental MR scan (the modality of choice in this type of cancer), should be available.
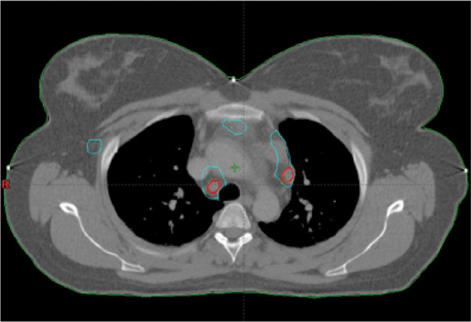
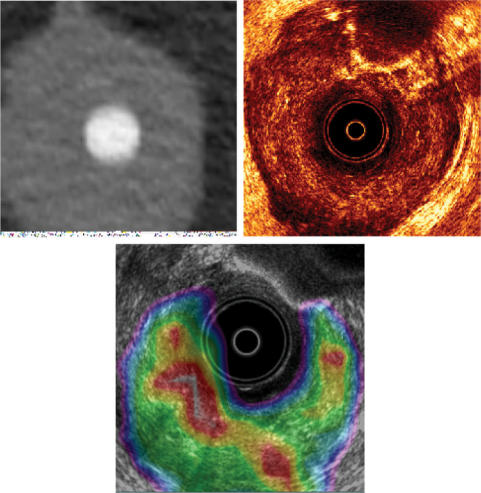
For patients with anal cancer a prosthesis similar to an ultrasound probe is placed so that fusion with a 3D ultrasound can later be completed. This form for combined scanning can further be utilised with needle placement in brachytherapy (Fig. 11).
Figure 11.
In the treatment for anal carcinoma, CT data (upper left) and ultrasonography (upper right) are fused with PET-CT data (lower). Note the ultrasound probe. The CT data with the GTV obtained from three different diagnostic methods are then used for the dose calculation.
CT or PET/CT scans are most useful for planning treatment for patients with lymphoma. The pre-chemotherapy GTV is used for therapy planning, and it is therefore important that the later treatment position is taken into account when performing the pre-chemotherapy diagnostic (PET)/CT scanning, to allow for optimal fusion of the images.
Patterns of failure
The concept of ‘highest probability of cure with lowest risk of complications’ is well known. It is the goal for every radiation oncologist. Ionising radiation does not selectively kill tumour cells. Late sequelae of radiotherapy are well known and radiation oncologists must try to reduce them. A PTV must be defined that gives the highest probability of cure with the lowest incidence of late complications. Reduction in PTV and irradiated volume reduces complication probability. The radiologist is therefore crucial in target volume definition.
Patterns of failure are the ultimate test of adequate target delineation. If all patients are locally cured with no side effects the target used was optimal. If local control is high but side effects are high, one might consider decreasing the PTV. If the cure rate is low and the late complication rate is low, then the PTV might be considered to be too small. In the follow-up of patients documentation of patterns of failure is very important. Patients should be seen by radiation oncologists interested in local tumour control as well as late complications.
Conclusions
Modern conformal radiotherapy can only be optimal if close collaboration is maintained between radiation oncologists and (diagnostic) imaging specialists. Adherence to definitions of volumes and stringent protocols for the different stages in planning and execution of treatment is mandatory if one wants to fully exploit the potential advantages of advanced modern radiotherapy techniques[33,34] for the benefit of the patient.
