Abstract
Melanocytic dysplastic nevi were first described in both patients and their relatives who had one or several cutaneous malignant melanomas. Most of these dysplastic lesions are biologically stable, but some of them have severe histological atypia and can progress further to melanomas. Although several studies have suggested the etiological importance of dysplastic nevi in the development of melanomas, comprehensive reviews of the molecular changes in these dysplastic lesions are still scarce. To remedy this issue, this article analyzes the available molecular information about dysplastic nevi and provides the current state of knowledge regarding the karyotypic abnormalities of the melanoma/dysplastic nevus trait and the involvement of allelic loss, tumor suppressor genes, mismatch repair proteins, microsatellite instability, oncogenes, extracellular matrix proteins, and growth factors in the genesis of these lesions. These studies suggest that although some of these lesions represent “genetic dead-ends,” others represent intermediate lesional steps in the melanoma tumorigenesis pathway.
Although the concept of the melanocytic dysplastic nevus as a risk factor for cutaneous malignant melanomas (CMMs) was introduced only recently, these lesions were observed long ago 1 (Figure 1) . In 1978, Clark et al 2 published the first report to advance melanocytic dysplastic nevi (MDN) as a separate pathological entity. In 1980, Greene and colleagues 3, 4, 5 applied the term “dysplastic nevus,” as the lesions have clinically, architecturally, and cytologically atypical features. These moles are categorized into sporadic and familial dysplastic nevi. 6, 7 The presence of MDN is associated with almost 100% and 60% of familial and sporadic CMMs, respectively. 8, 9, 10, 11
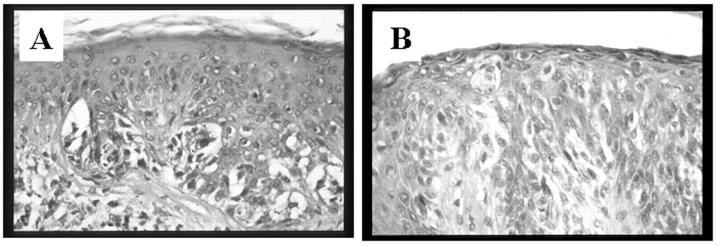
Figure 1.
Histological appearance of melanocytic dysplastic nevus (A) and cutaneous malignant melanoma (B).
Over the last decade, most of the relevant molecular analyses have focused on CMMs rather than on MDN. The underlying reasons include the relatively large size of CMMs, their direct lethal outcome and the feasibility of propagating and establishing corresponding CMM cell lines. In contrast, due to their relatively small size, variable criteria for histological diagnosis, controversial terminology, and difficulty in establishing in vitro cultures, MDN have hardly been studied. The limited studies on these lesions reported some genetic changes and suggested that evolution of some MDN may result in CMMs. Although genetic changes in CMMs have been analyzed in several review articles, reviews about these changes in MDN have remained scarce. To remedy this gap in the literature, this review seeks to examine genetic alterations in MDN.
Genetic Alterations in MDN
The genesis of MDN seems to be a complex process that involves poorly understood phenotypic and genotypic alterations. These alterations include loss of tumor suppressor genes (TSGs) and alterations of oncogenes, housekeeping genes, growth factors, and extracellular matrix proteins. Two models are currently proposed to explain the genesis of MDN. The first one considers that MDN arise by inactivation of one allele of melanoma suppressor genes, while the subsequent loss of the second allele leads to malignant transformation of the dysplastic nevic cells. 12 The second model relies on the presence of at least two genes working independently. Therefore, alterations of one of them cause dysplasia in the melanocytes while the other results in malignant transformation. 13 Both models rely on the “two-hit” hypothesis, suggesting that two genetic events are required for inactivation of TSGs. In familial forms of cancer, one mutation is believed to be germline and the other somatic, whereas in sporadic cancers, both mutations are somatic. 14
Genetic alterations accompany and drive the evolution of neoplasms and their subsequent progression to more malignant phenotypes. These alterations may either provide new potential for aggressive behavior of the tumor cells, such as by activation of oncogenes and alterations of housekeeping genes, or may release the tumor cells from regulatory effects through the loss of TSGs. We subdivided these genetic alterations into: karyotypic changes in CMM/MDN trait; allelic loss; alterations of tumor suppressor genes; alterations of mismatch repair protein expression; microsatellite instability; alterations of oncogenes; alterations of the extracellular proteins; and alterations of cytokines and growth factors.
Karyotypic Alterations
Chromosome 1p and CMM/MDN Trait
Linkage analysis studies mapped a susceptibility locus for CMM/MDN to chromosome 1p near the rhesus blood group locus (Rh). Subsequent linkage analysis studies supported a role for 1p but also excluded many candidate regions around this locus. 15, 16 In 1989, a CMM/MDN locus was mapped to chromosome 1p36 by Bale et al. 17 To determine the site of this locus, Bale and colleagues evaluated 99 relatives and 26 spouses in six families with CMM/MDN predisposition using 26 polymorphic markers on the 1p region. They analyzed the co-segregation of the CMM/MDN trait and mapped the trait susceptibility locus to be between an anonymous DNA marker (D1S47) and the gene locus for pronatrodilatin (PND) at the 1p36 region. 17 The failure of subsequent studies from 1989 to 1991 12, 18 to confirm linkage between a CMM/MDN locus and the 1p region may have been due to diagnostic, clinical, and genetic heterogeneity. 19, 20 .
In 1992, Goldstein et al 12, 18, 19, 21, 22, 23 incorporated the previous linkage analyses and performed three linkage analyses to examine the relationship between CMM/MDN and D1S47, PND, and D1S160 markers in several families. They demonstrated that the CMM/MDN susceptibility locus is located at 1p36 and linked to the D1S47 marker. 19 They also showed strong evidence for genetic heterogeneity in these lesions. In 1996, Goldstein and colleagues 20 simultaneously examined the 1p36 and 9p21 regions using two-trait-locus, two-marker-locus linkage analysis. Their work suggested the presence of two susceptibility loci at these regions, with the 1p locus contributing to both CMM and CMM/MDN and a 9p locus contributing mostly to CMM alone.
Chromosome 9p and CMM/MDN Trait
In 1991, Fountain et al and Petty et al 24, 25 presented papers at the eighth International Congress of Human Genetics proposing chromosome 9p as a possible location of melanoma susceptibility genes. In 1992, further support for this proposal came from the work of Nancarrow et al, 21 who carried out genetic linkage analysis in Australian kindreds with CMMs. This group found two major gaps in the exclusion map, located at 9p22 cen and 9q12-q32, as well as smaller regions at each telomere. Simultaneously, Cannon-Albright et al 26 examined eleven extended kindreds with 82 cases of CMMs using genetic markers for the 9p22–21 region. They assigned the susceptibility locus to 9p13-p22 and addressed the possibility that the locus functions as a tumor suppressor gene. 27 In 1993, Nancarrow et al 28 confirmed these findings by examining linkage analysis in 26 Australian CMMs families for IFNA and D9S126 markers in the 9p region. Subsequently, Goldstein et al 29 performed linkage analysis on 13 families previously investigated for linkage to chromosome 1p. 16, 21, 22, 28 They used IFNA/D9S126 markers and reported significant evidence for linkage to IFNA. In contrast, they found no evidence for linkage between CMM alone or CMM/MDN and D9S126.
