Abstract
We have used a continuous fluorescence monitoring method to assess cyclin D1 mRNA expression in a variety of hematological and non-hematological processes. We examined 14 cell lines, 11 reactive lymphoid tissues, and 57 primary hematopoietic neoplasms including mantle cell lymphoma (MCL) (n = 10), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (n = 11), acute lymphoblastic leukemia/lymphoma (n = 15), follicular lymphoma (n = 6), peripheral T-cell lymphoma (PTCL) (n = 3), anaplastic large cell lymphoma (n = 3), hairy cell leukemia (n = 3), Burkitt lymphoma (n = 1), Burkitt-like lymphoma (n = 4), and plasmacytoma (n = 1) for the expression of cyclin D1 mRNA using fluorescently labeled sequence-specific hybridization probes. Fluorescence (F) was plotted against cycle (C) number over 45 cycles. The log-linear portion of the F versus C graph identified a fractional cycle number for threshold fluorescence. A β-globin mRNA transcript with equivalent amplification efficiency to that of cyclin D1 was used for assessment of RNA integrity and normalization. In general, the MCLs demonstrated substantially higher levels of cyclin D1 mRNA than the other lymphoproliferative processes. Moderately high levels of cyclin D1 mRNA were detected in one PTCL. On average, the CLL/SLL cases showed cyclin D1 mRNA levels two to three orders of magnitude lower than observed in the MCLs. Cell lines derived from non-hematopoietic neoplasms such as fibrosarcoma, small cell carcinoma, and neuroblastoma showed comparable or higher levels of cyclin D1 mRNA than the MCLs. Our results indicate that quantitative real-time reverse transcription (RT) polymerase chain reaction is a simple, rapid, and accurate technique for assessing cyclin D1 expression, and while it is not specific, it can reliably be used in the distinction of MCL from CLL/SLL.
Mantle cell lymphoma (MCL) is a distinct clinicopathologic entity that is characterized by the presence of the t(11;14)(q13;q32) chromosomal translocation. 1, 2, 3 The t(11;14) results in juxtaposition of the bcl-1 locus in close proximity to the immunoglobulin heavy chain enhancer, resulting in deregulation and overexpression of cyclin D1, an important regulator of G1/S progression in the cell cycle. 4, 5
MCL shares some histological and immunophenotypic features with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and other low-grade B-cell lymphomas. Because MCL is a clinically aggressive neoplasm, its distinction from CLL/SLL and other low-grade B-cell lymphomas is important. In this regard, the detection of the t(11;14) has served as a good discriminator of MCL from other entities exhibiting similar histopathological features. The translocation can be detected in 90 to 95% of MCLs by fluorescence in situ hybridization, 6 70 to 80% by conventional cytogenetics, 7 60 to 70% by Southern blot hybridization, 8 and 30 to 40% using polymerase chain reaction (bcl-1, major translocation cluster/immunoglobulin joining). 9, 10 On the other hand, cyclin D1 protein expression is demonstrable in approximately 70% of cases of MCL by immunohistochemical methods. 11, 12, 13
While highly characteristic of MCL, elevated cyclin D1 protein has been demonstrated in other lymphoproliferative processes such as hairy cell leukemia, 11, 14, 15 plasma cell dyscrasias, 11, 12 rare cases of B-cell CLL/SLL, 11, 12 and epithelial malignancies. 16 Elevated cyclin D1 mRNA expression has been demonstrated by Northern blot 17, 18 and in situ hybridization analyses in MCLs. 19 Similarly, RNA expression studies have also shown elevated levels of cyclin D1 transcripts in the majority of MCLs and in a minority of other lymphoproliferative disorders by conventional end-point reverse trancription-polymerase chain reaction (RT-PCR)-based analyses. 13, 20 Such end-point PCR-based methods suffer the drawback of including the non-quantitative plateau phase of the amplification in the final determination of relative cyclin D1 expression levels. Furthermore, these methods are labor intensive, and may yield variable results. The purpose of this study, therefore, was to apply continuous fluorescence PCR monitoring, which reliably determines the onset of the exponential phase of PCR, for quantification of cyclin D1 mRNA levels. Using this methodology, we also sought to determine the sensitivity and specificity of cyclin D1 mRNA overexpression for the diagnosis of MCL.
Materials and Methods
Sample Selection
Archived snap-frozen tissue samples of a total of 57 cases of lymphoproliferative disorders including mantle cell lymphoma (n = 10), CLL/SLL (n = 11), follicular lymphoma (n = 6), peripheral T-cell lymphoma (n = 3), anaplastic large cell lymphoma (n = 3), acute lymphoblastic leukemia/lymphoma (n = 15), hairy cell leukemia (n = 3), Burkitt lymphoma (n = 1), Burkitt-like lymphoma (n = 4), and plasmacytoma (n = 1) were selected for study. Reactive follicular hyperplasia (n = 5) and healthy peripheral blood lymphocytes (n = 6) were also examined. All clinical samples were classified according to the Revised European American Lymphoma (REAL) classification, 3 and demonstrated the characteristic histological and immunophenotypic profiles of the diagnosis rendered. Fourteen cell lines (10 hematopoietic, 4 non-hematopoietic) were also evaluated for cyclin D1 mRNA expression (Table 1) .