References
- 1.Dische S, Saunders MI, Williams C, Hopkins A, Aird E. Precision in reporting the dose given in a course of radiotherapy. Radiother Oncol. 1993;29:287–93. doi: 10.1016/0167-8140(93)90146-y. [DOI] [PubMed] [Google Scholar]
- 2.ICRU. ICRU Report 29. Bethesda, MD: ICRU; 1978. International Commission on Radiation Units and Measurements. Dose specification for reporting external beam therapy with photons and electrons. [Google Scholar]
- 3.ICRU. ICRU Report 38. Bethesda, MD: ICRU; 1985. International Commission on Radiation Units and Measurements. Dose and volume specifications for reporting intracavitary therapy in gynecology. [Google Scholar]
- 4.ICRU. ICRU Report 50. Bethesda, MD: ICRU; 1993. International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy. [Google Scholar]
- 5.ICRU. ICRU Report 58. Bethesda, MD: ICRU; 1997. International Commission on Radiation Units and Measurements. Dose and volume specification for reporting interstitial therapy. [Google Scholar]
- 6.ICRU. ICRU Report 62. Bethesda, MD: ICRU; 1999. International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting photon beam therapy, Supplement to ICRU Report No. 50. [Google Scholar]
- 7.ICRU. ICRU Report 71 J ICRU 2004. 1 Vol. 4. International Commission on Radiation Units and Measurements. Prescribing, recording, and reporting electron beam therapy. [Google Scholar]
- 8.1997. UICC International Union against Cancer. [Google Scholar]
- 9.2002. UICC International Union against Cancer. [Google Scholar]
- 10.AJCC. 1997. [Google Scholar]
- 11.AJCC. American Joint Committee on Cancer. In: Greene FL, Page DL, Fleming ID, et al., editors. Manual for staging of cancer. 6th. New York, Berlin, Heidelberg: Springer; 2002. [Google Scholar]
- 12.Carey BM. Imaging for prostate cancer. Clin Oncol. 2005;17:553–9. doi: 10.1016/j.clon.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.DICOM. Digital imaging and communications in medicine. Supplement 11: Radiotherapy objects. 1997. Available at: htpp:/www.dclunie.com/dicom-status/status.html. [Google Scholar]
- 14.DAHANCA (Danish Head and Neck Cancer Study Group) 2003. Guidelines for radiation treatment of head and neck cancer. DAHANCA. ( http://www.dahanca.dk). [Google Scholar]
- 15.Martinez-Monge R, Fernandes PS, Gupta N, Gahbauer R. Cross-sectional nodal atlas: a tool for the definition of clinical target volumes in three-dimensional radiation therapy planning. Radiology. 1999;211:815–28. doi: 10.1148/radiology.211.3.r99jn40815. [DOI] [PubMed] [Google Scholar]
- 16.Grégoire V, Scalliet P, Ang KK, editors. Berlin: Springer Verlag; 2004. Clinical target volumes in conformal and intensity modulated radiation therapy. A clinical guide to cancer treatment. [Google Scholar]
- 17.BIR. Prepared by a Working Party of the British Institute of Radiology. 2003 . Geometric uncertainties in radiotherapy: defining the planning target volume. (ISBN 0-905749-53-7). [Google Scholar]
- 18.Gustavsson A, Eskilsson J, Landberg T, et al. Late cardiac effects after mantle radiotherapy in patients with Hodgkin's disease. Ann Oncol. 1990;1:355–63. doi: 10.1093/oxfordjournals.annonc.a057774. [DOI] [PubMed] [Google Scholar]
- 19.Gustavsson A, Eskilsson J, Landberg T, et al. Long term effects on pulmonary function after mantle treatment in patients with Hodgkin's disease. Ann Oncol. 1992;3:455–61. doi: 10.1093/oxfordjournals.annonc.a058234. [DOI] [PubMed] [Google Scholar]
- 20.Forsell-Aronsson E, Kjellén E, Mattsson S, Hellström M, for the Swedish Cancer Society Investigation Group. Medical imaging for improved tumour characterization, delineation and treatment verification. Acta Oncol. 2002;41:604–14. doi: 10.1080/028418602321028201. [DOI] [PubMed] [Google Scholar]