Chromosome 1p and 9p Involvement in CMM/MDN Trait
Despite their contributions, these studies failed to answer a challenging question: is there a single CMM/MDN locus or are there two tightly linked loci, one for CMM and the other for MDN? To address this question, Goldstein and colleagues 20 conducted two-trait-locus, two-marker-locus linkage analysis on 19 CMM/MDN kindreds (previously genotyped for one or more markers on the 9p and 1p regions) in 1996. This study suggested that two loci act separately in production of CMM or CMM/MDN, with substantially stronger evidence of CMM linkage to a 9p than to a 1p region. In contrast, another study presented comparable evidence for CMM/MDN linkage to both regions. Nevertheless, it is still possible that the 1p locus contributes to both CMM and CMM/MDN, whereas a 9p locus contributes more to CMM alone. 30
To summarize, most genetic analysis studies have suggested that CMMs and MDN may be pleiotropic manifestations of alterations of the same susceptibility gene. 31 A subset of kindreds showed evidence of linkage to a 1p region, another subset to a 9p region, and others to both 1p and 9p regions. Furthermore, the assignment of the locus for CMM/MDN to regions (1p and 9p) in the human genome that are usually involved with karyotypic abnormalities in CMMs raised the possibility that chromosomal deletions represent an important event in the evolution of the CMM/MDN trait. 32, 33
Allelic Loss at the 1p, 9p, and 17p Regions
The concept of TSGs implies that every living cell is potentially cancerous, but as long as it has functioning TSGs, it is somehow prevented from fulfilling its malignant potential. The evidence for the presence of tumor suppressor genes can be obtained from loss of heterozygosity (LOH) studies. Loss of heterozygosity can result from chromosomal deletion, mitotic recombination, non-disjunction, or unbalanced translocation. 34, 35
In MDN, few studies have examined the presence of LOH at the 1p, 9p, and 17p regions using polymerase chain reaction-based microsatellite assays (Figures 2and 3). 36, 37, 38 Previous studies indicated a close link between the sites of LOH and the location of TSGs. 39, 40 Interestingly, the TSGs at these chromosomal regions are commonly involved in a wide variety of tumors. 41, 42, 43, 44, 45, 46 It is unknown if these TSGs belong to the class of general TSGs that can be inactivated by various mechanisms in a variety of tumors. 45, 47 Although the allelic loss in MDN was much lower than that in CMMs, it was still similar in pattern. Therefore, it is conceivable that LOH at these (1p36 and 9p22–21) regions does play an early role in CMMs tumorigenesis. These observations may support the existence of a biological continuum between some MDN and CMMs 48, 49 and reinforce the need to follow up these dysplastic lesions.
Loss of Tumor Suppressor Genes
TSGs are genes which, when deleted, inactivated, or expressed at a reduced level contribute to carcinogenesis. So far, three TSGs have been examined in MDN, including p16/CDKN2A, TP53, and Melastatin genes.
p16/CDKN2A Gene (Cyclin Dependent Kinase Inhibitor 2A)
p16 (MTS1/multiple tumor suppressor 1) is a putative TSG 50, 51 located at 9p21 region and encoding for a p16INK4a protein or INK4a (inhibitor for kinase 4a). 52, 53, 54 This protein inhibits the activity of the cyclin D1-CDK4 complex. This complex phosphorylates the retinoblastoma (Rb) protein and therefore allows progression of the cells through the G1 cell cycle checkpoint. Therefore, p16 protein works as a TSG by exerting negative regulation of cell growth. 52 p16 is commonly deleted and mutated in a variety of neoplasms, such as bladder tumors, esophageal cancer 44, 45, 46 and CMMs, and in lymphoblastoid cell lines derived from patients with dysplastic nevus syndrome. 45
In MDN, mutational analysis of p16 revealed contrasting results. Therefore, while some groups asserted the absence of these mutations, 55 others acknowledged the presence of point mutations in these dysplastic lesions. 36 p16 mutations were not of the type commonly involved in CMMs, raising the possibility that they could represent artifacts of polymerase chain reaction. 54
On the immunohistochemical level, p16 protein was expressed in nearly all MDN at levels similar to those in benign nevi. 56, 57, 58 Keller-Melchior et al 58 reported a uniform labeling pattern in almost 86% and 59% of the cells of MDN and CMMs, respectively. Whether p16/INK4a alterations play an important role in the evolution of MDN and development of CMMs is still unclear.
TP53 gene
TP53 is a stress response gene, located at the 17p13.1 region, that encodes a 53-kd oncosuppressive nuclear protein with an Mr of 53,000. 59, 60, 61, 62, 63, 64, 65, 66 Its main functions include maintenance of genomic stability and induction of apoptosis in response to DNA damage. 67, 68, 69 Loss of these functions leads to increased genomic instability and gene amplification and change in DNA ploidy. 70, 71 A combination of these alterations is associated with transformation in vitro and development of neoplasms in vivo. 72, 73 This critical role of p53 in tumorigenesis is evidenced by the fact that TP53 is involved in more than 50% of human malignancies. 72, 74, 75
Several methods have been used to detect p53 gene alterations in MDN, including single stranded conformation polymorphism of exons 5 through 8 (the hot spots for mutations). 76 In MDN, the frequency of TP53 gene mutations is much lower (∼0% to 18%) than that in CMMs. These mutations include the presence of C:G to T:A transition-type mutations related to UV irradiation 36 and silent mutations. 77, 78 Of note, most TP53 mutation-positive nevi were found in patients who previously had cutaneous moles and a family and/or personal history of CMMs. 78 It is still unclear whether the presence of these mutations in MDN implies that TP53 gene mutations play a role early in the evolution of CMMs. 36
In MDN, immunohistochemistry has been used to detect alterations in p53 protein expression. This method can detect the altered p53 protein with an increased half-life. 76 Accumulation of p53 protein in MDN has been reported by a few groups. 79, 80, 81, 82 However, in most of these reports, the overall frequency of p53 immunoreactivity was much lower (∼5% to 15%) when compared to CMMs. 83 Similarly, the p53 staining in these lesions was heterogeneous, with a considerably reduced percentage of positively stained cells (less than 1%) when compared to CMMs. 83, 84 These observations raise two notions: the difference in p53 protein expression between MDN and CMMs might be related to the differences in their biological behavior and the rare p53 positivity in MDN may merely reflect cell cycle fluctuations of p53 protein at the checkpoints, not underlying TP53 gene defects. 79, 85, 86, 87
Melastatin
Located at 15q13-q14 regions, Melastatin is a novel suppressor of metastasis gene identified in murine and human CMMs cells. The expression of this melanocyte-specific gene is down-regulated with CMM progression and is inversely related to tumor thickness. In MDN, in situ hybridization revealed diffuse Melastatin mRNA expression, although the exact role of this molecule is still unknown. 88, 89, 90
Alterations of Mismatch Repair Protein Expression
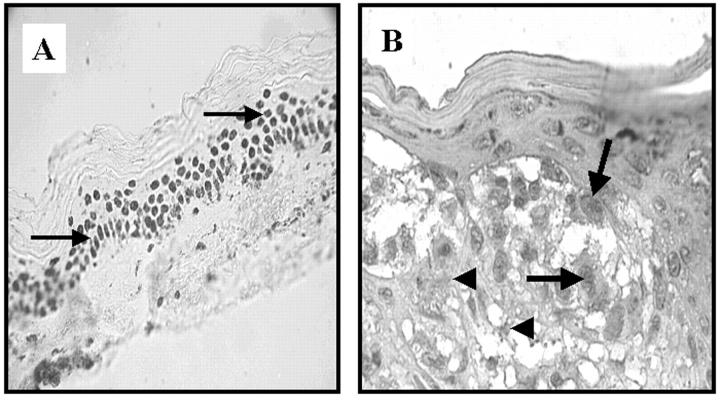
The mismatch repair (MMR) system is responsible for the repair of mismatched bases during DNA replication. 91 In humans, its enzymatic components include hMSH2 (MutS homolog 2), hMLH1 (MutL homolog 1), hPMS1 and hPMS2 (human postmeiotic segregation 1 and 2), and GTBP (GT binding protein). 92, 93, 94 These genes are located on 2p16, 3p21–23, 2q31–33, 7p22, and 2p16 chromosomal regions, respectively 95, 96 and function similarly to TSGs. 97 Therefore, loss of both alleles causes rapid accumulation of mutations, altered expression of the corresponding MMR proteins, and microsatellite instability. In some tumors, such as those of the urinary bladder and lung, these alterations have diagnostic and prognostic ramifications. 98, 99, 100, 101, 102 In MDN, examination of the expression patterns of the repair proteins using immunoperoxidase-staining methods revealed that the vast majority of MDN had strong immunopositivity (Figure 4) . 103 Interestingly, the repair protein expression values in MDN were intermediate between those of benign melanocytic nevi and CMMs. In this respect, these findings are consistent with the hypothesis of Clark et al 48, 103 that MDN represent intermediate lesions in the evolution of CMMs.