Table 1.
Cyclin D1 mRNA Expression Normalized to β-Globin and Relative to Follicular Hyperplasia: Cell Lines
| Sample | Diagnosis | Cyclin D1 mRNA overexpression | Cyclin D1 normalized quantity* |
|---|---|---|---|
| Granta | Mantle cell lymphoma | + | 15.35 |
| NCEB | Mantle cell lymphoma | − | 0.82 |
| SUPB-15 | B-cell ALL | − | 0.30 |
| K-562 | Chronic myelogenous leukemia | − | 0.52 |
| Molt-4 | Precursor T-cell ALL | − | 0.31 |
| REH | Precursor B-cell ALL | − | 0.25 |
| 697 | Precursor B-cell ALL | − | 0.20 |
| NB-4 | Acute promyelocytic leukemia | + | 1.56 |
| Kasumi-1 | Acute myeloid leukemia | − | 0.20 |
| Karpas 299 | t(2;5) positive T-cell lymphoma | − | 0.22 |
| SW1271 | Small cell carcinoma | + | 59.30 |
| HT1080 | Fibrosarcoma | + | 290.02 |
| PA-1 | Ovary teratocarcinoma | + | 390.72 |
| SK-N-SH | Neuroblastoma | + | 18.77 |
Samples with a normalized cyclin D1 expression level greater than 1.00 were defined as overexpressing cyclin D1 MRNA.
Cyclin D1 normalized quantity = 
CT = crossing threshold.
+, positive.
−, negative.
E = efficiency of reaction.
Immunohistochemical Studies
Immunohistochemistry for cyclin D1 was performed on formalin-fixed, paraffin-embedded tissue sections of all tissue samples, but not on cell lines. Antigen retrieval was performed using microwave heat pretreatment. 21 An avidin-biotin peroxidase method was performed using an automated immunostainer (Ventana Medical System, Tuscon, AZ). We used a commercially available antibody against cyclin D1 (Neomarkers, Union City, CA).
RNA Extraction and Reverse Transcription
Total RNA was extracted from archived fluid and tissue samples taken from patients from the University of Utah Health Sciences Center, Salt Lake City, Utah, and from the Sunnybrook and Women’s College Health Sciences Center, Toronto, Ontario, Canada. RNA was extracted using Trizol (Gibco BRL, Life Technologies, Rockville, MD) or the RNeasy Mini RNA extraction kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Reverse transcription was performed using Moloney murine leukemia virus (MMLV) reverse transcriptase (Gibco BRL, Life Technologies, Rockville, MD) and random hexamers (Promega, Madison, WI) in the presence of RNase inhibitor (Amersham-Pharmacia, Piscataway, NJ). cDNA quantity was assessed using absorbance at 260 nm.
Primer and Probe Design
The primers and probes used for the cyclin D1 (GenBank Accession no. Z23022) and β-globin (GenBank Accession no. AF181989) RT-PCR are summarized in Table 2 , and were designed using Primer Designer software version 4.0 (Sci-Ed Software, Durham, NC).
Table 2.
Oligonucleotide Primer and Probe Sequences for Fluorescent Cyclin D1 and β-Globin PCR
| Primers and probes | Sequence | GenBank accession no. | Bases |
|---|---|---|---|
| β-Globin | |||
| βG-55 | 5′-GAGAAGTCTGCCGTTACTGC-3′ | AF181989 | 55–74 |
| βG-268c | 5′-GGTGAGCCAGGCCATACTA-3′ | AF181989 | 249–268 |
| βG-151-F | 5′-CAGAGGTTCTTTGAGTCCTTTGGGGATCTG-fluorescein-3′ | AF181989 | 151–180 |