- 21.Daisne J-F, Duprez T, Weynand B, et al. Tumour volume in pharyngolaryngeal squamous cell carcinoma: comparison at CT, MR imaging, and FDG PET and validation with surgical specimen. Radiology. 2004;233:93–100. doi: 10.1148/radiol.2331030660. [DOI] [PubMed] [Google Scholar]
- 22.Girinsky T, van der Maazen R, Specht L, et al. Involved node radiotherapy (INRT) in patients with early Hodgkin lymphoma: concepts and recommendations. Radiother Oncol. 2006;79:270–7. doi: 10.1016/j.radonc.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 23.RTOG 0225. Protocol for a phase II study of intensity modulated radiation therapy (IMRT) +/– chemotherapy for nasopharyngeal cancer. http://www.rtog.org. [Google Scholar]
- 24.Hamilton CS, Ebert MA. Volumetric uncertainty in radiotherapy. Clin Oncol (R Coll Radiol) 2005;17:456–64. doi: 10.1016/j.clon.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Leunens G, Menten J, Weltens C, Verstraet J, van der Scheuren E. Quality assessment of medical decision making in radiation oncology: variability in target volume delineation for brain tumours. Radiother Oncol. 1993;29:169–175. doi: 10.1016/0167-8140(93)90243-2. [DOI] [PubMed] [Google Scholar]
- 26.Rasch C, Steenbakkers R, van Herk M. Target definition in prostate, head, and neck. Semin Radiat Oncol. 2005;15:136–45. doi: 10.1016/j.semradonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Tai P, Van Dyk J, Yu E, Battista J, Stitt L, Coad T. Variability of target volume delineation in cervical esophageal cancer. Int J Radiat Oncol Biol Phys. 1998;42:277–88. doi: 10.1016/s0360-3016(98)00216-8. [DOI] [PubMed] [Google Scholar]
- 28.Hurkmans CW, Borger JH, Pieters BR, Russell NS, Jansen EP, Mijnheer BJ. Variability in target volume delineation on CT scans of the breast. Int J Radiat Oncol Biol Phys. 2001;50:1366–72. doi: 10.1016/s0360-3016(01)01635-2. [DOI] [PubMed] [Google Scholar]
- 29.Van de Steene J, Linthout N, de Mey J, et al. Definition of gross tumour volume in lung cancer: inter-observer variability. Radiother Oncol. 2002;62:37–49. doi: 10.1016/s0167-8140(01)00453-4. [DOI] [PubMed] [Google Scholar]
- 30.Villeirs GM, Van Vaerenbergh K, Vakaet L, et al. Interobserver delineation variation using CT versus combined CT+MRI in intensity-modulated radiotherapy for prostate cancer. Strahlenther Onkol. 2005;181:424–30. doi: 10.1007/s00066-005-1383-x. [DOI] [PubMed] [Google Scholar]
- 31.Steenbakkers RJ, Duppen JC, Fitton I, et al. Reduction of observer variation using matched CT-PET for lung cancer delineation: a three-dimensional analysis. Int J Radiat Oncol Biol Phys. 2006;64:435–48. doi: 10.1016/j.ijrobp.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 32.Riegel AC, Berson AM, Destian S, et al. Variability of gross tumour volume delineation in head-and-neck cancer using CT and PET/CT fusion. Int J Radiat Oncol Biol Phys. 2006;65:726–32. doi: 10.1016/j.ijrobp.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 33.Royal College of Radiologists. 2006. Recommendations for cross-sectional imaging in cancer management. http://www.rcr.ac.uk. [Google Scholar]
- 34.Royal College of Radiologists. 2004. Imaging for oncology, Faculty of Clinical Oncology, Royal College of Radiologists. (ISBN 1 872599 99 0). [Google Scholar]
- 35.Withers HR, Taylor JMG, Maciejewski B. Treatment volume and tissue tolerance. Int J Radiat Oncol Biol Phys. 1988;14:751–9. doi: 10.1016/0360-3016(88)90098-3. [DOI] [PubMed] [Google Scholar]
- 36.Källman P, Ågren A, Brahme A. Tumour and normal tissue responses to fractionated nonuniform dose delivery. Int J Radiat Biol. 1992;62:249–62. doi: 10.1080/09553009214552071. [DOI] [PubMed] [Google Scholar]
- 37.Deasy JO, Blanco AI, Clark VH. CERR: a computational environment for radiotherapy research. Med Phys. 2003;30:979–85. doi: 10.1118/1.1568978. [DOI] [PubMed] [Google Scholar]