Figure 4.
Immunohistochemical staining of MMR proteins in melanocytic dysplastic nevi (MDN). A: hMLH1 expression in the normal epidermis. Note the nuclear expression pattern (arrow): B: hMSH2 expression in MDN. Positively (arrow) and negatively (arrowhead) stained cells are observed in both MDN and CMM.
Microsatellite Instability
Microsatellites are sequences made up of single sequence motifs no more than six bases long that are arranged in a head-to-tail manner. These sequences are repetitively scattered throughout the human genome with the most common class being in the form of (CA)n. 104 The variation in microsatellite pattern length between tumorous and matching non-tumorous tissues is referred to as microsatellite instability (MSI), and the tumors demonstrating this phenomenon are labeled as tumors with MSI (Figure 2) . 104, 105, 106 According to the level of instability, tumors with MSI were categorized into two groups: MSI-H and MSI-L (for high and low instability). The group with instability at >30% of tested markers (ie, MSI-H pattern), such as human non-polyposis colorectal cancer (HNPCC), 107 was found to have mutations in MMR genes. 92, 108, 109 The other group of tumors, with instability at <30% of tested markers (ie, MSI-L pattern), arises through unknown mechanisms. 107, 110
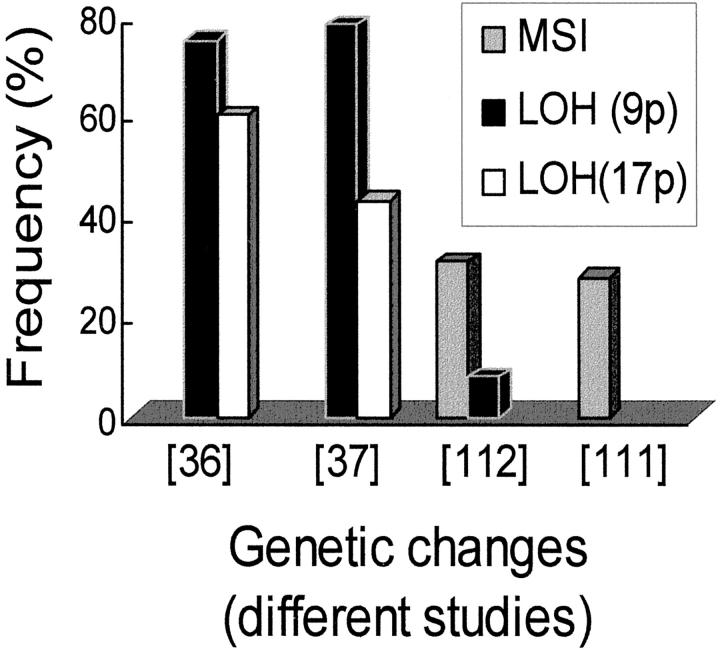
Figure 2.
The reported frequency of microsatellite instability (MSI) and loss of heterozygosity (LOH) on several chromosomal arms in melanocytic dysplastic nevi. The bars compare the frequency of these genetic changes among the different studies. 36 37 111 112
In MDN, MSI-L pattern was found at the 1p and 9p regions (Figures 2 and 3) . 111, 112 These chromosomal regions are well known for their karyotypic abnormalities in CMMs and harbor significant cancer genes such as p16 (9p22–21), p73, and p58 (1p36). 113 The incidence of MSI was statistically significant in MDN as compared to benign melanocytic nevi, and there was a statistically significant difference in the prevalence of MSI between both MDN with severe and moderate atypia when compared to those with mild atypia. The finding of MSI-L pattern in MDN suggests that MSI is acquired early during CMMs development and supports the notion that some MDN represent an early stage of this process. The presence of MSI-L in these lesions may be explained by: the variable expression of MMR genes with weakly penetrant mutations and attenuated phenotype; 114 inherent intrinsic instability of these loci; and the inactivation of non-MMR genes or additional MMR genes other than those encountered in HNPCC, such as the hMSH6 gene. 111, 115, 116
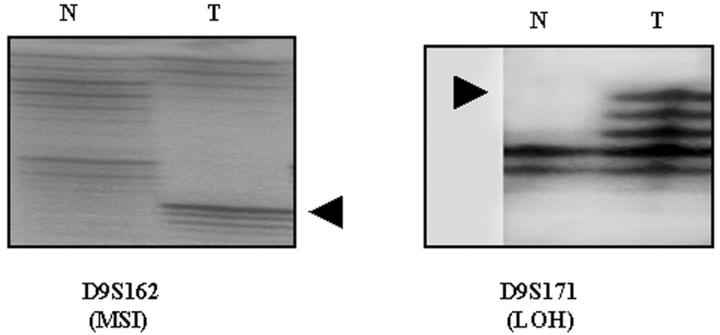
Figure 3.
Genetic changes in melanocytic dysplastic nevi. Left: Microsatellite instability (MSI), with the arrowhead indicating the appearance of a novel band in the tumor (T) DNA as compared to DNA from the normal tissues (N). Right: Loss of heterozygosity (LOH) in the tumor DNA, with the arrowhead indicating loss of upper allele in the tumor.
Oncogenes
Proto-oncogenes have critical roles in the regulation of both cell growth and differentiation and after alteration can confer a malignant potential and become oncogenes. 117 So far, few studies have examined the role of oncogenes, such as ras and myc, in the pathogenesis of MDN. The ras family of proto-oncogenes encodes small GTP-binding proteins involved in intracellular signal transduction of mitogenic signals arising from activation of growth factor receptors. 54 Although mutations of ras genes are relatively common (5% to 24%) in CMMs, 118, 119 they are only occasionally found in MDN. It is still unclear if the infrequent ras mutations in MDN indicate involvement of this gene in the initiation or progression of these atypical lesions. 120
The myc oncogene is a cellular proto-oncogene that codes for a nuclear phosphoprotein. Its functions include regulation of G0/G1 cell cycle transition and control of cellular differentiation. myc overexpression has been reported in a variety of tumors. 121, 122, 123 In CMMs, high c-myc expression has been found in primary and metastatic tumors. 122 Using interphase fluorescence in situ hybridization, Kraehn et al reported c-myc gain in relation to the centromere 8-copy number in advanced CMMs and the absence of a similar gain in nevi. 123
Alterations of the Extracellular Proteins
The extracellular matrix (ECM) represents a network of proteins that can interact with tumor cells and thereby modulate their proliferation and migration. 124 In CMMs, the expression of these molecules gradually increases with the progression of the tumor. Morphologically, MDN are characterized by the presence of peculiar stromal reactions, ie, fibroplasia. 5 On the molecular level, altered expression of ECM proteins (interstitial collagens type I, III, and VI, tenascin, and fibronectin) was found in the stroma surrounding dysplastic nevic cells, suggesting that alteration of these molecules may create a suitable microenvironment for the progression of these lesions. 125, 126, 127, 128, 129, 130
Alterations of Cytokines and Growth Factors
Cytokines are a group of polypeptides that has modulatory actions on the growth and proliferation of cells. Over the last decade, several lines of evidence have suggested that these substances are critical for the uncontrolled growth of tumor cells in vitro and potentially have the same effect in vivo. 131, 132 In vitro experiments have established the role of these peptides in the abnormal growth of CMMs. 133 Basic fibroblast growth factor (bFGF) is a 17.5-kd-polypeptide autocrine growth factor. It is produced by several tissues and is involved in angiogenesis and mutagenesis. 134, 135 In vitro, it is produced by CMMs cell lines 136 but not by normal melanocytes. 137 In MDN, bFGF was found to be differentially expressed; however, its exact role in these dysplastic lesions is still unknown. 132
Conclusions
In the multi-step melanoma tumorigenesis pathway that culminates with the metastatic phase, early steps appear to involve mutations of the melanocytes of the MDN. A review of the changes involved in the pathogenesis of MDN (Table 1) reveals four key points: the molecular changes in MDN are complex and involve allelic loss, MSI, and alterations of TSGs, MMR proteins, oncogenes, ECM proteins, and some growth factors; some of these genetic alterations are shared between MDN and CMMs, suggesting that some MDN represent intermediate steps or sequential phases in CMM tumorigenesis; the association of some MDN with CMMs may reflect pleiotropic and divergent manifestations of these genetic changes rather than sequential phases in multi-step melanoma tumorigenesis; and although significant information is accumulating about molecular changes in CMMs, little is available about MDN. Therefore, our understanding of the genetic changes in MDN is limited, and much work is needed to expand it. Finally, the sense of confidence afforded by histological evaluation of the melanocytic lesions and especially CMMs, “the fully developed tumor among the melanocytic lesions,” is short-lived, and conclusively predicting their biology is beyond the scope of light microscopy and immunohistochemical or molecular markers available to date. In the face of these inadequacies, unfolding studies regarding gene expression profiling in CMMs, a strategy that can establish the expression of thousands of individual genes in the tissue sample, seem to have promising investigative, diagnostic, and prognostic ramifications. In this sense, expression profiling in CMMs can separate these tumors into distinct subsets and relate these subsets to definitive stages in the course of melanocytic transformation. 138, 139, 140
Table 1.