| βG-181-705 | 5′-LCRed705-TCCACTCCTGATGCTGTTAT-phosphate-3′ | AF181989 | 181–200 |
| Cyclin D1 | |||
| D1-1109 | 5′-CCTCCTCTCCGGAGCATTT-3′ | Z23022 | 1109–1127 |
| D1-1315c | 5′-CTGTAGCACAACCCTCCTCC-3′ | Z23022 | 1296–1315 |
| D1-1140-F | 5′-GGAAAGCTTCATTCTCCTTGTTGTTGGTTG-fluorescein-3′ | Z23022 | 1140–1169 |
| D1-1171-640 | 5′-LCRed640-TTTTTCCTTTGCTCTTTCCC-phosphate-3′ | Z23022 | 1171–1190 |
Fluorescence RT-PCR
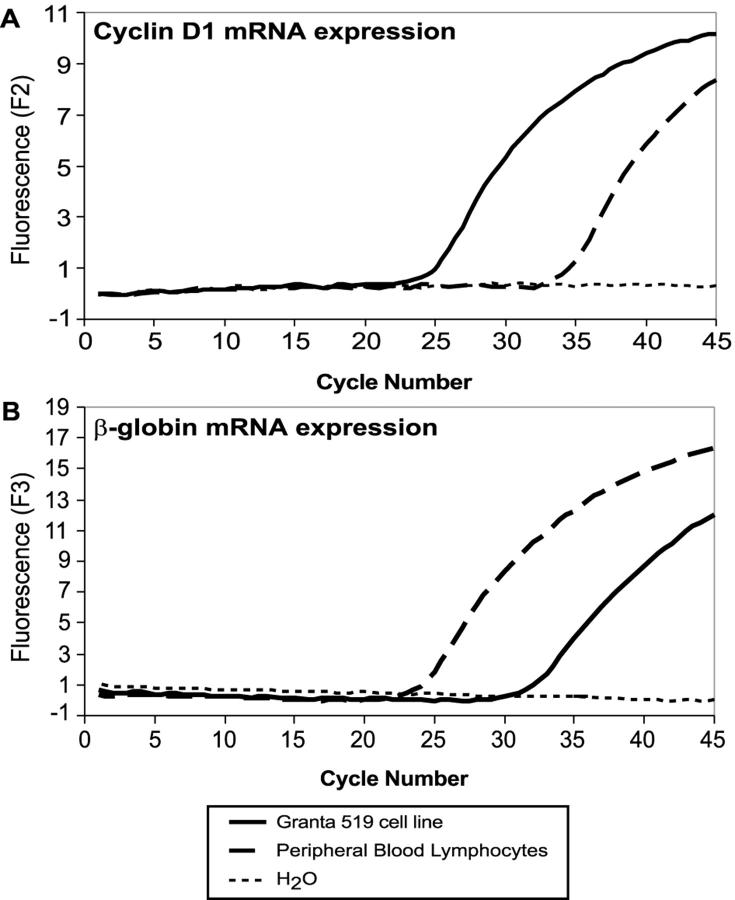
Quantitative PCR was performed using sequence-specific hybridization probes for cyclin D1 and β-globin mRNA transcripts. Rapid cycle amplification was performed using a thermal cycler integrated with a fluorimeter (Lightcycler, Roche Molecular Biochemicals, Indianapolis, IN). Fifty ng of template cDNA were amplified in a 10 μl reaction containing 1X PCR buffer (50 mmol/L Tris [pH 8.3], 3.0 mmol/L MgCl2, and 500 μg/ml bovine serum albumin), deoxynucleotide triphosphates at 200 mmol/L each, and 0.4 units Promega Taq polymerase (Madison, WI) with 11 ng/μl of TaqStart antibody (ClonTech, Palo Alto, CA). The primers specific for cyclin D1 were used at a concentration of 0.5 μmol/L per reaction, while the β-globin primers were used at 0.2 μmol/L per reaction (Table 2) . Reactions also included a fluorescein isothiocyanate (FITC)-labeled probe and a LightCycler Red (LCRed) 640-labeled probe specific for cyclin D1, and a FITC-labeled probe and a LCRed705 labeled-probe specific for β-globin. FITC-labeled probes were used at a 0.1 μmol/L concentration, while the LCRed- labeled probes were used at 0.2 μmol/L (Table 2) . The reaction mixture was subjected to rapid PCR amplification consisting of denaturation at 95°C for 0 seconds, annealing at 55°C for 10 seconds, and extension at 72°C for 10 seconds. The fluorescence readings were plotted against the cycle number over 45 cycles. The log-linear portion of this graph was used todetermine a fractional cycle number for threshold fluorescence (Figure 1) .
Figure 1.
Real-time PCR for determination of cyclin D1 and β-globin cycle thresholds. Fluorescence versus cycle number graphs obtained using the hybridization probe format. Real-time RT-PCR was performed using sequence-specific hybridization probes for quantification of cyclin D1 and β-globin transcripts. In these assays, samples containing abundant pre-amplification amounts of a particular target show an early rise in fluorescence. A: Shows an earlier onset (cycle 24) of amplification of cyclin D1 in the Granta-519 cell line (solid black line), as compared to the peripheral B-lymphocyte mRNA which exhibits a log-linear cycle threshold at cycle 35 (dashed black line). B: Shows the cycle thresholds obtained using primer/probes specific for β-globin. The reactive peripheral B-lymphocyte sample shows an earlier cycle threshold, indicating a greater initial concentration of cDNA. The dotted line represents the no template (H2O) control.