Molecular Changes in MDN
| Genetic changes | Chromosomal regions | References |
|---|---|---|
| Karyotypic alterations | 1p and 9p | 60, 67, 75, 76 |
| Allelic loss | 1p, 9p and 17p | 79, 80, 81, 152 |
| Loss of tumor suppressor genes | ||
| TP53 gene | 17p.13 | 79, 80, 101 |
| p16/CDKN2A gene | 9p22-21 | 80, 92, 93 |
| Melastatin | 15q13-q14 | 131, 132, 133 |
| Alterations of MMR protein expression | 54 | |
| Microsatellite Instability | 1p32-36 and 9p22-21 | 53, 152 |
| Oncogenes | ||
| ras gene | 19q13.3-qter | 158, 159 |
| myc gene | 8q24, 12q24.13 | 161, 162, 163 |
| Alterations of the extracellular proteins | ||
| Interstitial collagens type I, III, and VI | 17q21, 2q31, 21q22 | 166, 167, 168, 169 |
| Tenascin and fibronectin | 6p21.3, 2q34 | 165, 169, 170 |
| Alterations of growth factors | ||
| Basic fibroblast growth factor (bFGF) | 4q25-27 | 171, 172 |
Acknowledgments
We thank Ms. Anna Midtling for critical suggestions to improve the manuscript, Drs. Anita Gilliam, Sahar Ismail, and George Reizner for figure procurement, and Ms. Kathryn Kleckner and Mr. Jeffery Root for figure preparation.
Address reprint requests to Gary S. Wood, M.D., Department of Medicine (Dermatology), University of Wisconsin and William S. Middleton Memorial Veteran Hospital, Madison, WI 53705. E-mail: gsw@medicine.wisc.edu.
Footnotes
Supported by Merit Review Funding from the Department of Veteran Affairs and National Institutes of Health grant AR 01326.
References
- 1.Munro DD: Multiple active junctional naevi with family history of malignant melanoma. Proc R Soc Med 1974, 67:594-595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark WH, Jr, Reimer RR, Greene M, Ainsworth AM, Mastrangelo MJ: Origin of familial malignant melanomas from heritable melanocytic lesions: “the B-K mole syndrome.”. Arch Dermatol 1978, 114:732-738 [PubMed] [Google Scholar]
- 3.Greene MH, Clark WH, Jr, Tucker MA, Elder DE, Kraemer KH, Fraser MC, Bondi EE, Guerry D, Tuthill R, Hamilton R, LaRossa D: Precursor naevi in cutaneous malignant melanoma: a proposed nomenclature. Lancet 1980, 2:1024. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JW: Primary cutaneous malignant melanoma: diagnosis and management. Aust Fam Physician 1986, 15:855–856, 858–859 [PubMed]
- 5.: NIH Consensus Conference: Diagnosis and treatment of early melanoma. JAMA 1992, 268:1314-1319 [DOI] [PubMed] [Google Scholar]
- 6.Elder DE, Jucovy PM, Tuthill RJ, Clark WH, Jr: The classification of malignant melanoma. Am J Dermatopathol 1980, 2:315-320 [DOI] [PubMed] [Google Scholar]
- 7.Rahbari H, Mehregan AH: Sporadic atypical mole syndrome: a report of five nonfamilial B-K mole syndrome-like cases and histopathologic findings. Arch Dermatol 1981, 117:329-331 [DOI] [PubMed] [Google Scholar]
- 8.Greene MH, Clark WH, Jr, Tucker MA, Kraemer KH, Elder DE, Fraser MC: High risk of malignant melanoma in melanoma-prone families with dysplastic nevi. Ann Intern Med 1985, 102:458-465 [DOI] [PubMed] [Google Scholar]
- 9.Carey WP, Jr, Thompson CJ, Synnestvedt M, Guerry DT, Halpern A, Schultz D, Elder DE: Dysplastic nevi as a melanoma risk factor in patients with familial melanoma. Cancer 1994, 74:3118-3125 [DOI] [PubMed] [Google Scholar]
- 10.Elder DE, Goldman LI, Goldman SC, Greene MH, Clark WH, Jr: Dysplastic nevus syndrome: a phenotypic association of sporadic cutaneous melanoma. Cancer 1980, 46:1787-1794 [DOI] [PubMed] [Google Scholar]
- 11.Rhodes AR, Harrist TJ, Day CL, Mihm MC, Jr, Fitzpatrick TB, Sober AJ: Dysplastic melanocytic nevi in histologic association with 234 primary cutaneous melanomas. J Am Acad Dermatol 1983, 9:563-574 [DOI] [PubMed] [Google Scholar]
- 12.Kefford RF, Salmon J, Shaw HM, Donald JA, McCarthy WH: Hereditary melanoma in Australia: variable association with dysplastic nevi and absence of genetic linkage to chromosome 1p. Cancer Genet Cytogenet 1991, 51:45-55 [DOI] [PubMed] [Google Scholar]
- 13.Weiss J, Garbe C, Buttner P, Jung EG: Dysplastic nevi-dysplastic nevus syndromes: clinical features and genetic aspects. Recent Results Cancer Res 1993, 128:101-118 [DOI] [PubMed] [Google Scholar]
- 14.Knudson AG, Jr: Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci USA 1971, 68:820-823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene MH, Goldin LR, Clark WH, Jr, Lovrien E, Kraemer KH, Tucker MA, Elder DE, Fraser MC, Rowe S: Familial cutaneous malignant melanoma: autosomal dominant trait possibly linked to the Rh locus. Proc Natl Acad Sci USA 1983, 80:6071-6075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun-Falco O, Landthaler M, Ryckmanns F: BK-mole syndrome. Fortschr Med 1979, 97:1489-1494 [PubMed] [Google Scholar]
- 17.Bale SJ, Dracopoli NC, Tucker MA, Clark WH, Jr, Fraser MC, Stanger BZ, Green P, Donis-Keller H, Housman DE, Greene MH: Mapping the gene for hereditary cutaneous malignant melanoma-dysplastic nevus to chromosome 1p (published erratum appears in N Engl J Med 1991, 324: 925). N Engl J Med 1989, 320:1367-1372 [DOI] [PubMed] [Google Scholar]
- 18.van Haeringen A, Bergman W, Nelen MR, van der Kooij-Meijs E, Hendrikse I, Wijnen JT, Khan PM, Klasen EC, Frants RR: Exclusion of the dysplastic nevus syndrome (DNS) locus from the short arm of chromosome 1 by linkage studies in Dutch families. Genomics 1989, 5:61-64 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein AM, Dracopoli NC, Ho EC, Fraser MC, Kearns KS, Bale SJ, McBride OW, Clark WH, Jr, Tucker MA: Further evidence for a locus for cutaneous malignant melanoma-dysplastic nevus (CMM/DN) on chromosome 1p, and evidence for genetic heterogeneity. Am J Hum Genet 1993, 52:537-550 [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein AM, Goldin LR, Dracopoli NC, Clark WH, Jr, Tucker MA: Two-locus linkage analysis of cutaneous malignant melanoma/dysplastic nevi. Am J Hum Genet 1996, 58:1050-1056 [PMC free article] [PubMed] [Google Scholar]
- 21.Nancarrow DJ, Walker GJ, Weber JL, Walters MK, Palmer JM, Hayward NK: Linkage mapping of melanoma (MLM) using 172 microsatellite markers. Genomics 1992, 14:939-947 [DOI] [PubMed] [Google Scholar]
- 22.Nancarrow DJ, Palmer JM, Walters MK, Kerr BM, Hafner GJ, Garske L, McLeod GR, Hayward NK: Exclusion of the familial melanoma locus (MLM) from the PND/D1S47 and MYCL1 regions of chromosome arm 1p in seven Australian pedigrees. Genomics 1992, 12:18-25 [DOI] [PubMed] [Google Scholar]
- 23.Gruis NA, Bergman W, Frants RR: Locus for susceptibility to melanoma on chromosome lp (letter; comment). N Engl J Med 1990, 322:853-854 [DOI] [PubMed] [Google Scholar]
- 24.Fountain JW, Karayiorgou M, Ernstoff MS, Kirkwood JM, Vlock DR, Titus-Ernstoff L, Bouchard B, Vijayasaradhi S, Houghton AN, Lahti J, Kidd VJ, Housman DE, Dracopoli NC: Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci USA 1992, 89:10557-10561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petty RE, Southwood TR, Baum J, Bhettay E, Glass DN, Manners P, Maldonado-Cocco J, Suarez-Almazor M, Orozco-Alcala J, Prieur AM: Revision of the proposed classification criteria for juvenile idiopathic arthritis: Durban, 1997. J Rheumatol 1998, 25:1991-1994 [PubMed] [Google Scholar]
- 26.Cannon-Albright LA, Goldgar DE, Meyer LJ, Lewis CM, Anderson DE, Fountain JW, Hegi ME, Wiseman RW, Petty EM, Bale AE, Olopade OI, Diaz MO, Kwiatkowski DJ, Piepkorn MW, Zone JJ, Skolnick MH: Assignment of a locus for familial melanoma, MLM, to chromosome 9p13–p22. Science 1992, 258:1148-1152 [DOI] [PubMed] [Google Scholar]
- 27.Jonasson J, Povey S, Harris H: The analysis of malignancy by cell fusion: VII. cytogenetic analysis of hybrids between malignant and diploid cells and of tumours derived from them. J Cell Sci 1977, 24:217-254 [DOI] [PubMed] [Google Scholar]
- 28.Nancarrow DJ, Mann GJ, Holland EA, Walker GJ, Beaton SC, Walters MK, Luxford C, Palmer JM, Donald JA, Weber JL, Fountain JW, Kefford RF, Hayward NK: Confirmation of chromosome 9p linkage in familial melanoma. Am J Hum Genet 1993, 53:936-942 [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein AM, Dracopoli NC, Engelstein M, Fraser MC, Clark WH, Jr, Tucker MA: Linkage of cutaneous malignant melanoma/dysplastic nevi to chromosome 9p, and evidence for genetic heterogeneity. Am J Hum Genet 1994, 54:489-496 [PMC free article] [PubMed] [Google Scholar]
- 30.Bergman W, Gruis NA, Sandkuijl LA, Frants RR: Genetics of seven Dutch familial atypical multiple mole-melanoma syndrome families: a review of linkage results including chromosomes 1 and 9. J Invest Dermatol 1994, 103:122S-125S [DOI] [PubMed] [Google Scholar]
- 31.Bale SJ, Chakravarti A, Greene MH: Cutaneous malignant melanoma and familial dysplastic nevi: evidence for autosomal dominance and pleiotropy. Am J Hum Genet 1986, 38:188-196 [PMC free article] [PubMed] [Google Scholar]
- 32.Balaban GB, Herlyn M, Clark WH, Jr, Nowell PC: Karyotypic evolution in human malignant melanoma. Cancer Genet Cytogenet 1986, 19:113-122 [DOI] [PubMed] [Google Scholar]
- 33.Cowan JM, Halaban R, Francke U: Cytogenetic analysis of melanocytes from premalignant nevi and melanomas. J Natl Cancer Inst 1988, 80:1159-1164 [DOI] [PubMed] [Google Scholar]
- 34.Marshall CJ: Tumor suppressor genes. Cell 1991, 64:313-326 [DOI] [PubMed] [Google Scholar]
- 35.Cavenee WK, Dryja TP, Phillips RA, Benedict WF, Godbout R, Gallie BL, Murphree AL, Strong LC, White RL: Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature 1983, 305:779-784 [DOI] [PubMed] [Google Scholar]
- 36.Lee JY, Dong SM, Shin MS, Kim SY, Lee SH, Kang SJ, Lee JD, Kim CS, Kim SH, Yoo NJ: Genetic alterations of p16INK4a and p53 genes in sporadic dysplastic nevus. Biochem Biophys Res Commun 1997, 237:667-672 [DOI] [PubMed] [Google Scholar]
- 37.Park WS, Vortmeyer AO, Pack S, Duray PH, Boni R, Guerami AA, Emmert-Buck MR, Liotta LA, Zhuang Z: Allelic deletion at chromosome 9p21(p16) and 17p13(p53) in microdissected sporadic dysplastic nevus. Hum Pathol 1998, 29:127-130 [DOI] [PubMed] [Google Scholar]
- 38.Boni R, Zhuang Z, Albuquerque A, Vortmeyer A, Duray P: Loss of heterozygosity detected on 1p and 9q in microdissected atypical nevi (letter). Arch Dermatol 1998, 134:882-883 [DOI] [PubMed] [Google Scholar]
- 39.Yeh SH, Chen PJ, Chen HL, Lai MY, Wang CC, Chen DS: Frequent genetic alterations at the distal region of chromosome 1p in human hepatocellular carcinomas. Cancer Res 1994, 54:4188-4192 [PubMed] [Google Scholar]
- 40.Kuroki T, Fujiwara Y, Tsuchiya E, Nakamori S, Imaoka S, Kanematsu T, Nakamura Y: Accumulation of genetic changes during development and progression of hepatocellular carcinoma: loss of heterozygosity of chromosome arm 1p occurs at an early stage of hepatocarcinogenesis. Genes Chromosomes Cancer 1995, 13:163-167 [DOI] [PubMed] [Google Scholar]
- 41.Schleiermacher G, Peter M, Michon J, Zucker JM, Thomas G, Magdelenat H, Delattre O: A multiplex PCR assay for routine evaluation of deletion of the short arm of chromosome 1 in neuroblastoma. Eur J Cancer 1995, 4:535-538 [DOI] [PubMed] [Google Scholar]
- 42.Nagai H, Negrini M, Carter SL, Gillum DR, Rosenberg AL, Schwartz GF, Croce CM: Detection and cloning of a common region of loss of heterozygosity at chromosome 1p in breast cancer. Cancer Res 1995, 55:1752-1757 [PubMed] [Google Scholar]
- 43.Di Vinci A, Infusini E, Peveri C, Sciutto A, Geido E, Risio M, Rossini FP, Giaretti W: Correlation between 1p deletions and aneusomy in human colorectal adenomas. Int J Cancer 1998, 75:45-50 [DOI] [PubMed] [Google Scholar]
- 44.Zhou X, Tarmin L, Yin J, Jiang HY, Suzuki H, Rhyu MG, Abraham JM, Meltzer SJ: The MTS1 gene is frequently mutated in primary human esophageal tumors. Oncogene 1994, 9:3737-3741 [PubMed] [Google Scholar]
- 45.Gruis NA, Weaver-Feldhaus J, Liu Q, Frye C, Eeles R, Orlow I, Lacombe L, Ponce-Castaneda V, Lianes P, Latres E, Skolnick M, Cordon-Cardo C, Kamb A: Genetic evidence in melanoma and bladder cancers that p16 and p53 function in separate pathways of tumor suppression. Am J Pathol 1995, 146:1199-1206 [PMC free article] [PubMed] [Google Scholar]
- 46.Igaki H, Sasaki H, Kishi T, Sakamoto H, Tachimori Y, Kato H, Watanabe H, Sugimura T, Terada M: Highly frequent homozygous deletion of the p16 gene in esophageal cancer cell lines. Biochem Biophys Res Commun 1994, 203:1090-1095 [DOI] [PubMed] [Google Scholar]
- 47.Heim S: Chromosome aberrations in solid tumors: cytogenetic examinations are now available in Norway. Tidskr Nor Laegeforen 1996, 116:2295-2296 [PubMed] [Google Scholar]
- 48.Clark WH, Jr, Elder DE, Guerry DT, Epstein MN, Greene MH, Van Horn M: A study of tumor progression: the precursor lesions of superficial spreading and nodular melanoma. Hum Pathol 1984, 15:1147-1165 [DOI] [PubMed] [Google Scholar]
- 49.Marras S, Faa G, Dettori T, Congiu L, Vanni R: Chromosomal changes in dysplastic nevi. Cancer Genet Cytogenet 1999, 113:177-179 [DOI] [PubMed] [Google Scholar]
- 50.Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, III, Johnson BE, Skolnick MH: A cell cycle regulator potentially involved in genesis of many tumor types. Science 1994, 264:436-440 [DOI] [PubMed] [Google Scholar]