Quantitative Fluorescence PCR
The second derivative maximum function included in the LightCycler software was used to determine the fractional cycle numbers used for quantification. 22
Determination of Relative Cyclin D1 Transcript Quantity
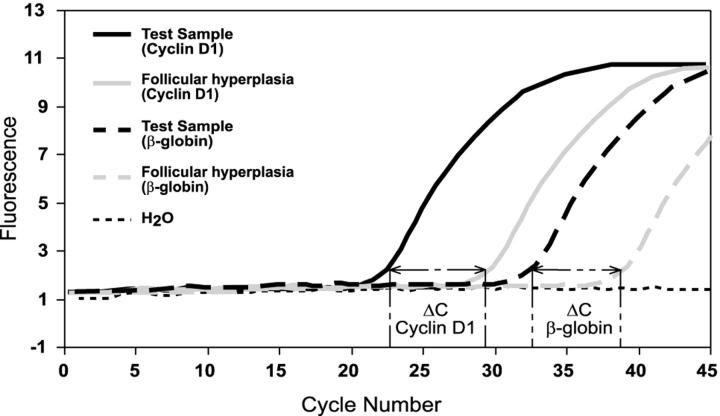
The exponential phase of a PCR amplification is described by the equation Tn = T0En, where Tn is the amount of target sequence at a particular cycle number (n), T0 is the initial target quantity, E represents the efficiency of the amplification reaction and n is cycle number. The log-linear equivalent of the above equation: log Tn = logT0 + n*logE, permits determination of E from the standard curves. Efficiency was calculated from LightCycler software plots using log E = −1/slope. 22 Our relative quantification assay using β-globin as an external standard was configured such that the PCR amplifications for both cyclin D1 (E = 2 ± 0.1) and β-globin (E = 2 ± 0.1) yielded very similar amplification efficiencies (data not shown). Quantification of mRNA was accomplished by analysis of fluorescence curves and determination of crossing threshold (the cycle at which the fluorescent signal rises above background) for each sample. Samples with a higher pre-amplification target concentration show an earlier cycle threshold (Figure 2) . The difference in cycle threshold obtained for samples with high levels of cyclin D1 mRNA and those samples with normal levels of cyclin D1 mRNA is used to calculate a relative quantity of cyclin D1 mRNA. This value is normalized to a β-globin transcript to adjust for differences in the amount of mRNA present in the sample. The cyclin D1 quantity relative to β-globin andnormalized to the cyclin D1 quantity of follicular hyperplasia was calculated using the following equation:
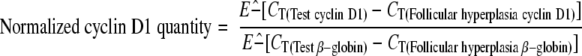 |
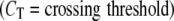 |
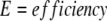 |
The cut-off value for overexpression of cyclin D1 was defined as greater than the highest normalized transcript level for cyclin D1 obtained for reactive follicular hyperplasia.
Figure 2.
Determination of cyclin D1 mRNA expression level normalized to β-globin and relative to follicular hyperplasia. Schematic representation of the cycle shifts using cyclin D1 and β-globin primer/probes. Quantification of mRNA expression is accomplished by analysis of the fluorescence curves from each sample. Samples with a higher pre-amplification target concentration show an earlier cycle threshold. The difference in cycle threshold obtained for samples with high levels of cyclin D1 mRNA and those samples with normal levels of cyclin D1 mRNA is used to calculate a relative quantity of cyclin D1 mRNA transcripts. β-globin is used to normalize for the amount of mRNA present in the sample. The differences in cycle threshold were used to calculate the normalized cyclin D1 mRNA expression level as indicated in the text. ΔC = cycle threshold difference.
Analytical Sensitivity
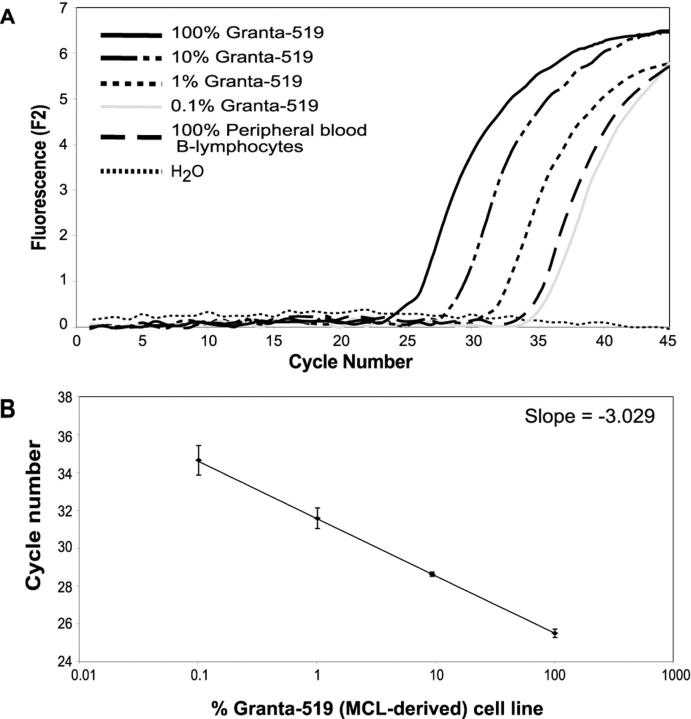
cDNA extracted from the Granta-519 cell line was serially diluted into cDNA obtained from peripheral blood lymphocytes. The samples were analyzed using our fluorescent RT-PCR method and the normalized quantities of cyclin D1 mRNA were calculated (Figure 3) .
Figure 3.