- 51.Nobori T, Miura K, Wu DJ, Lois A, Takabayashi K, Carson DA: Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature 1994, 368:753-756 [DOI] [PubMed] [Google Scholar]
- 52.Serrano M, Hannon GJ, Beach D: A new regulatory motif in cell cycle control causing specific inhibition of cyclin D/CDK4. Nature 1993, 366:704-707 [DOI] [PubMed] [Google Scholar]
- 53.Stone S, Jiang P, Dayananth P, Tavtigian SV, Katcher H, Parry D, Peters G, Kamb A: Complex structure and regulation of the P16 (MTS1) locus. Cancer Res 1995, 55:2988-2994 [PubMed] [Google Scholar]
- 54.Piepkorn M: Melanoma genetics: an update with focus on the CDKN2A(p16)/ARF tumor suppressors. J Am Acad Dermatol 2000, 42:705–722; quiz 723–726 [DOI] [PubMed]
- 55.Healy E, Sikkink S, Rees JL: Infrequent mutation of p16INK4 in sporadic melanoma. J Invest Dermatol 1996, 107:318-321 [DOI] [PubMed] [Google Scholar]
- 56.Grover R, Chana JS, Wilson GD, Richman PI, Sanders R: An analysis of p16 protein expression in sporadic malignant melanoma. Melanoma Res 1998, 8:267-272 [DOI] [PubMed] [Google Scholar]
- 57.Reed JA, Loganzo F, Jr, Shea CR, Walker GJ, Flores JF, Glendening JM, Bogdany JK, Shiel MJ, Haluska FG, Fountain JW, Albino AP: Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res 1995, 55:2713-2718 [PubMed] [Google Scholar]
- 58.Keller-Melchior R, Schmidt R, Piepkorn M: Expression of the tumor suppressor gene product p16INK4 in benign and malignant melanocytic lesions. J Invest Dermatol 1998, 110:932-938 [DOI] [PubMed] [Google Scholar]
- 59.Soussi T, Caron de Fromentel C, Sturzbecher HW, Ullrich S, Jenkins J, May P: Evolutionary conservation of the biochemical properties of p53: specific interaction of Xenopus laevis p53 with simian virus 40 large T antigen and mammalian heat shock proteins 70. J Virol 1989, 63:3894-3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeLeo AB, Jay G, Appella E, Dubois GC, Law LW, Old LJ: Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci USA 1979, 76:2420-2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dippold WG, Jay G, DeLeo AB, Khoury G, Old LJ: p53 transformation-related protein: detection by monoclonal antibody in mouse and human cells. Proc Natl Acad Sci USA 1981, 78:1695-1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stretch JR, Gatter KC, Ralfkiaer E, Lane DP, Harris AL: Expression of mutant p53 in melanoma. Cancer Res 1991, 51:5976-5979 [PubMed] [Google Scholar]
- 63.Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB: Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci USA 1992, 89:7491-7495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B: Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature 1993, 362:857-860 [DOI] [PubMed] [Google Scholar]
- 65.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B: WAF1, a potential mediator of p53 tumor suppression. Cell 1993, 75:817-825 [DOI] [PubMed] [Google Scholar]
- 66.Papp T, Jafari M, Schiffmann D: Lack of p53 mutations and loss of heterozygosity in non-cultured human melanocytic lesions. J Cancer Res Clin Oncol 1996, 122:541-548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lane DP: Cancer: a death in the life of p53. Nature 1993, 362:786-787 [DOI] [PubMed] [Google Scholar]
- 68.Lane DP: Cancer. p53, guardian of the genome. Nature 1992, 358:15-16 [DOI] [PubMed] [Google Scholar]
- 69.Hartwell LH, Kastan MB: Cell cycle control and cancer. Science 1994, 266:1821-1828 [DOI] [PubMed] [Google Scholar]
- 70.Livingstone LR, White A, Sprouse J, Livanos E, Jacks T, Tlsty TD: Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 1992, 70:923-935 [DOI] [PubMed] [Google Scholar]
- 71.Yin Y, Tainsky MA, Bischoff FZ, Strong LC, Wahl GM: Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell 1992, 70:937-948 [DOI] [PubMed] [Google Scholar]
- 72.Levine AJ, Momand J, Finlay CA: The p53 tumour suppressor gene. Nature 1991, 351:453-456 [DOI] [PubMed] [Google Scholar]
- 73.Oren M: p53: the ultimate tumor suppressor gene? FASEB J 1992, 6:3169-3176 [DOI] [PubMed] [Google Scholar]
- 74.Hollstein M, Sidransky D, Vogelstein B, Harris CC: p53 mutations in human cancers. Science 1991, 253:49-53 [DOI] [PubMed] [Google Scholar]
- 75.Lee JM, Abrahamson JL, Bernstein A: DNA damage, oncogenesis and the p53 tumour-suppressor gene. Mutat Res 1994, 307:573-581 [DOI] [PubMed] [Google Scholar]
- 76.Prokocimer M, Rotter V: Structure and function of p53 in normal cells and their aberrations in cancer cells: projection on the hematologic cell lineages. Blood 1994, 84:2391-2411 [PubMed] [Google Scholar]
- 77.Papp T, Pemsel H, Zimmermann R, Bastrop R, Weiss DG, Schiffmann D: Mutational analysis of the N-ras, p53, p16INK4a, CDK4, and MC1R genes in human congenital melanocytic naevi. J Med Genet 1999, 36:610-614 [PMC free article] [PubMed] [Google Scholar]
- 78.Levin DB, Wilson K, Valadares de Amorim G, Webber J, Kenny P, Kusser W: Detection of p53 mutations in benign and dysplastic nevi. Cancer Res 1995, 55:4278-4282 [PubMed] [Google Scholar]
- 79.Lassam NJ, From L, Kahn HJ: Overexpression of p53 is a late event in the development of malignant melanoma. Cancer Res 1993, 53:2235-2238 [PubMed] [Google Scholar]
- 80.McGregor JM, Yu CC, Dublin EA, Barnes DM, Levison DA, MacDonald DM: p53 immunoreactivity in human malignant melanoma and dysplastic naevi. Br J Dermatol 1993, 128:606-611 [DOI] [PubMed] [Google Scholar]
- 81.Gelsleichter L, Gown AM, Zarbo RJ, Wang E, Coltrera MD: p53 and mdm-2 expression in malignant melanoma: an immunocytochemical study of expression of p53, mdm-2, and markers of cell proliferation in primary versus metastatic tumors. Mod Pathol 1995, 8:530-535 [PubMed] [Google Scholar]
- 82.Radhi JM: Malignant melanoma arising from nevi, p53, p16, and Bcl-2: expression in benign versus malignant components. J Cutan Med Surg 1999, 3:293-297 [DOI] [PubMed] [Google Scholar]
- 83.Cristofolini M, Boi S, Girlando S, Zumiani G, Cristofolini P, Dalla Palma P, Doglioni C, Barbareschi M: p53 protein expression in nevi and melanomas. Arch Dermatol 1993, 129:739-743 [PubMed] [Google Scholar]
- 84.Kaleem Z, Lind AC, Humphrey PA, Sueper RH, Swanson PE, Ritter JH, Wick MR: Concurrent Ki-67 and p53 immunolabeling in cutaneous melanocytic neoplasms: an adjunct for recognition of the vertical growth phase in malignant melanomas? Mod Pathol 2000, 13:217-222 [DOI] [PubMed] [Google Scholar]