Dilutional analysis for cyclin D1 mRNA quantification using sequence-specific hybridization probes. A: cDNA from the mantle cell lymphoma cell line, Granta-519, was diluted into cDNA obtained from peripheral B-lymphocytes in log dilutions. When Granta-519 cDNA was diluted in peripheral blood lymphocyte-derived cDNA in a 1:10 ratio, it was still possible to demonstrate an earlier fractional cycle for the 10% Granta-519 sample when compared to 100% peripheral blood lymphocyte cDNA. H2O, no template control. B: Standard curve generated using dilutions of Granta-519. The error bars represent the crossing threshold standard deviation (SD) of three replicates for each dilution (100% Granta: SD = 0.22; 10% Granta: SD = 0.12; 1% Granta: SD = 0.54; and 0.1% Granta: SD = 0.78).
Statistical Analyses
The statistical tool included in the Microsoft Excel program (Microsoft, Redwood, WA) was used for the determination of p-values (paired two-tailed Students’ t-test) for the normalized quantities of cyclin D1 in the MCLs in comparison to the CLL/SLLs, and the mean values of normalized quantities of cyclin D1 transcripts.
Results
Immunohistochemical Studies
Immunohistochemical reactivity for cyclin D1 was demonstrated in 8 of 10 MCLs. These immunohistochemically positive samples corresponded to those identified by RT-PCR analysis for cyclin D1 mRNA. No reactivity for cyclin D1 was demonstrable in all other (n = 57) biopsy samples (data not shown).
Quantitative Fluorescence RT-PCR
The hybridization probe assay results are summarized in Tables 1 and 3 . The cyclin D1 mRNA expression level normalized to β-globin and relative to the highest cyclin D1 quantity obtained for a follicular hyperplasia sample was determined as described in the methods section. Samples with a normalized cyclin D1 quantity greater than 1.00 were defined as overexpressing cyclin D1 mRNA. By this criterion, cyclin D1 mRNA overexpression was detected in 8 of 10 MCLs, 1 of 6 T-cell lymphomas, 1 acute promyelocytic leukemia derived cell line (NB-4), and all 4 of 4 non-hematopoietic cell lines. All CLL/SLL cases (n = 11) showed relatively low levels of cyclin D1 transcript expression (generally two to three orders of magnitude less than MCL). Using our method, none of the cases of hairy-cell leukemia (0 of 3) showed elevated cyclin D1 transcript levels (Table 3) .
Table 3.
Cyclin D1 mRNA Expression Normalized to β-globin and Relative to Follicular Hyperplasia: Biopsy Samples
| Diagnosis | Number of samples | % of samples showing cyclin D1 mRNA overexpression | Range of relative normalized cyclin D1 quantity |
|---|---|---|---|
| Mantle cell lymphoma | 10 | 80% | 0.611–62.25 |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma | 11 | 0% | 0.00064–0.395 |
| Hairy cell leukemia | 3 | 0% | 0.0063–0.0105 |
| Follicular lymphoma (grades I & II) | 6 | 0% | 0.066–0.702 |
| Anaplastic large cell lymphoma | 3 | 0% | 0.014–0.0415 |
| Peripheral T-cell lymphoma | 3 | 33% | 0.110–6.63 |
| Other non-Hodgkin’s lymphomas | 6 | 0% | 0.0091–0.165 |
| Precursor acute lymphoblastic leukemia/lymphoma | 15 | 0% | 9.02E-07–0.078 |
| Reactive follicular hyperplasia | 5 | 0% | 0.0204–1.00 |
| Reactive peripheral blood lymphocytes | 6 | 0% | 9.64E-05–0.00012 |
Samples with a normalized cyclin D1 expression level greater than 1.00 were defined as over-expressing cyclin D1 mRNA.
Other Non-Hodgkin’s lymphomas included Burkitt (n = 1), Burkitt-like lymphoma (n = 4), and plasmacytoma (n = 1).
Analytical Sensitivity
Dilutional analysis using Granta-519 cell line cDNA diluted into cDNA obtained from peripheral blood lymphocytes (PBL) revealed that cyclin D1 mRNA expression was higher in a 10% dilution of the Granta-519 cell line cDNA, when compared to the cyclin D1 expression in 100% PBL cDNA (Figure 3) . This suggests that cyclin D1 mRNA overexpression may only be distinguished when the sample analyzed contains at least 10% cyclin D1 overexpressing tumor cells. The within run standard deviation (three replicates each) for the fractional threshold cycles for the cyclin D1 quantitative PCR using the Granta-519 cell line at 100%, 50%, and 10% dilutions into cDNA derived from unstimulated peripheral blood lymphocyte-derived mRNA were 0.22, 0.15, and 0.12, respectively.
Statistical Analyses
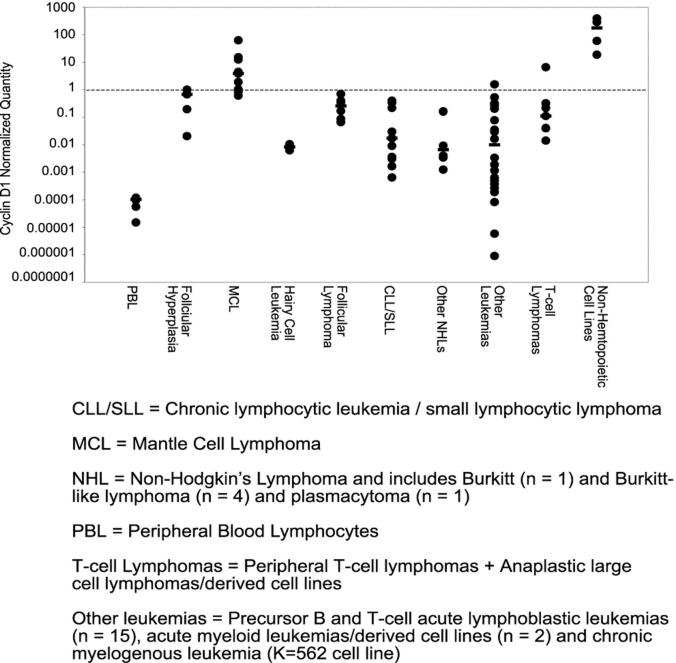
Cyclin D1 mRNA overexpression clearly distinguished MCL from CLL/SLL (p = 0.07) (paired two-tailed Student’s t-tests), but was less discriminatory in the other lymphoproliferative disorders (Figure 4) .
Figure 4.
Comparison of normalized cyclin D1 mRNA expression levels in mantle cell lymphoma and other non-Hodgkin’s lymphomas, leukemias, and cell lines derived from hematopoietic and non-hematopoietic neoplasms. The difference in cycle threshold between the cyclin D1 and β-globin reactions was calculated and then normalized against the cycle threshold difference for reactive hyperplasia. Samples with a normalized cyclin D1 quantity greater than 1.00 (dashed line) were defined as overexpressing cyclin D1 mRNA. The horizontal black bars indicate the median expression level for each sample type.
Discussion
Several methods have been described for the quantitative analysis of nucleic acid sequences. In general, these methods have been based on end-point or competitive PCR analyses measuring band intensities in ethidium bromide stained gels or hybridization-based approaches using radioisotopic labeling and densitometry. 23, 24, 25, 26
Higuchi et al 27, 28 introduced fluorescence monitoring at each cycle for quantitative PCR analysis using ethidium bromide to monitor DNA synthesis. Since then, real-time PCR has gained increasing application in molecular diagnostics. Fluorescence real-time PCR is advantageous over traditional end-point methods in that it permits product quantification in a “kinetic” fashion. Conventional methods, on the other hand, require multiple amplification reactions in several tubes, which are labor intensive and could lead to inaccurate results.
The hybridization probe chemistry used in our current study is advantageous when compared to assays using non-specific double-stranded DNA binding dyes because the labeled probes serve as an additional parameter for verification of the identity of the product amplified by PCR. This is particularly important for cyclin D1 as the gene shares substantial sequence homology to cyclins D2 and D3 20 which could skew the results of such quantitative assessments for cyclin D1 using non-specific dsDNA binding dyes (eg, SYBR Green I). In this study, we sought to address cyclin D1 mRNA expression levels using a real-time fluorescence probe-based PCR method as opposed to conventional methods, which are “end-point” assays. We configured our assay so that both the test and reference genes had comparable amplification efficiencies, thus allowing us to perform quantification of the cyclin D1 mRNA relative to β-globin. Our studies reveal that while cyclin D1 mRNA overexpression distinguishes MCL from CLL/SLL, it may be of less utility in discriminating MCL from rare peripheral T-cell lymphomas (PTCLs). We also show that the mean level of cyclin D1 expression in MCL is two to three orders of magnitude greater than in CLL/SLL. For the purpose of distinguishing MCL from CLL/SLL by cyclin D1 quantification, our results are comparable to those of previous studies that use end-point PCR-based assays. 13
The ability to objectively quantify cyclin D1 expression levels facilitates comparisons with “normal” cellular populations, and the definition of a threshold for increased expression of specific mRNA species. Thus we were able to define a threshold of cyclin D1 expression in reactive follicular hyperplasia above which cyclin D1 overexpression was scorable (Tables 1 and 3) . Hence by our criteria, cyclin D1 overexpression mRNA was detected in 9 of 12 (75%) MCLs and derived cell lines, 1 acute promyelocytic leukemia derived cell line (NB-4), and 1 of 7 (14%) T-cell lymphomas (Figure 4) . Thus, for the ancillary diagnosis of MCL, real-time cyclin D1 mRNA quantification represents an improvement on the detection rates for the t(11;14) by PCR (40%), or immunohistochemical detection of cyclin D1 expression (70% of MCLs) as demonstrated in the study. We believe that the detection rates of our quantitative RT-PCR assay for MCL could be further improved by microdissection, 29, 30 as partial involvement of a lymph node or extra-nodal organs could dilute the detection of cyclin D1 overexpression in the MCL. Conversely, in independent experiments not included in this study, we noted that higher levels of cyclin D1 transcripts were detected in the two reactive tonsils in which tonsillar epithelium was present. This result is consistent with the fact that basal epithelial cells in particular are known to constitutively express cyclin D1. 31
In conclusion, the wide dynamic range of real-time PCR enables simultaneous quantitative analysis of samples with highly different starting concentrations. Our studies show that cyclin D1 mRNA is expressed in a wide range of hematological and non-hematological disorders. We have also shown that quantitative fluorescence RT-PCR for cyclin D1 can be useful in the diagnosis of mantle cell lymphoma. The detection of cyclin D1 mRNA overexpression in the absence of cyclin D1 protein expression in rare cases of PTCL in this study and reported in some cases of hairy cell leukemia 14 suggests that there may be differential post-transcriptional regulation of cyclin D1 expression levels in different cells and tissues.