- 85.Barnhill RL, Castresana JS, Rubio MP, Martin MT, Idoate M, Vazquez JJ, Thor AD: p53 expression in cutaneous malignant melanoma: an immunohistochemical study of 87 cases of primary, recurrent, and metastatic melanoma. Mod Pathol 1994, 7:533-535 [PubMed] [Google Scholar]
- 86.Goding CR: Melanocyte development and malignant melanoma. Forum (Genova) 2000, 10:176-187 [PubMed] [Google Scholar]
- 87.Poremba C, Yandell DW, Metze D, Kamanabrou D, Bocker W, Dockhorn-Dworniczak B: Immunohistochemical detection of p53 in melanomas with rare p53 gene mutations is associated with mdm-2 overexpression. Oncol Res 1995, 7:331-339 [PubMed] [Google Scholar]
- 88.Fang D, Setaluri V: Expression and up-regulation of alternatively spliced transcripts of melastatin, a melanoma metastasis-related gene, in human melanoma cells. Biochem Biophys Res Commun 2000, 279:53-61 [DOI] [PubMed] [Google Scholar]
- 89.Hunter JJ, Shao J, Smutko JS, Dussault BJ, Nagle DL, Woolf EA, Holmgren LM, Moore KJ, Shyjan AW: Chromosomal localization and genomic characterization of the mouse melastatin gene (Mlsn1). Genomics 1998, 54:116-123 [DOI] [PubMed] [Google Scholar]
- 90.Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA, Tepper RI, Shyjan AW: Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 1998, 58:1515-1520 [PubMed] [Google Scholar]
- 91.Eshleman JR, Markowitz SD: Mismatch repair defects in human carcinogenesis. Hum Mol Genet 1996, 5:1489-1494 [DOI] [PubMed] [Google Scholar]
- 92.Fishel R, Lescoe MK, Rao MR, Copeland NG, Jenkins NA, Garber J, Kane M, Kolodner R: The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer (published erratum appears in Cell 1994, 77: 167). Cell 1993, 75:1027-1038 [DOI] [PubMed] [Google Scholar]
- 93.Palombo EA, Bishop RF: Genetic and antigenic characterization of a serotype G6 human rotavirus isolated in Melbourne, Australia. J Med Virol 1995, 47:348-354 [DOI] [PubMed] [Google Scholar]
- 94.Palombo F, Gallinari P, Iaccarino I, Lettieri T, Hughes M, D’Arrigo A, Truong O, Hsuan JJ, Jiricny J: GTBP, a 160-kilodalton protein essential for mismatch-binding activity in human cells. Science 1995, 268:1912-1914 [DOI] [PubMed] [Google Scholar]
- 95.Leach FS, Nicolaides NC, Papadopoulos N, Liu B, Jen J, Parsons R, Peltomaki P, Sistonen P, Aaltonen LA, Nystrom-Lahti M, Guan XY, Zhang J, Meltzer PS, Yu JW, Kao FT, Chen DJ, Cerosaletti KM, Fournier REK, Todd S, Lewis T, Leach RJ, Naylor SL, Weissenbach J, Mecklin JP, Jarvinen H, Petersen GM, Hamilton SR, Green J, Jass J, Watson P, Lynch HT, Trent JM, de la Chapelle A, Kinzler KW, Vogelstein B: Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell 1993, 75:1215-1225 [DOI] [PubMed] [Google Scholar]
- 96.Lindblom A, Tannergard P, Werelius B, Nordenskjold M: Genetic mapping of a second locus predisposing to hereditary non-polyposis colon cancer. Nat Genet 1993, 5:279-282 [DOI] [PubMed] [Google Scholar]
- 97.Liu B, Farrington SM, Petersen GM, Hamilton SR, Parsons R, Papadopoulos N, Fujiwara T, Jen J, Kinzler KW, Wyllie AH, Vogelstein B, Dunlop MG: Genetic instability occurs in the majority of young patients with colorectal cancer. Nat Med 1995, 1:348-352 [DOI] [PubMed] [Google Scholar]
- 98.Mao L, Lee DJ, Tockman MS, Erozan YS, Askin F, Sidransky D: Microsatellite alterations as clonal markers for the detection of human cancer. Proc Natl Acad Sci USA 1994, 91:9871-9875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mao L, Sidransky D: Cancer screening based on genetic alterations in human tumors. Cancer Res 1994, 54:1939s-1940s [PubMed] [Google Scholar]
- 100.Thibodeau SN, French AJ, Roche PC, Cunningham JM, Tester DJ, Lindor NM, Moslein G, Baker SM, Liskay RM, Burgart LJ, Honchel R, Halling KC: Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res 1996, 56:4836-4840 [PubMed] [Google Scholar]
- 101.Wei Q, Guan Y, Cheng L, Radinsky R, Bar-Eli M, Tsan R, Li L, Legerski RJ: Expression of five selected human mismatch repair genes simultaneously detected in normal and cancer cell lines by a nonradioactive multiplex reverse transcription-polymerase chain reaction. Pathobiology 1997, 65:293-300 [DOI] [PubMed] [Google Scholar]
- 102.Leung SY, Yuen ST, Chung LP, Chu KM, Chan AS, Ho JC: hMLH1 promoter methylation and lack of hMLH1 expression in sporadic gastric carcinomas with high-frequency microsatellite instability. Cancer Res 1999, 59:159-164 [PubMed] [Google Scholar]
- 103.Hussein MR, Roggero E, Sudilovsky EC, Tuthill RJ, Wood GS, Sudilovsky O: Alterations of mismatch repair protein expression in benign melanocytic nevi, melanocytic dysplastic nevi, and cutaneous malignant melanomas. Am J Dermatopathol 2001, 23:308-314 [DOI] [PubMed] [Google Scholar]
- 104.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Powell SM, Jen J, Hamilton SR, Petersen GM, Kinzler KW, Vogelstein B, de la Chapelle A: Clues to the pathogenesis of familial colorectal cancer. Science 1993, 260:812-816 [DOI] [PubMed] [Google Scholar]
- 105.Thibodeau SN, Bren G, Schaid D: Microsatellite instability in cancer of the proximal colon. Science 1993, 260:816-819 [DOI] [PubMed] [Google Scholar]
- 106.Peltomaki P, Aaltonen LA, Sistonen P, Pylkkanen L, Mecklin JP, Jarvinen H, Green JS, Jass JR, Weber JL, Leach FS, Petersen GM, Hamilton SR, de la Chapelle A, Vogelstein B: Genetic mapping of a locus predisposing to human colorectal cancer. Science 1993, 260:810-812 [DOI] [PubMed] [Google Scholar]
- 107.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN, Srivastava S: A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998, 58:5248-5257 [PubMed] [Google Scholar]
- 108.Bronner CE, Baker SM, Morrison PT, Warren G, Smith LG, Lescoe MK, Kane M, Earabino C, Lipford J, Lindblom A, Tannergard P, Bollag RJ, Godwin AR, Ward DC, Nordenskjold M, Fishel R, Kolodner R, Liskay RM: Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature 1994, 368:258-261 [DOI] [PubMed] [Google Scholar]
- 109.Nicolaides NC, Papadopoulos N, Liu B, Wei YF, Carter KC, Ruben SM, Rosen CA, Haseltine WA, Fleischmann RD, Fraser CM, Adams MD, Venter JC, Dunlop MG, Hamilton SR, Petersen GM, de la Chapelle A, Vogelstein B, Kinzler KW: Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature 1994, 371:75-80 [DOI] [PubMed] [Google Scholar]
- 110.Perucho M: Cancer of the microsatellite mutator phenotype. Biol Chem 1996, 377:675-684 [PubMed] [Google Scholar]
- 111.Hussein MR, Sun M, Tuthill RJ, Roggero E, Monti JA, Sudilovsky EC, Wood GS, Sudilovsky O: Comprehensive analysis of 112 melanocytic skin lesions demonstrates microsatellite instability in melanomas and dysplastic nevi, but not in benign nevi. J Cutan Pathol 2001, 28:343-350 [DOI] [PubMed] [Google Scholar]
- 112.Birindelli S, Tragni G, Bartoli C, Ranzani GN, Rilke F, Pierotti MA, Pilotti S: Detection of microsatellite alterations in the spectrum of melanocytic nevi in patients with or without individual or family history of melanoma. Int J Cancer 2000, 86:255-261 [DOI] [PubMed] [Google Scholar]
- 113.Tsao H, Zhang X, Majewski P, Haluska FG: Mutational and expression analysis of the p73 gene in melanoma cell lines. Cancer Res 1999, 59:172-174 [PubMed] [Google Scholar]
- 114.New L, Liu K, Crouse GF: The yeast gene MSH3 defines a new class of eukaryotic MutS homologues. Mol Gen Genet 1993, 239:97-108 [DOI] [PubMed] [Google Scholar]
- 115.Papadopoulos N, Nicolaides NC, Liu B, Parsons R, Lengauer C, Palombo F, D’Arrigo A, Markowitz S, Willson JKV, Kinzler KW, Jiricny J, Vogelstein B: Mutations of GTBP in genetically unstable cells. Science 1995, 268:1915-1917 [DOI] [PubMed] [Google Scholar]
- 116.Parc YR, Halling KC, Wang L, Christensen ER, Cunningham JM, French AJ, Burgart LJ, Price-Troska TL, Roche PC, Thibodeau SN: HMSH6 alterations in patients with microsatellite instability-low colorectal cancer Cancer Res 2000, 60:2225-2231 [PubMed] [Google Scholar]
- 117.Bishop JM: The molecular genetics of cancer. Science 1987, 235:305-311 [DOI] [PubMed] [Google Scholar]
- 118.Lowe DG, Capon DJ, Delwart E, Sakaguchi AY, Naylor SL, Goeddel DV: Structure of the human and murine R-ras genes, novel genes closely related to ras proto-oncogenes. Cell 1987, 48:137-146 [DOI] [PubMed] [Google Scholar]
- 119.Albino AP, Nanus DM, Mentle IR, Cordon-Cardo C, McNutt NS, Bressler J, Andreeff M: Analysis of ras oncogenes in malignant melanoma and precursor lesions: correlation of point mutations with differentiation phenotype. Oncogene 1989, 4:1363-1374 [PubMed] [Google Scholar]
- 120.Shukla VK, Hughes DC, Hughes LE, McCormick F, Padua RA: ras mutations in human melanotic lesions: K-ras activation is a frequent and early event in melanoma development. Oncogene Res 1989, 5:121-127 [PubMed] [Google Scholar]
- 121.Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, Aaronson S, Leder P: Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci USA 1982, 79:7837-7841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schlagbauer-Wadl H, Griffioen M, van Elsas A, Schrier PI, Pustelnik T, Eichler HG, Wolff K, Pehamberger H, Jansen B: Influence of increased c-Myc expression on the growth characteristics of human melanoma. J Invest Dermatol 1999, 112:332-336 [DOI] [PubMed] [Google Scholar]
- 123.Kraehn GM, Utikal J, Udart M, Greulich KM, Bezold G, Kaskel P, Leiter U, Peter RU: Extra c-myc oncogene copies in high risk cutaneous malignant melanoma and melanoma metastases. Br J Cancer 2001, 84:72-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uitto J, Olsen DR, Fazio MJ: Extracellular matrix of the skin: 50 years of progress. J Invest Dermatol 1989, 92:61S-77S [DOI] [PubMed] [Google Scholar]
- 125.Skow LC, Adkison L, Womack JE, Beamer WG, Taylor BA: Mapping of the mouse fibronectin gene (Fn-1) to chromosome 1: conservation of the Idh-1-Cryg-Fn-1 synteny group in mammals. Genomics 1987, 1:283-286 [DOI] [PubMed] [Google Scholar]
- 126.Solomon E, Swallow D, Burgess S, Evans L: Assignment of the human acid alpha-glucosidase gene (alphaGLU) to chromosome 17 using somatic cell hybrids. Ann Hum Genet 1979, 42:273-281 [DOI] [PubMed] [Google Scholar]
- 127.Emanuel BS, Cannizzaro LA, Seyer JM, Myers JC: Human alpha 1(III) and alpha 2(V) procollagen genes are located on the long arm of chromosome 2. Proc Natl Acad Sci USA 1985, 82:3385-3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Weil D, Mattei MG, Passage E, N′Guyen VC, Pribula-Conway D, Mann K, Deutzmann R, Timpl R, Chu ML: Cloning and chromosomal localization of human genes encoding the three chains of type VI collagen. Am J Hum Genet 1988, 42:435-445 [PMC free article] [PubMed] [Google Scholar]
- 129.Van Duinen CM, Fleuren GJ, Bruijn JA: The extracellular matrix in pigmented skin lesions: an immunohistochemical study. Histopathology 1994, 24:33-40 [DOI] [PubMed] [Google Scholar]
- 130.Tee MK, Thomson AA, Bristow J, Miller WL: Sequences promoting the transcription of the human XA gene overlapping P450c21A correctly predict the presence of a novel, adrenal-specific, truncated form of tenascin-X. Genomics 1995, 28:171-178 [DOI] [PubMed] [Google Scholar]
- 131.Fukushima Y, Byers MG, Fiddes JC, Shows TB: The human basic fibroblast growth factor gene (FGFB) is assigned to chromosome 4q25. Cytogenet Cell Genet 1990, 54:159-160 [DOI] [PubMed] [Google Scholar]
- 132.Reed JA, McNutt NS, Albino AP: Differential expression of basic fibroblast growth factor (bFGF) in melanocytic lesions demonstrated by in situ hybridization: implications for tumor progression. Am J Pathol 1994, 144:329-336 [PMC free article] [PubMed] [Google Scholar]
- 133.Krasagakis K, Garbe C, Zouboulis CC, Orfanos CE: Growth control of melanoma cells and melanocytes by cytokines. Recent Results Cancer Res 1995, 139:169-182 [DOI] [PubMed] [Google Scholar]
- 134.Folkman J, Klagsbrun M: Angiogenic factors. Science 1987, 235:442-447 [DOI] [PubMed] [Google Scholar]
- 135.Thomas KA: Fibroblast growth factors. FASEB J 1987, 1:434-440 [DOI] [PubMed] [Google Scholar]
- 136.Rodeck U, Becker D, Herlyn M: Basic fibroblast growth factor in human melanoma. Cancer Cells 1991, 3:308-311 [PubMed] [Google Scholar]
- 137.Halaban R, Langdon R, Birchall N, Cuono C, Baird A, Scott G, Moellmann G, McGuire J: Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol 1988, 107:1611-1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ladanyi M, Chan WC, Triche TJ, Gerald WL: Expression profiling of human tumors: the end of surgical pathology? J Mol Diagn 2001, 3:92-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bittner M, Meltzer P, Chen Y, Jiang Y, Seftor E, Hendrix M, Radmacher M, Simon R, Yakhini Z, Ben-Dor A, Sampas N, Dougherty E, Wang E, Marincola F, Gooden C, Lueders J, Glatfelter A, Pollock P, Carpten J, Gillanders E, Leja D, Dietrich K, Beaudry C, Berens M, Alberts D, Sondak V: Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature 2000, 406:536-540 [DOI] [PubMed] [Google Scholar]
- 140.Brown PO, Botstein D: Exploring the new world of the genome with DNA microarrays. Nat Genet 1999, 21:33-37 [DOI] [PubMed] [Google Scholar]