Address reprint requests to Kojo S. J. Elenitoba-Johnson, M.D., Division of Anatomic Pathology, University of Utah School of Medicine, 50 North Medical Drive, Salt Lake City, UT 84132. E-mail: kojo.elenitobaj@path.utah.edu.
Footnotes
Supported by the ARUP Institute for Clinical and Experimental Pathology and National Institutes of Health grant CA83984.
References
- 1.Banks PM, Chan J, Cleary ML, Delsol G, De Wolf-Peeters C, Gatter K, Grogan TM, Harris NL, Isaacson PG, Jaffe ES, Mason D, Pileri S, Ralfkiaer E, Stein H, Warnke RA: Mantle cell lymphoma: a proposal for unification of morphologic, immunologic, and molecular data. Am J Surg Pathol 1992, 16:637-640 [DOI] [PubMed] [Google Scholar]
- 2.Campo E, Raffeld M, Jaffe ES: Mantle-cell lymphoma. Semin Hematol 1999, 36:115-127 [PubMed] [Google Scholar]
- 3.Harris N, Jaffe E, Stein H, Banks P, Chan J, Cleary M, Delsol G, De Wolf-Peeters C, Falini B, Gatter K, Grogan T, Isaacson P, Knowles D, Mason D, Muller-Hermelink H-K, Pileri S, Piris M, Ralfkiaer E, Warnke R: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Yunis J, Onorato-Showe L, Erikson J, Nowell PC, Croce CM: Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science 1984, 224:1403-1406 [DOI] [PubMed] [Google Scholar]
- 5.Tsujimoto Y, Jaffe E, Cossman J, Gorham J, Nowell PC, Croce CM: Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature 1985, 315:340-343 [DOI] [PubMed] [Google Scholar]
- 6.Vaandrager JW, Schuuring E, Zwikstra E, de Boer CJ, Kleiverda KK, van Krieken JH, Kluin-Nelemans HC, van Ommen GJ, Raap AK, Kluin PM: Direct visualization of dispersed 11q13 chromosomal translocations in mantle cell lymphoma by multicolor DNA fiber fluorescence in situ hybridization. Blood 1996, 88:1177-1182 [PubMed] [Google Scholar]
- 7.Cuneo A, Bigoni R, Rigolin GM, Roberti MG, Bardi A, Piva N, Milani R, Bullrich F, Veronese ML, Croce C, Birg F, Dohner H, Hagemeijer A, Castoldi G: Cytogenetic profile of lymphoma of follicle mantle lineage: correlation with clinicobiologic features. Blood 1999, 93:1372-1380 [PubMed] [Google Scholar]
- 8.Williams ME, Meeker TC, Swerdlow SH: Rearrangement of the chromosome 11 bcl-1 locus in centrocytic lymphoma: analysis with multiple breakpoint probes. Blood 1991, 78:493-498 [PubMed] [Google Scholar]
- 9.Luthra R, Hai S, Pugh WC: Polymerase chain reaction detection of the t(11;14) translocation involving the bcl-1 major translocation cluster in mantle cell lymphoma. Diagn Mol Pathol 1995, 4:4-7 [DOI] [PubMed] [Google Scholar]
- 10.Pinyol M, Campo E, Nadal A, Terol MJ, Jares P, Nayach I, Fernandez PL, Piris MA, Montserrat E, Cardesa A: Detection of the bcl-1 rearrangement at the major translocation cluster in frozen and paraffin-embedded tissues of mantle cell lymphomas by polymerase chain reaction. Am J Clin Pathol 1996, 105:532-537 [DOI] [PubMed] [Google Scholar]
- 11.Zukerberg LR, Yang WI, Arnold A, Harris NL: Cyclin D1 expression in non-Hodgkin’s lymphomas: detection by immunohistochemistry. Am J Clin Pathol 1995, 103:756-760 [DOI] [PubMed] [Google Scholar]
- 12.Vasef MA, Medeiros LJ, Koo C, McCourty A, Brynes RK: Cyclin D1 immunohistochemical staining is useful in distinguishing mantle cell lymphoma from other low-grade B-cell neoplasms in bone marrow. Am J Clin Pathol 1997, 108:302-307 [DOI] [PubMed] [Google Scholar]
- 13.Aguilera NS, Bijwaard KE, Duncan B, Krafft AE, Chu WS, Abbondanzo SL, Lichy JH, Taubenberger JK: Differential expression of cyclin D1 in mantle cell lymphoma and other non-Hodgkin’s lymphomas. Am J Pathol 1998, 153:1969-1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch F, Campo E, Jares P, Pittaluga S, Munoz J, Nayach I, Piris MA, Dewolf-Peeters C, Jaffe ES, Rozman C, Montserrat E, Cardesa A: Increased expression of the PRAD-1/CCND1 gene in hairy cell leukaemia. Br J Haematol 1995, 91:1025-1030 [DOI] [PubMed] [Google Scholar]
- 15.Miranda RN, Briggs RC, Kinney MC, Veno PA, Hammer RD, Cousar JB: Immunohistochemical detection of cyclin D1 using optimized conditions is highly specific for mantle cell lymphoma and hairy cell leukemia. Mod Pathol 2000, 13:1308-1314 [DOI] [PubMed] [Google Scholar]
- 16.Donnellan R, Chetty R: Cyclin D1 and human neoplasia. Mol Pathol 1998, 51:1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosch F, Jares P, Campo E, Lopez-Guillermo A, Piris MA, Villamor N, Tassies D, Jaffe ES, Montserrat E, Rozman C, Cardesa A: PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood 1994, 84:2726-2732 [PubMed] [Google Scholar]
- 18.de Boer CJ, van Krieken JH, Kluin-Nelemans HC, Kluin PM, Schuuring E: Cyclin D1 messenger RNA overexpression as a marker for mantle cell lymphoma. Oncogene 1995, 10:1833-1840 [PubMed] [Google Scholar]
- 19.Williams ME, Nichols GE, Swerdlow SH, Stoler MH: In situ hybridization detection of cyclin D1 mRNA in centrocytic/mantle cell lymphoma. Ann Oncol 1995, 6:297-299 [DOI] [PubMed] [Google Scholar]
- 20.Uchimaru K, Taniguchi T, Yoshikawa M, Asano S, Arnold A, Fujita T, Motokura T: Detection of cyclin D1 (bcl-1, PRAD1) overexpression by a simple competitive reverse transcription-polymerase chain reaction assay in t(11;14)(q13;q32)-bearing B-cell malignancies and/or mantle cell lymphoma. Blood 1997, 89:965-974 [PubMed] [Google Scholar]
- 21.Bhan A: Immunoperoxidase. ed 2 Colvin R Bhan A McCluskey R eds. Diagnostic Immunopathology, 1995, :711-723 Raven Press, New York [Google Scholar]
- 22.Rasmussen R: Quantification on the LightCycler. Meuer S Wittwer CT Nakagawara K eds. Rapid Cycle Real-Time PCR. 2001, :21-34 Germany, Springer, Mannheim [Google Scholar]
- 23.Wang AM, Doyle MV, Mark DF: Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci USA 1989, 86:9717-9721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raeymaekers L: A commentary on the practical applications of competitive PCR. Genome Res 1995, 5:91-94 [DOI] [PubMed] [Google Scholar]
- 25.Ferre F: Quantitative or semi-quantitative PCR: reality versus myth. PCR Methods Appl 1992, 2:1-9 [DOI] [PubMed] [Google Scholar]
- 26.Clementi M, Menzo S, Bagnarelli P, Manzin A, Valenza A, Varaldo PE: Quantitative PCR and RT-PCR in virology. PCR Methods Appl 1993, 2:191-196 [DOI] [PubMed] [Google Scholar]
- 27.Higuchi R, Dollinger G, Walsh PS, Griffith R: Simultaneous amplification and detection of specific DNA sequences. Biotechnology (N Y) 1992, 10:413-417 [DOI] [PubMed] [Google Scholar]
- 28.Higuchi R, Fockler C, Dollinger G, Watson R: Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology (N Y) 1993, 11:1026-1030 [DOI] [PubMed] [Google Scholar]
- 29.Emmert-Buck MR, Bonner RF, Smith PD, Chuaqui RF, Zhuang Z, Goldstein SR, Weiss RA, Liotta LA: Laser capture microdissection. Science 1996, 274:998-1001 [DOI] [PubMed] [Google Scholar]
- 30.Bijwaard KE, Aguilera NS, Monczak Y, Trudel M, Taubenberger JK, Lichy JH: Quantitative real-time reverse transcription-PCR assay for cyclin D1 expression: utility in the diagnosis of mantle cell lymphoma. Clin Chem 2001, 47:195-201 [PubMed] [Google Scholar]
- 31.Liu SC, Sauter ER, Clapper ML, Feldman RS, Levin L, Chen SY, Yen TJ, Ross E, Engstrom PF, Klein-Szanto AJ: Markers of cellproliferation in normal epithelia and dysplastic leukoplakiasof the oral cavity. Cancer Epidemiol Biomarkers Prev 1998, 7:597-603 [PubMed] [Google Scholar]